Abstract
Objective
We investigated the effect of docosahexaenoic acid (DHA) on the invasion and metastasis of ovarian cancer cells (A2780, HO8910, and SKOV-3).
Methods
Cytotoxicity assay was performed to determine the optimal doses of DHA in this experiment. The effects of DHA on invasion ability were assessed by invasion assay. The expressions of messenger RNA and/or proteins associated with invasion or metastasis were detected by quantitative Real Time-Polymerase Chain Reaction or Western blot. The effect of DHA on cell metastasis was assessed in xenograft model of zebrafish.
Results
Docosahexaenoic acid and α-linolenic acid could reduce the cell vitalities in dose-dependent manner. However, DHA inhibited the invasion and metastasis of ovarian cancer cells, but α-linolenic acid did not (**P < 0.01). Docosahexaenoic acid could downregulate the expressions of WAVE3, vascular endothelial cell growth factor, and MMP-9, and upregulate KISS-1, TIMP-1, and PPAR-γ, which negatively correlated with cell invasion and metastasis (*P < 0.05). Docosahexaenoic acid restrained the development of subintestinal vessels and cancer cell metastasis in xenograft model of zebrafish (**P < 0.01).
Conclusions
Docosahexaenoic acid inhibited the invasion and metastasis of ovarian cancer cells in vitro and in vivo through the modulation of NF-κB signaling pathway, suggesting that DHA is a promising candidate for ovarian cancer therapy.
Key Words: Docosahexaenoic acid, Invasion, Metastasis, Ovarian cancer, Zebrafish
Ovarian cancer is the most lethal gynecological malignancy. Because the symptoms of ovarian cancer are not obvious, it is not easy to be detected in the early stage. Approximately 70% of patients are diagnosed at advanced stage,1 which raised challenge to the subsequent therapy. One of the main characteristics of ovarian cancer is easy metastasis and recurrence even after the tumor tissue was removed, which severely worsened the prognosis of patients. Despite of the improvements of treatment methods in recent years, patients with advanced ovarian cancer still lack effective therapy. So suppression of tumor metastasis and recurrence might be a promising therapeutic strategy for improving living quality of these patients. Many researches are concentrating on effective therapy methods through adjusting lifestyle, such as nutrient supplement.2
Epidemiological studies show that a diet rich in ω-3 polyunsaturated fatty acids (ω-3 PUFAs) was correlated with reduced risk of cancers.3–5 ω-3 PUFAs include α-linolenic acid (ALA), docosahexaenoic acid (DHA), and eicosapentaenoic acid. They are essential fatty acids and the main component of cell membrane phospholipids. They cannot be synthesized by mammals and must be obtained from dietary sources, such as cold-water fish, certain seeds (flax), and nuts (walnuts). Previous studies reported that DHA could inhibit the invasion and metastasis of a variety of cancer cells, such as hepatocellular carcinoma,2,6,7 breast cancer,8–10 colorectal cancer,11 pancreatic cancer,12 and stomach cancer.13 However, the effects of DHA on invasion and metastasis in ovarian cancer were scarcely reported. In this study, we investigated the effect of DHA on in vitro and in vivo inhibition of invasion and metastasis in 3 kinds of ovarian cancer cells (A2780, HO8910, and SKOV-3).
MATERIALS AND METHODS
Cell, Chemicals, and Reagents
The human ovarian cancer cell lines A2780, HO8910, and SKOV-3 were purchased from Enogene Biotech Co, Ltd (Jiangsu, China). The 1640 medium (RPMI 1640) and fetal bovine serum (FBS) was purchased from Gibco (United States). A2780, HO8910, and SKOV-3 were routinely cultivated in RPMI 1640 medium supplied with 10% FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin. Cells were routinely grown in a 75-mm flask at 37°C in a humidified environment containing 5% CO2. Horseradish peroxidase–linked secondary antibody was purchased from Nanjing Enogene Biotech Co, Ltd (Jiangsu, China). Matrigel was purchased from BD Biosciences (United States). α-Linolenic acid, DHA, and DMSO were obtained from Sigma-Aldrich (St Louis, MO). RT-PCR primers were designed and synthesized by GenScript Co, Ltd (Nanjing, China). CM-DiI (Invitrogen, United States), 1-phenyl-2-thiourea (PTU; Sigma, United States), and polyvinyl pyrrolidone (PVP; BioDee, Japan) were used according to manufacturer.
Cell Viability Assay
To determine the optimal doses of ALA and DHA on A2780, HO8910, and SKOV-3, viability assay was performed by cell counting kit-8 (CCK-8) in these 3 kinds of ovarian cancer cell lines. Briefly, cells at logarithmic phase were seeded into 96-well plates (1000 cells/well). After 24-hour incubation, the medium was removed and then treated with fresh medium containing different doses of ALA and DHA, each concentration was replicated in 3 wells. α-Linolenic acid was used as isotype control. After 72-hour treatment, cells were incubated in medium containing 10 μL CCK-8 at 37°C for 4 hours, and the colorimetric change was measured with an enzyme-linked immunosorbent assay reader at 450 nm. The absorbance in the control group was regarded as 100% cell viability. The results were expressed as the percentage of inhibition in the form of absorbance. The maximum safe dosage for ALA and DHA (survival rates of cancer cells were more than 95%) was used as optimal dose of ALA and DHA in 3 cancer cells.
Invasion Assays
The effects of ALA and DHA on the invasion ability of 3 kinds of ovarian cancer cells were determined by invasion assays using 24-well transwell chamber (8 μm, BD Biosciences), coated with 50 μL Matrigel. A2780 cells were pretreated with 64 μM ALA, 32 μM and 64 μM DHA, HO8910 cells with 120 μM ALA, 60 μM, and 120 μM DHA, and SKOV-3 cells with 60 μM ALA, 30 μM, and 60 μM DHA for 72 hours, respectively. α-Linolenic acid was used as isotype control. Two hundred microliters of serum-free RPMI 1640 medium containing 4 × 104 A2780 cells was placed in the upper chamber, and 600 μL of RPMI 1640 supplemented with 10% FBS was added to the lower chamber. The plates were incubated at 37°C in 5% CO2 incubator for 48 hours. Then cells on the upper side of the filters were removed with cotton-tipped swabs, and the filters were washed with Phosphate buffer saline. Cells were fixed in 4% paraformaldehyde for 15 minutes and stained with 0.05% crystal violet for 30 minutes. Cells on the lower surface of the filters were counted from 4 fields at 200 magnification under a microscope. The invasive abilities of DHA on HO8910 and SKOV-3 cells were determined according to the same method, but the migrated cells were counted from 4 fields at 100 magnification. Each experiment was repeated at least 3 times. The cell line with the strongest invasive ability among the 3 cell lines was used to do the subsequent in vivo test.
RNA Extraction and Quantitative RT-PCR Analysis
To investigate the effect of DHA on cancer cell invasion after DHA treatment, some genes associated with invasion were chosen and performed by RT-PCR.14–20 Cells treated with ALA were use as a control. After 3 kinds of ovarian cancer cells were pretreated with DHA for 72 hours, total RNA from cells was extracted using RNAiso Reagent (Takara, Japan). Concentrations of RNA were determined and quantified by measuring the absorbance at 260 and 280 nm on a spectrophotometer. Complementary cDNA was synthesized from total RNA using the PrimeScript RT Reagent Kit (TaKaRa, Dalian, China) following the manufacturer’s instructions. A real-time PCR analysis was performed using the StepOnePlus Real-Time PCR System (Applied Biosystems, United States) with SYBR Premix Ex Taq II (TaKaRa, Dalian, China) to determine each messenger RNA (mRNA) expression. The cycling conditions were as follows: 95°C for 10 minutes, followed by 40 cycles including 95°C for 15 seconds and 60°C for 1 minute. GAPDH was used as the reference gene. The relative levels of gene expression were represented as ΔCt = Ct gene − Ct reference, and the fold change of gene expression was calculated by the 2-ΔΔCT method. Experiments were repeated in triplicate. The primer sequences for KISS-1, vascular endothelial cell growth factor (VEGF), MMP-9, TIMP-1, WAVE3, PPAR-γ, and GAPDH are listed as follows:
KISS-1 forward, 5′-GCTTCTCCTCTGTGTGGCCTC-3′
KISS-1 reverse, 5′-AGCCCCAGGCTTGCTCTC-3′
VEGF forward, 5′-GCAGGGGACAGAGGGACTTG-3′
VEGF reverse, 5′-GAGGCCATCGCTGCACTCA-3′
MMP-9 forward, 5′-CCTCTGGAGGTTCGACGTGA-3′
MMP-9 reverse, 5′-TAGGCTTTCTCTCGGTACTGGAA-3′
TIMP-l forward, 5′-CTGGCTTCTGGCATCCTGTT-3′
TIMP-l reverse, 5′-CCCTAAGGCTTGGAACCCTTT-3′
WAVE3 forward, 5′-TGCCTTTAGTGAAGAGGAACA-3′
WAVE3 reverse, 5′-ATTCGAATAGCAGCGAGGAG-3′
PPAR-γ forward, 5′-GACCACTCCCACTCCTTTGA-3′
PPAR-γ reverse, 5′-AGGCTCCACTTTGATTGCAC-3′
GAPDH forward, 5′-AGGTGAAGGTCGGAGTCAAC-3′
GAPDH reverse, 5′-CGCTCCTGGAAGATGGTGAT-3′
Western Blot Analysis
According to the changes in gene expression, some proteins associated with cell invasion were detected by Western blot. A2780 cells were treated with 32 and 64 μM DHA; HO8910 cells were treated with 60 and 120 μM DHA; and SKOV-3 cells were treated with 30 and 60 μM DHA. After 72 hours, cells were washed with ice-cold PBS and cracked in RIPA lysis buffer (Beyotime, Haimen, China) containing protease inhibitor (Complete, Roche Diagnostics, Mannheim, Germany). Cell lysate was centrifuged at 13,000 rpm for 10 minutes at 4°C; the supernatant was collected and boiled at 100°C for 10 minutes. Protein concentrations were measured using the BCA protein assay kit. Then, 50 μg of proteins was electrophoresed in SDS-PAGE, and transferred onto PVDF (polyvinylidene difluoride) membranes in a wet-transfer apparatus. Membranes were blocked with 5% bovine serum albumin in TBS-Tween for 1 hour and then incubated with a specific primary antibody (VEGF, COX-2, MMP-3, MMP-9, N-Ca, WAVE3, STAT3, and NF-κB) overnight at 4°C. Membranes were washed 3 times with TBS-Tween (TBS containing 0.1% Tween 20) and incubated with horseradish peroxidase–conjugated secondary antibodies for 1 hour at room temperature, and blots were developed with enhanced chemiluminescence reagents (PerkinElmer Life Sciences, Waltham, MA) and exposed to x-ray film. The expression levels were normalized to GAPDH.
Zebrafish Model
Transgenic zebrafishes (fli-1:EGFP) were purchased from the Model Animal Research Center of Nanjing University, raised by strict control of light and temperature in the laboratory. Day and night time ratio is 14:10 hours. The pH is 7.0 ± 0.2, and temperature is approximately 28.0°C ± 1.0°C in water. Zebrafishes were fed with brine shrimp once and prawn crackers twice every day. Adult transgenic zebrafishes younger than 1 year were used in the experiments. The day before fertilization, individual female zebrafishes were placed in mating tanks with 2 males. The next morning, mating was initiated by light stimuli, followed by collection of fertilized eggs. The eggs were incubated in egg water (0.2 g/L Instant Ocean Salt in demi water) at 28.5°C. All protocols were approved by the Institutional Animal Care and Use Committee at Nanjing Tech University.
DHA Microinjection
At 1 day after fertilization (dpf), embryos were positioned on a 10-cm Petri dish coated with 1% agarose. One-microgram ALA and DHA preparations were injected into the yolk sac of transgenic zebrafishes, respectively. Transgenic zebrafishes injected with the same dose of PBS was used as a control. At 2 dpf, images were taken using fluorescence microscope to examine the effects of ALA and DHA on the growth of the subintestinal vessels of zebrafishes. Angiogenic perimeter (pixel) was measured using the ImagePro plus software, and vascular branch number was calculated. Ten embryos per group were used in our study, and each experiment was carried out in 3 independent replicates.
Human Ovarian Cancer Cell Implantation
HO8910 cells were pretreated with 120 μM ALA and DHA for 72 hours, respectively. Before microinjection, cells were incubated with cell tracker CM-Dil at a final concentration of 2.5 μg/mL for 5 minutes at 37°C, then followed by 15 minutes at 4°C. Cells were washed twice to remove unincorporated dye with PBS and resuspended with 2% PVP as described previously (2 × 107 cells/mL).21 In general, PVP is used as pharmaceutic adjuvant to prevent cell clumping and needle clogging. At 2 dpf, embryos were anesthetized with 0.003% tricaine (Sigma-Aldrich Corp) and positioned on a 10-cm Petri dish coated with 1% agarose. Then the avascular region of the yolk sac was injected with 10 nL cell suspension containing approximately 200 cells using a glass needle, Narishige IM-31 injection system (Narishige, Tokyo, Japan) under the dissecting microscope (Nikon SMZ745, Japan). The glass needles used to inject the cells were prepared from G-100 glass capillaries (Narishige, Tokyo, Japan) using a P-97 flaming/brown micropipette puller (Sutter Instrument Co, United States). Injection of 2% PVP was used as a control. After the implantation, zebrafishes were raised by standard methods as described previously,22,23 and the embryos were kept in egg water at 32°C containing 1% PTU to inhibit melanin production. This temperature was chosen as an intermediate between 37°C (optimal cells temperature) and 28°C (optimal fishes temperature), which allowed for normal development of the embryo and not impaired ovarian cancer cells. At 1 dpi, embryos with cells injected into the circulation were discarded. At 5 dpi, images were taken using fluorescence microscope (IX71, Olympus, Japan). The red florescence of ovarian cancer cells was examined to determine metastasis. We used 30 embryos per group in our study, and each experiment was carried out in 3 independent replicates.
Statistical Analysis
The measurement data were presented as mean ± SD and were processed by using GraphPad Prism 5.0 (GraphPad Software Inc, San Diego, CA) and SPSS 17.0 statistical software package (SPSS, Illinois). Intergroup differences were analyzed by t test, and multiple comparisons among several groups were conducted by analysis of variance. *P < 0.05 was considered to be statistically significant.
RESULTS
The Effects of DHA on Cell Viability in A2780, HO8910, and SKOV-3
As shown in Figure 1, significant inhibition was noted in A2780, HO8910, or SKOV-3 after treatment with ALA and DHA, and in a dose-dependent manner. In fact, the ratio of 50% inhibition was approximately at a concentration of 128 μM of ALA and DHA in A2780 and SKOV-3 cell lines except HO8910 whose proliferation was 50% inhibited at 256 μM. It is seemed that HO8910 was resistant to the inhibitory effects of ALA and DHA and need a much higher concentration of ALA and DHA compared with A2780 and SKOV-3. The maximum safe dosage for ALA and DHA in HO8910 was 120 μM (survival rates of cancer cells were more than 95%). According to the low growth-inhibitory effect, A2780 cells pretreated with 64 μM ALA, 32 μM and 64 μM DHA, HO8910 cells with 120 μM ALA, 60 μM, and 120 μM DHA, and SKOV-3 cells with 60 μM ALA, 30 μM, and 60 μM DHA for 72 hours were selected in subsequent experiment, respectively.
FIGURE 1.
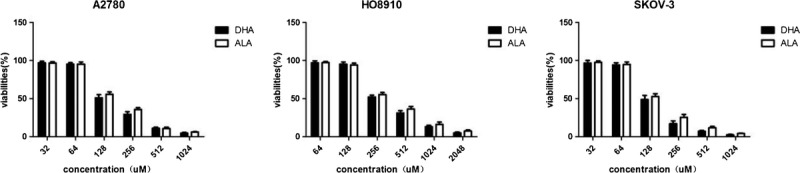
The cytotoxicity of ALA and DHA in A2780, HO8910, and SKOV-3. The effects of ALA and DHA on cell viability in A2780, HO8910, and SKOV-3 were determined by CCK-8 assay. Each column shows cell viability (%) mean ± SD of 3 independent experiments performed in triplicate. ALA was used as isotype control.
DHA Inhibited Invasive Abilities of Ovarian Cancer Cell
The invasion assay was performed to test the effects of ALA and DHA on 3 kinds of ovarian cancer cells. A2780, HO8910, and SKOV-3 cells were pretreated with 64 μM, 120 μM, and 60 μM of ALA and 32/64 μM, 60/120 μM, and 30/60 μM of DHA and 0.1% DMSO for 72 hours, respectively. Cells were considered to be invasive if they could get through the layer of Matrigel. As shown in Figure 2, HO8910 showed the strongest aggressiveness with maximum number of cells (approximately 133 cells), but A2780 showed the weakest invasion ability (approximately 50 cells) among 3 kinds cell lines. Therefore, HO8910 was chosen to do the subsequent in vivo metastasis experiment. With the increasing concentrations of DHA, the number of cells got through the Matrigel was obviously decreased in 3 kinds of cell lines (P < 0.005). However, compared with 0.1% DMSO groups, there were not significantly differences in 3 kinds of cells treated with the same concentration of ALA (P > 0.05). It is seemed that DHA suppressed the invasive abilities of ovarian cancer cells in dose-dependent manner.
FIGURE 2.

The effect of ALA and DHA on invasion abilities in A2780, HO8910, and SKOV-3 cells. A2780, HO8910, and SKOV-3 cells were pretreated with ALA (64 μM, 120 μM, and 60 μM), DHA (32/64 μM, 60/120 μM, and 30/60 μM), or without DHA for 72 hours, respectively. Images of A2780 were acquired at 200 magnification, and images of HO8910 and SKOV-3 were acquired at 100 magnification under an inverted microscope. Each column shows the mean of 3 independent experiments performed in triplicate. The asterisks labeled above the error bars indicated significant differences compared with the group treated with ALA or 0.1% DMSO (***P < 0.005 compared with the control). ALA was used as isotype control.
DHA Downregulated mRNA Expression or Proteins Expression Associated with Invasion and Metastasis in Human Ovarian Cancer Cells
To qualitatively detect the effects of DHA on mRNA or proteins expression associated with invasion and metastasis, we investigated mRNA or proteins associated with invasion and metastasis using cancer cells treated with DHA (32/64 μM, 60/120 μM, and 30/60 μM) for 72 hours, respectively. As shown in Figure 3A, compared with the control, the levels of genes expression associated with invasion and metastasis (VEGF, WAVE3, MMP-9) were decreased, but anti-invasion or metastasis genes (KISS-1, PPAR-γ, TIMP-1) were increased obviously. As shown in Figure 3B, compared with 0.1% DMSO group, the level of expression (NF-κB, VEGF, MMP-9, MMP-3, STAT3, WAVE3, COX-2) was low in A2780, HO8910, or SKOV-3 treated with DHA, respectively, except N-Ca protein, though the expression of some proteins were not detected (the blanks were shown).
FIGURE 3.

The effect of DHA on expression of genes or proteins associated with invasion and metastasis (A) A2780, HO8910, and SKOV-3 cells were respectively pretreated with (64 μM, 120 μM, and 60 μM) or without DHA for 72 hours. RT-PCR analysis was used to assess the relative expression of KISS-1, VEGF, MMP-9, TIMP-1, WAVE3, and PPAR-γ. The fold change of gene expression was calculated by the 2-ΔΔCT method. GAPDH was used as the reference gene. Cells with ALA treatment were used as the respective control (***P < 0.005, **P < 0.01, *P < 0.05). B, A2780, HO8910, and SKOV-3 cells were pretreated with DHA (32 and 64 μM, 60 and 120 μM, 30 and 60 μM) for 72 hours, respectively. Western blot analysis was performed using antibodies against NF-κB, VEGF, MMP-9, MMP-3, STAT3, WAVE3, COX-2, and N-Ca. The expressions of some genes or proteins were not detected by RT-PCR or Western bolt (the blanks were shown).
DHA Inhibited the Development of Subintestinal Vessels in Transgenic Zebrafish
To elucidate the vascular development affected by DHA, angiogenesis, which was considered to be an essential element of solid tumor metastasis, was assessed. We examined the changes in subintestinal vessels after ALA and DHA treatment by transgenic zebrafishes. The development of subintestinal vessels of transgenic zebrafishes was significantly suppressed at 48 hpi after microinjection with DHA. Either the vascular perimeter or the vascular branch number in the DHA group was less than that in the control group (P < 0.01). However, this inhibitory effect was not obvious in the ALA group (Fig. 4).
FIGURE 4.

The effects of ALA and DHA on the development of vascular system in transgenic zebrafishes. One microgram of ALA and DHA was injected into the yolk sac of transgenic zebrafish at 24 hpf, respectively. The vascular branch number and the vascular perimeter were calculated in each fish at 48 hpi. Results showed 1 of 3 independent experiments (**P < 0.01, *P < 0.05 compared with the control). The same dose of PBS was used as a control. ALA was used as isotype control.
DHA Inhibited Metastasis of HO8910 Cells in Model of Transgenic Zebrafishes
To further elucidate the effect of ALA and DHA on metastasis of human ovarian cancer cells, the aggressive ability of HO8910 cells was assayed. We evaluated the optimal number (200 cells injected per fish). The cancer cell dissemination was considered as migration if the cells were beyond the boundaries with the heart cavity frontally, on top of the swim bladder dorsally and beyond the urogenital opening caudally. After 5 days, approximately 40% of the zebrafishes injected by HO8910 cells displayed metastasis phenotype, and approximately 35% of the zebrafishes injected by HO8910 cells pretreated with 120 μM ALA displayed metastasis phenotype (Fig. 5B). As shown in Figure 5A, HO8910 cells untreated with DHA migrated to the head, blood vessels, and the tail part; while pretreated with 120 μM DHA for 72 hours, cells were jailed in the injection site or within the boundaries without migration phenotype. The 5 days survival rate of zebrafishes injected cells were both approximately 85% in groups treated with or without ALA or DHA, however, 90% in 2% PVP group. There was no statistically significant difference in survival rate among groups; however, there was statistically significant difference in the metastasis rate between the groups treated with or without DHA (P < 0.005) (Fig. 5B).
FIGURE 5.
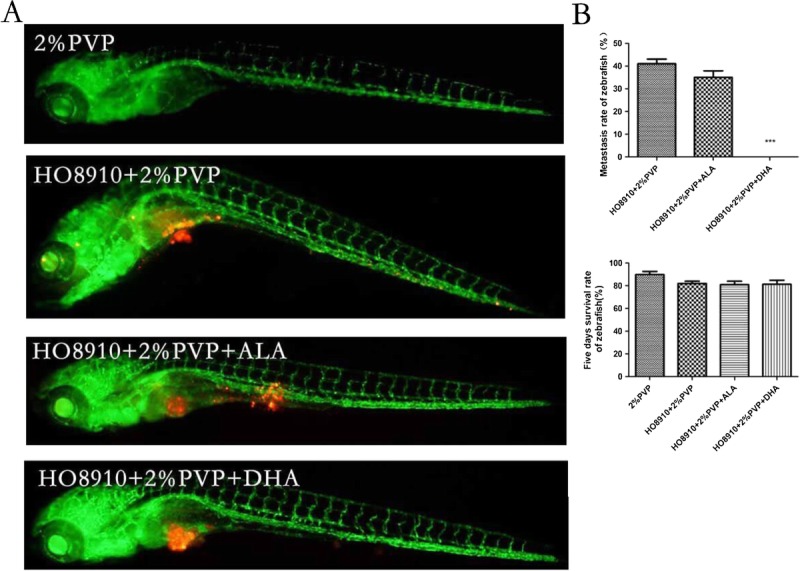
The effects of ALA and DHA on metastasis of HO8910 cells in model of transgenic zebrafishes. A, 200 cells were injected into the yolk sac of transgenic zebrafishes at 48 hpf per fish, which were pretreated with 120 μM ALA and DHA for 72 hours. A fluorescence microscopy was used to observe dissemination of HO8910 cells in zebrafishes 5 dpi. Green fluorescence shows vasculature of transgenic zebrafish, red fluorescence representing HO8910 cells. B, The metastasis rate and survival rate of zebrafishes was shown by histogram. These experiments were performed in triplicate, and the results showed 1 of 3 independent experiments (***P < 0.005 compared with the control). ALA was used as isotype control. Two percent PVP was used as pharmaceutic adjuvant to prevent cell clumping and needle clogging, and also as a control.
DISCUSSION
Cancer invasion is started and maintained by signaling pathways that control cytoskeletal dynamics in tumor cells.24 It is also the first step of cancer metastasis, suggesting that effective inhibition of tumor cells invasion can also effectively inhibit tumor metastasis.25 In the present study, we observe that DHA administration could significantly reduce the number of cells that got through the basement membrane in a dose-dependent manner (Fig. 2). All of these suggest that DHA had a beneficial effect on inhibition of invasion in human ovarian cancer cells.
Tumor metastasis, the spread of cancer from the primary tissue or origin and subsequent growth in distant organs or tissues,25 is recognized to be one of the major causes of failure in cancer therapy. Vascular growth is often regulated by angiogenic cytokines, growth factors, and integrins.26 Vascular endothelial cell growth factor plays an important role in endothelial cell proliferation, angiogenesis, tumor invasion, tumor metastasis, and often highly expressed in many malignant tumors, such as gastric cancer, bladder cancer, and ovarian cancer. In this study, we find that DHA administration could suppress both VEGF mRNA and/or protein expression of A2780 and HO8910 (Fig. 3). Our results suggest that DHA might directly or indirectly affect invasion ability of A2780 and HO8910 through downregulation expression of VEGF gene or protein.
It is known that KISS-1 is an important tumor metastasis suppressor gene in a variety of cancer cells.14 Overexpression of KISS-1 reduces invasion and migration of breast cancer cells15 and loss of KISS-1 results in metastasis.16 KISS-1 can also inhibit tumor metastasis through inhibiting MMP-9 activity by decreasing NF-κB expression, which binds to the MMP-9 promoter.27 NF-κB, a transcription factor widely existing in organisms, is involved in cell proliferation, inflammation, and tumor invasion and metastasis. NF-κB can promote cancer invasion and metastasis by inducing tumor cell Epithelial Mesenchymal Transformation28 and upregulate angiogenic factors expression.29 The key changes in this process are the downregulation of the E-cadherin expression and upregulation of the N-cadherin expression.30 Gravaghi et al reported that DHA could suppress tumor cells invasion and metastasis through reducing the NF-κB mRNA transcription and protein expression.31 Stöcker et al also showed that DHA could inhibit tumor invasion and metastasis through downregulation COX-2 expression, downstream target of NF-κB.32 In this study, the KISS-1 is upregulated in 3 kinds of ovarian cancer cells (Fig. 3A). In the NF-κB protein and its downstream target COX-2, E-Ca protein expression is downregulated in both HO8910 and SKOV-3 treated with DHA for 72 hours (Fig. 3B). With the increasing of DHA concentration, the expression of N-Ca protein is increased in SKOV-3 and HO8910. It suggests that DHA may inhibit ovarian cancer cell invasion and metastasis through upregulating KISS-1 and downregulating NF-κB.
MMP-9 is also a major NF-κB target gene and is one of the most crucial members of the family of MMPs, which are named for their dependence on metal ions for catalytic activity and their ability to degrade ECM,33 which can promote EMT, activate growth factor receptor, and promote angiogenesis to facilitate the migration and invasion of cancer cells.17 Furthermore, DHA could downregulate the MMP-9 expression or enzyme activity to inhibit prostate cancer cells invasion and metastasis, in a dose-dependent fashion.18 In our study, we find that the expression of TIMP-1 of A2780 and HO8910 was significantly upregulated after treatment with DHA for 72 hours (Fig. 3A), but MMP-9 expression of gene or protein was downregulated. It is seemed that DHA inhibited invasion and metastasis of ovarian cancer through increasing expression of TIMP-1 (MMP-9 inhibitor) or inhibiting expression of MMP-9, as a result of downregulation of upstream signaling molecule NF-κB. Furthermore, we find that the WAVE3 gene and protein expressions were downregulated in A2780 treated with DHA (Fig. 3).
Various animal models have been established to research the role of drug on antiangiogenesis and antitumor invasion and metastasis before the advent of zebrafishes model. The most common application model is the transgenic zebrafish, in which vascular endothelial cells were transfected with green fluorescent protein (fil-1:EGFP).19 Its blood vessel system shows green fluorescence observation under the fluorescence microscope. Using the advantage of the optical transparency of zebrafish, we can detect tumor cell progression within 1 week after injection and provide live imaging of fluorescently labeled cancer cells.20,34 In this study, compared with the control group, the development of subintestinal vessels is significantly inhibited in terms of branch number and angiogenic perimeter in transgenic zebrafishes injected with DHA (Fig. 4). When the dose of DHA is reduced to 500 ng or observation time is shorted to 24 hours, there is no obvious change in subintestinal vessel development between the 2 groups treated with or without DHA, suggesting that DHA inhibits angiogenesis in dose- and time-dependent manner.
We find that there is no metastasis phenotype in our zebrafishes model, meanwhile HO8910 cells continued to jail in the injection site after treatment with 120 μM DHA for 72 hours (Fig. 5A). However approximately 40% of zebrafishes show metastasis phenotype (injected cells was transferred to head, tail, and blood vessel) in zebrafishes injected HO8910 without DHA (Fig. 5B). It demonstrates that DHA could significantly inhibit the metastasis of HO8910 in zebrafishes.
In conclusion, DHA inhibits angiogenesis, invasion, and metastasis of ovarian cancer cells in vitro and in vivo, but ALA (another important member of ω-3 PUFAs family) does not inhibit in the study. The inhibition effect is modulated via NF-κB signaling pathways directly or indirectly, as well as other signaling molecules, such as VEGF and MMP-9. However, these in vitro and in vivo studies cannot accurately show the integrative nature of DHA in human. Further studies should be carried out to verify the antitumor activity of DHA in clinic experiments rather than data obtained from zebrafish. Next, we are going to test levels of ω-3 polyunsaturated fatty acids, particularly DHA, stored in adipose tissue of patients before treatment. As previously mentioned, diet is the main supply source of ω-3 PUFA to tissues. Because adipose tissue is a biomarker of the past 2 to 3 years of intake of dietary lipids, it was hypothesized that supplementing patients with these lipids could improve treatment outcome.
Footnotes
Supported by grants from National Science Foundation (81473636); Jiangsu Government Scholarship “333” Plan, Jiangsu Key Medical Personnel (RC2011091); and Chinese Post-Doctor Program (2013M542578).
The authors declare no conflicts of interest.
REFERENCES
- 1.Ozols RF. Treatment goals in ovarian cancer. Int J Gynecol Cancer. 2005;15(Suppl 1):3–11. [DOI] [PubMed] [Google Scholar]
- 2.Hagi A, Nakayama M, Miura Y, et al. Effects of a fish oil-based emulsion on rat hepatoma cell invasion in culture. Nutrition. 2007;23:871–877. [DOI] [PubMed] [Google Scholar]
- 3.Khankari NK, Bradshaw PT, Steck SE, et al. Polyunsaturated fatty acid interactions and breast cancer incidence: a population-based case-control study on Long Island, New York. Ann Epidemiol. 2015;25:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiyabu GY, Inoue M, Saito E, et al. Fish, n - 3 polyunsaturated fatty acids and n - 6 polyunsaturated fatty acids intake and breast cancer risk: the Japan Public Health Center-based prospective study. Int J Cancer. 2015;137:2915–2926. [DOI] [PubMed] [Google Scholar]
- 5.Song M, Chan AT, Fuchs CS, et al. Dietary intake of fish, ω-3 and ω-6 fatty acids and risk of colorectal cancer: a prospective study in U.S. men and women. Int J Cancer. 2014;135:2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun SN, Jia WD, Chen H, et al. Docosahexaenoic acid (DHA) induces apoptosis in human hepatocellular carcinoma cells. Int J Clin Exp Pathol. 2013;6:281–289. [PMC free article] [PubMed] [Google Scholar]
- 7.Brown MD, Hart C, Gazi E, et al. Influence of omega-6 PUFA arachidonic acid and bone marrow adipocytes on metastatic spread from prostate cancer. Br J Cancer. 2010;102:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandal CC, Ghosh-Choudhury T, Yoneda T, et al. Fish oil prevents breast cancer cell metastasis to bone. Biochem Biophys Res Commun. 2010;402:602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanckaert V, Ulmann L, Mimouni V, et al. Docosahexaenoic acid intake decreases proliferation, increases apoptosis and decreases the invasive potential of the human breast carcinoma cell line MDA-MB-231. Int J Oncol. 2010;36:737–742. [DOI] [PubMed] [Google Scholar]
- 10.Andrade-Vieira R, Han JH, Marignani PA. Omega-3 polyunsaturated fatty acid promotes the inhibition of glycolytic enzymes and mTOR signaling by regulating the tumor suppressor LKB1. Cancer Biol Ther. 2013;14:1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasano E, Serini S, Piccioni E, et al. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim Biophys Acta. 2012;1822:1762–1772. [DOI] [PubMed] [Google Scholar]
- 12.D’Eliseo D, Manzi L, Merendino N, et al. Docosahexaenoic acid inhibits invasion of human RT112 urinary bladder and PT45 pancreatic carcinoma cells via down-modulation of granzyme B expression. J Nutr Biochem. 2012;23:452–457. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Meng X, Han J, et al. Anti-cancer activity of DHA on gastric cancer–an in vitro and in vivo study. Tumour Biol. 2013;34:3791–3800. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Berk M, Singh LS, et al. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin Exp Metastasis. 2005;22:369–376. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57:2384–2387. [PubMed] [Google Scholar]
- 16.Lee JH, Miele ME, Hicks DJ, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. [DOI] [PubMed] [Google Scholar]
- 17.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. [DOI] [PubMed] [Google Scholar]
- 18.Li CC, Hou YC, Yeh CL, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid on prostate cancer cell migration and invasion induced by tumor-associated macrophages. PLoS One. 2014;9:e99630. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Lin C, Wu M, Dong J. Quercetin-4′-O-β-D-glucopyranoside (QODG) inhibits angiogenesis by suppressing VEGFR2-mediated signaling in zebrafish and endothelial cells. PLoS One. 2012;7:e31708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He S, Lamers GE, Beenakker JW, et al. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J Pathol. 2012;227:431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spaink HP, Cui C, Wiweger MI, et al. Robotic injection of zebrafish embryos for high-throughput screening in disease models. Methods. 2013;62:246–254. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C, Wang X, Zhao Y, et al. A novel xenograft model in zebrafish for high-resolution investigating dynamics of neovascularization in tumors. PLoS One. 2011;6:e21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao C, Yang H, Shi H, et al. Distinct contributions of angiogenesis and vascular co-option during the initiation of primary microtumors and micrometastases. Carcinogenesis. 2011;32:1143–1150. [DOI] [PubMed] [Google Scholar]
- 24.Ou Y, Zheng X, Gao Y, et al. Activation of cyclic AMP/PKA pathway inhibits bladder cancer cell invasion by targeting MAP4-dependent microtubule dynamics. Urol Oncol. 2014;32:47.e21–48.e21. [DOI] [PubMed] [Google Scholar]
- 25.Caino MC, Chae YC, Vaira V, et al. Metabolic stress regulates cytoskeletal dynamics and metastasis of cancer cells. J Clin Invest. 2013;123:2907–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunapuli P, Chitta KS, Cowell JK. Suppression of the cell proliferation and invasion phenotypes in glioma cells by the LGI1 gene. Oncogene. 2003;22:3985–3991. [DOI] [PubMed] [Google Scholar]
- 28.Ahmad A, Biersack B, Li Y, et al. Targeted regulation of PI3K/Akt/mTOR/NF-κB signaling by indole compounds and their derivatives: mechanistic details and biological implications for cancer therapy. Anticancer Agents Med Chem. 2013;13:1002–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuo Y, Sawai H, Ochi N, et al. Proteasome inhibitor MG132 inhibits angiogenesis in pancreatic cancer by blocking NF-kappaB activity. Dig Dis Sci. 2010;55:1167–1176. [DOI] [PubMed] [Google Scholar]
- 30.Takai M, Terai Y, Kawaguchi H, et al. The EMT (epithelial-mesenchymal-transition)-related protein expression indicates the metastatic status and prognosis in patients with ovarian cancer. J Ovarian Res. 2014;7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novak TE, Babcock TA, Jho DH, et al. NF-kappa B inhibition by omega -3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol. 2003;284:L84–L89. [DOI] [PubMed] [Google Scholar]
- 32.Gravaghi C, La Perle KM, Ogrodwski P, et al. Cox-2 expression, PGE(2) and cytokines production are inhibited by endogenously synthesized n-3 PUFAs in inflamed colon of fat-1 mice. J Nutr Biochem. 2011;22:360–365. [DOI] [PubMed] [Google Scholar]
- 33.Stöcker W, Grams F, Baumann U, et al. The metzincins—topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci. 1995;4:823–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drabsch Y, He S, Zhang L, et al. Transforming growth factor-β signalling controls human breast cancer metastasis in a zebrafish xenograft model. Breast Cancer Res. 2013;15:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]


