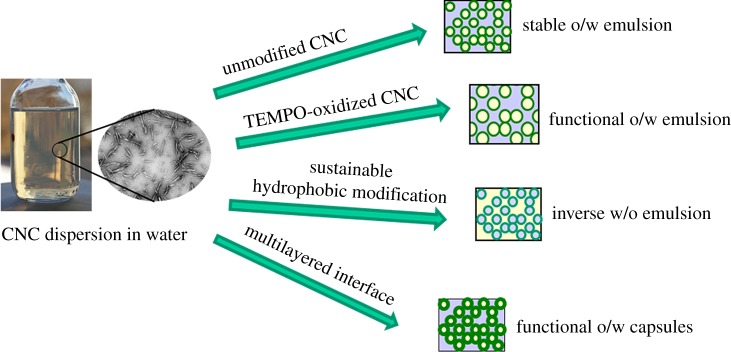
Abstract
Cellulose nanocrystals (CNCs) are negatively charged colloidal particles well known to form highly stable surfactant-free Pickering emulsions. These particles can vary in surface charge density depending on their preparation by acid hydrolysis or applying post-treatments. CNCs with three different surface charge densities were prepared corresponding to 0.08, 0.16 and 0.64 e nm−2, respectively. Post-treatment might also increase the surface charge density. The well-known TEMPO-mediated oxidation substitutes C6-hydroxyl groups by C6-carboxyl groups on the surface. We report that these different modified CNCs lead to stable oil-in-water emulsions. TEMPO-oxidized CNC might be the basis of further modifications. It is shown that they can, for example, lead to hydrophobic CNCs with a simple method using quaternary ammonium salts that allow producing inverse water-in-oil emulsions. Different from CNC modification before emulsification, modification can be carried out on the droplets after emulsification. This way allows preparing functional capsules according to the layer-by-layer process. As a result, it is demonstrated here the large range of use of these biobased rod-like nanoparticles, extending therefore their potential use to highly sophisticated formulations.
This article is part of the themed issue ‘Soft interfacial materials: from fundamentals to formulation’.
Keywords: colloidal particle, cellulose nanocrystal, nanorod, functional emulsion, capsule, layer-by-layer
1. Introduction
Emulsion stabilization is conventionally achieved by the addition of amphiphilic surfactants to reduce interfacial tension. However, it is now well established that surfactant-free dispersions can be stabilized by dispersed solid particles to form the so-called Pickering emulsions [1–4] for which colloidal-size particles may be irreversibly anchored at the oil–water interface. Pickering emulsions typically require an interfacial solid material that exhibits an affinity for the two phases of the emulsion [3,5] and present the double advantage of being extremely stable and requiring a very small quantity of particles. Research efforts are currently focused on the development of environmentally friendly renewable materials [6–8]. Cellulose constitutes the most abundant renewable polymer resource available today. Therefore, solid cellulosic particles with their low carbon footprint and low density are of particular interest for various applications such as composites, cosmetics, pharmaceutics or medical implants.
As a chemical raw material, cellulose has been used in the form of fibres or derivatives for nearly 150 years for a wide spectrum of products and materials in daily life. In the 1950s, it was demonstrated that cellulose fibres are composed of microfibrils that can be defibrillated and produce long semi-crystalline wires constituted of crystalline rod-like residues alternating with less ordered amorphous domains. The solid crystalline part may be recovered from preferential hydrolysis of the amorphous regions of cellulose fibres. This hydrolysis leads to highly crystalline solid rod-like particles known as cellulose nanocrystals (CNCs) [9,10]. In the materials science community, CNCs have reached a high level of attention that does not appear to be diminishing. These biopolymeric assemblies warrant such attention not only because of their inherent biodegradability, renewability and sustainability, in addition to their abundance, but also because of their impressive physical and chemical properties.
Nanocrystals of various dimensions can be obtained depending on the source. The most common sources include, among others, cellulose fibres from cotton, ramie, hemp, flax, hardwood and cotton linter pulp, microcrystalline cellulose, bacterial cellulose and tunicates [11–13]. The CNCs generally used are obtained from wood and cotton through sulfuric acid hydrolysis. This hydrolysis results in charged nanoparticles with a length of approximately 160–200 nm and a cross section of approximately 7–25 nm, making them natural anisotropic rods that can be used as platforms for various modifications. This platform can appear under various more or less sulfated forms depending on the hydrolysis condition. It may even be totally desulfated which leads to more aggregated systems as revealed by visual opacification of the suspension [14,15].
CNCs have recently been used as emulsion stabilizers by taking advantage of their self-assembling ability at the oil–water interface, similar to particle-stabilized Pickering systems [16,17]. They thus lead to the formation of ultrastable and monodispersed oil-in-water emulsions. Depending on the biological source, CNCs can form crystals with various aspect ratios, up to several micrometres long, but with a section that is always around 10–30 nm. They all present the ability to stabilize oil-in-water emulsions [13]. However, depending on the morphology and the quantity of nanocrystals involved, they can be used to prepare either individual droplets or three-dimensional networks with interconnected droplets, as well as emulsions of varying coverage, thereby modulating the porosity of the interface and visco-elasticity of the emulsion. CNC is a crystalline colloid; it is then a perfectly ordered solid particle with well-defined faces. The stabilization at the interface is attributed to the less hydrophilic crystalline plane of the crystal. This plane is of minor importance in terms of surface area as it is located at the corner of the cellulosic crystals. Defined as (2 0 0) crystalline plane for cotton Iβ allomorph, it is considered flat, exposing at the surface a repetition of the CH groups of the glucosyl moieties without hydroxyl functions [17,18]. Neutron scattering experiments showed clearly that rigid nanoparticles can be densely adsorbed at the oil–water interface without deforming it at the nanoscale confirming the postulate that interactions involve only the crystal surface and oil, the particle residing in water only [19]. Above these dimension variations, CNCs are versatile solid platforms for significant chemical reaction by covalent and non-covalent surface modification as reported in several reviews [20,21].
This work focuses on the modulation of the surface chemistry of CNCs and their chemical transformations for emulsion processing. It shows that their amphiphilic character is maintained after various modifications. Several ways are illustrated for CNC stabilized emulsions providing versatile surfactant-free functional emulsions in order to control the droplet dispersion in formulations.
2. Experimental procedure
(a). Sample preparation
Sulfated cotton CNCs were prepared from Whatman filters (grade 20 Chr), based on a previous process using sulfuric acid hydrolysis at two concentrations (58 or 64%) and different conditions. The slightly sulfated CNC-S was hydrolysed with 58% H2SO4 and kept at 65°C under stirring for 20 min. The medium sulfated CNC-M and the highly sulfated CNC-H samples were hydrolysed with 58% and 64% H2SO4, respectively, and kept under stirring at 70°C for 20–30 min. After hydrolysis, the prepared suspensions were systematically washed by centrifugation, dialysed to neutrality against Milli-Q water, and deionized using mixed bed resin (TMD-8). The final dispersions were sonicated for 10 min (ultrasonic Qsonica Q700; Misonix, Inc., NY, USA), filtered and stored at 4°C.
TEMPO-mediated oxidation of CNCs was performed using 4-acetamido-2,2,6,6-tetramethyl-1-piperinidyloxy radical (TEMPO) as a catalyst, according to the method described by Saito et al. [22]. The CNCs were suspended in water containing TEMPO (0.075 mmol g−1 of cellulose) and sodium bromide (1.25 mmol g−1 of cellulose). The TEMPO oxidation was started by adding the desired amount of sodium hypochlorite solution (3.6 mmol g−1 of cellulose) and was continued at room temperature while stirring. The pH was kept at 10 by adding sodium hydroxide. The TEMPO-oxidized CNCs were subsequently thoroughly washed with water by centrifugation, dialysis and filtration.
Hydrophobic surface modification of CNCs was done with quaternary ammonium salts. The pH of the CNC suspension (0.1 wt%) was adjusted to 10 using NaOH aqueous solution in order to have the carboxyl groups on the surface of the CNCs fully dissociated. The CNC suspension was then added dropwise into desired amount of stearyltrimethylammonium chloride solution (0.1 wt%). The suspension was kept at 60°C for 3 h, and stirred at room temperature overnight. The suspension was then dialysed against Milli-Q water to remove NaCl formed during the adsorption and unbound quaternary ammonium salts. The final suspension was freeze dried and redispersed in toluene using an ultrasonic treatment for 1 min. The suspensions were then centrifuged for 10 min at 20 000g to remove possible aggregated excess of quaternary ammonium salts. The resulting pellet was easily redispersed in the desired amount of organic solvent with an ultrasonic treatment.
(b). Characterization of the cellulose nanocrystals
Conductometric titrations were performed to determine the surface charge density according to Kalashnikova et al. [17] with a few modifications. Titration was performed on a total of 10 ml of a cellulose suspension at 10 g l−1 in water for CNC sulfated and neutrals, and in 5 mM HCl for CNC-TEMPO, with freshly prepared NaOH. Sulfate groups being only positioned at the surface, results in millimole per gram were calculated from dimensions obtained by microscopies and given as an average amount of elementary charge per square nanometre (e nm−2).
Dimensions were determined by image analysis. For transmission electron microscopy (TEM), the suspensions were sonicated just before deposition on a substrate of a freshly glow-discharged carbon-coated copper grid and negatively stained with phosphotungstic acid at 1%w/v adjusted to pH 6. The grids were observed with a Jeol JEM 1230 TEM at 80 kV. For atomic force microscopy (AFM), a drop of CNC suspension freshly sonicated and filtered at 0.1 g l−1 was deposited on freshly cleaved mica covered with a positive polyelectrolyte (poly(allylamine hydrochloride), PAH). The sample dried under ambient conditions was analysed using tapping mode AFM (Innova AFM, Bruker, Santa Barbara, CA, USA).
Transmission wide-angle X-ray scattering (WAXS) diffractograms of samples lyophilized and dried in a desiccator were recorded with a Bruker D8 Discover diffractometer (Madison, WI, USA) using Cu Kα1 radiation (λCu Kα1=1.5405 Å) produced by a sealed tube at 40 kV and 40 mA. The average dimension of the crystal perpendicular to the diffracting planes with hkl Miller indices, Dhkl, was evaluated using Scherrer’s expression from the width at half-height of intensity.
(c). Emulsion preparation
Oil-in-water (o/w) emulsions were prepared using hexadecane as oil phase and a 20/80 oil/aqueous phase ratio. Practically, emulsions were sonicated with an ultrasonic device with a dipping titanium probe close to the surface (pulsed 2 W ml−1 applied power). The emulsions were all visualized after dilution by light microscopy (BX51 Olympus). Average droplet diameter was measured by image analysis using ImageJ software and compared with the drop size distribution determined by laser light diffraction using a Malvern 2000 granulometer equipped with a HeNe laser (Malvern Instruments, UK). The diameter was expressed as surface mean diameter D(3,2) (the Sauter diameter). Similar results were obtained via granulometer and ImageJ analysis. Inverse water-in-oil (w/o) emulsions were prepared at a 20/80 water/hexadecane ratio mixed using an ultrasonic device.
Scanning electron microscopy (SEM) images were prepared as previously described [13] from 20/80 styrene/water emulsions obtained by sonication and degassed with nitrogen before polymerization. The resulting beads were washed by repeated centrifugation to reduce the amount of artefactual small droplets appearing during curing. Dried beads were metalized with platinum and visualized with a JEOL 6400F instrument (IMN-Nantes).
3. Results and discussion
(a). Cellulose nanocrystals modulated in surface charge density
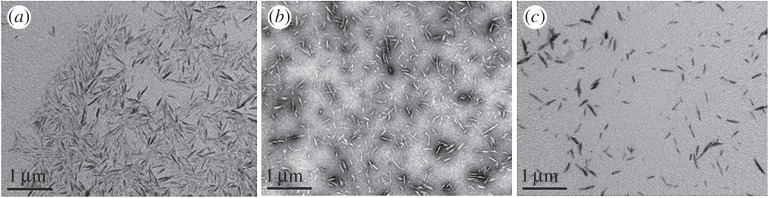
When mixing an aqueous suspension of colloidal CNCs and an oil phase, an ultrastable Pickering emulsion is produced. CNCs are known to serve as platform for several modifications but it is not possible to predict whether or not they might still allow preparing stable emulsions once modified. Basically, CNCs are obtained by acid hydrolysis using sulfuric acid. According to the hydrolysis parameters (mainly concentration in acid, reaction temperature and time), various degrees of substitution might be reached where the hydroxyl groups exposed at the surface are replaced by sulfate ester groups. The surface-modified samples are generally characterized by conductometric titration for the resulting surface charge density. Samples with different sulfate charge densities were prepared and characterized. As a result, three samples were prepared, a slightly sulfated one (CNC-S), one with medium substitution degree (CNC-M) and a third one highly substituted (CNC-H). They were characterized for their surface charge, size and crystallinity (table 1). Some aggregations were observed for CNC-S as illustrated in figure 1, but no clear size variation was noticed for the different samples. The three CNC samples had length of 156±53 nm and width of 16±8 nm giving a similar aspect ratio around 10 (mainly from 7 to 14) whatever the acidic treatment. Since the sulfate charges are known to be responsible for crystal destructuration, these post-hydrolysis treatments were followed by WAXS to evaluate an eventual variation at a crystal level. WAXS diagrams showed a high crystallinity of 85±3% for the three samples. As a result, the various treatments maintained the same crystalline organization.
Table 1.
Characteristics of CNCs with three surface charge densities. Crystalline plane dimensions and crystallinity for the elementary unit as determined by WAXS, according to the Miller index maximum and the dimensions of the nanoparticles as determined by AFM.
| slightly sulfated CNC-S | medium sulfated CNC-M | highly sulfated CNC-H | |
|---|---|---|---|
| sulfate surface | 0.08 e nm−2 | 0.16 e nm−2 | 0.64 e nm−2 |
| charge density | (0.062 mmol g−1) | (0.248 mmol g−1) | |
| ζ potential (mV) | −30±0.5 | −43±1 | −60±0.7 |
| crystalline elementary unit dimensions | |||
| (1–10) thickness (nm) | 5.1 | 4.4 | 4.1 |
| (110) width (nm) | 5.7 | 6.6 | 5.7 |
| (200) edge (nm) | 6.5 | 6.4 | 6.3 |
| crystallinity (%) | 83 | 82 | 89 |
Figure 1.
TEM images of (a) slightly, (b) medium and (c) highly charged CNCs.
These three CNCs were tested for their ability to stabilize emulsions. As a result, in pure water, repulsion between the charged CNC prevented a sufficient surface density of CNC to stabilize the oil–water emulsions; instead coalescence occurs and the oil and water phases separate. However adding salt, the different samples were all able to efficiently stabilize o/w emulsions with same drop diameters for more than a year (figure 2). The diameter decreased with mp at low CNC concentration in accordance with the limited coalescence process. The drop diameter is then controlled by the amount of CNC available to stabilize the interface. This domain is followed by stabilization of the diameter values at higher mp. When the same values are plotted as 1/D versus mp, a linear behaviour appears at low mp and a deviation arises at a critical concentration of 7 mg ml−1 of hexadecane [19]. This change indicates a coverage variation that is possibly due to the limited flexibility of the particles hindering the decrease in radius of curvature and thereby in drop diameter. It results in a denser coverage of the drops.
Figure 2.
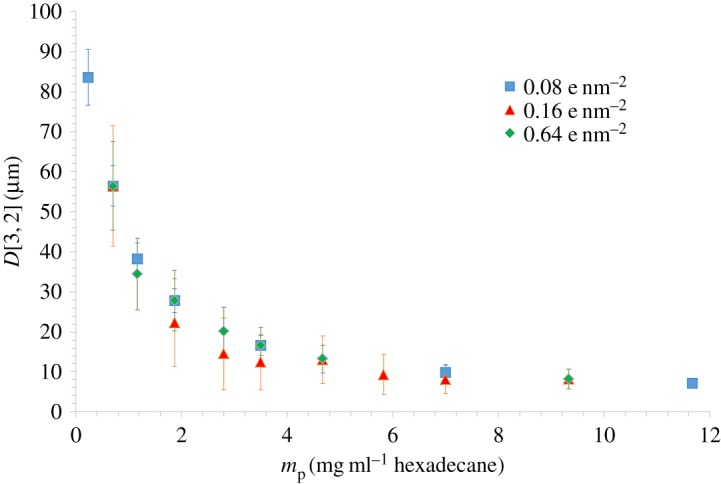
Mean D[3,2] Sauter diameter with the concentration of particles given in milligrams of CNC per millilitre of hexadecane, the dispersed phase, for the three charged CNCs at a 30/70 hexadecane/water ratio.
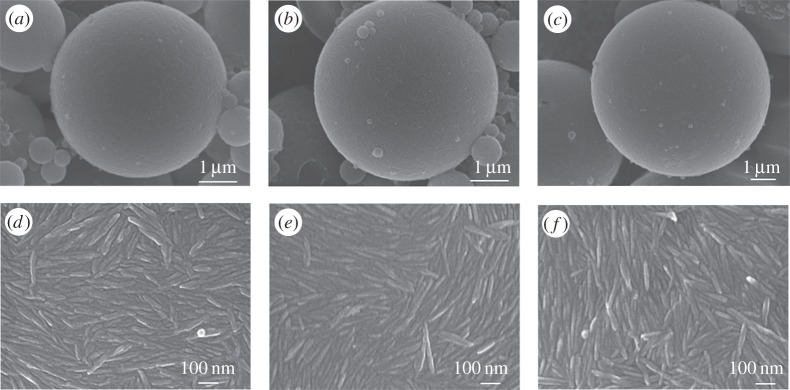
Styrene-in-water emulsions with 0.05 M NaCl were prepared with CNCs bearing various surface charge densities and polymerized. SEM images of the resulting beads are shown in figure 3. Some artefactual small droplets arising during polymerization are visible. However, the average size and size distribution of the larger beads is similar to the hexadecane-in-water emulsions allowing comparison. These images confirmed the identical organization at the surface of the individual droplets, showing the CNC curved along the droplets creating a dense armoured layer in all cases.
Figure 3.
Scanning electron microscope images of polymerized PS/W Pickering emulsion droplets stabilized by slightly sulfated CNC-S (a,d), medium sulfated CNC-M (b,e) and highly sulfated CNC-H (c,f).
Based on the previous WAXS results, four characteristic planes of cellulose I, namely (1-10), (110), (102/012) and (200) [11,17,23] were identified. We proposed that the process of stabilization involves this crystalline regular organization of CNCs forming a faceted surface. The (2 0 0) edge planes do not bear charges since they are only composed of CH groups, maintaining the more hydrophobic surface available for interface stabilization [18]. It indicates similar exposure of the crystalline planes, regardless of the charge density. The present results illustrate the low susceptibility of surface charge modulation of CNCs on their ability to adsorb at the interface. It differs from the tendency to aggregation generally observed at low surface charge density such as that illustrated in figure 1, important aggregation seems not to occur on a two-dimensional organization just varying the amount of charges. It appears then possible to modify the surface chemistry of CNCs and subsequently of droplets, without changing any other interfacial parameter.
(b). Surface modification of cellulose nanocrystal for functional droplets
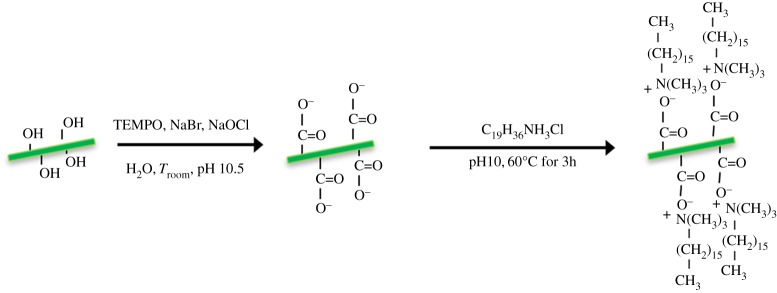
These CNCs might then further be used as a platform for subsequent modifications. As previously described, the sulfuric acid used for the preparation results in sulfate moieties at the surface of the CNCs. Hydroxyl groups have low reactivity, and sulfate groups might be used for chemical modification but they are unfortunately rather labile, being, in particular, readily removed under mild alkaline conditions. Oxidation of the CNCs, whether sulfated or not, with TEMPO results in high anionic surface charges with high densities (1.7 glucose units nm−2) arising from dissociated C6-carboxyl groups formed on the surface [24,25]. The use of this technique has been the subject of a number of studies since the first reports of De Nooy et al. in the 1990s, who showed that only the hydroxymethyl groups of polysaccharides were oxidized, whereas the secondary hydroxyls remained unaffected (figure 4).
Figure 4.
Schematic of a two-step modification of CNCs.
When submitting the TEMPO-mediated oxidized CNC with an oil phase to emulsification, it appeared clearly that such carboxylated CNC kept stabilizing efficiently oil–water interfaces. Figure 5 shows an example of emulsions stabilized with both unmodified and TEMPO-oxidized CNCs at 5 g l−1 in the aqueous phase in the presence of NaCl to reduce repulsions between the CNCs, with a 20/80 (oil/water) ratio. The average diameter increased from 5 μm for sulfated CNCs to 15 μm using TEMPO-oxidized CNCs. Such modified CNCs are not aggregated due to the highly charged surface, the reason is then attributed to unadsorbed CNCs maintained in suspension in the continuous phase. However, a stable emulsion was prepared. TEMPO oxidation improves chemical modification of cellulose through the presence of carboxylate groups. This opportunity to produce highly functionalized droplets reveals that a much larger range of functional emulsions might be reached as one could be inspired by examples of modification described in several issues dealing with more or less sophisticated modifications [21,26,27].
Figure 5.
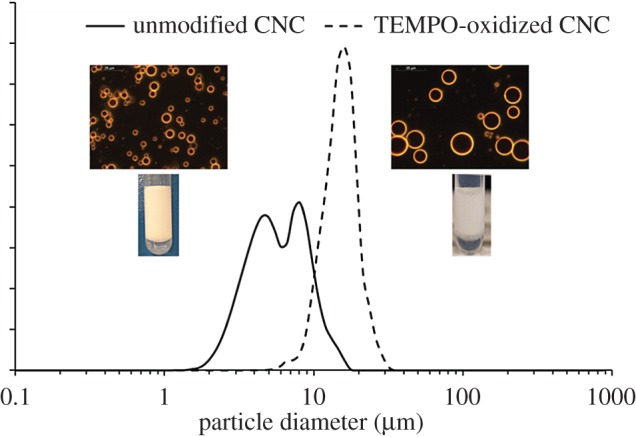
Compared Sauter diameter distribution, optical microscopy images and photographs of 80/20 hexadecane/water (v/v) emulsions stabilized by unmodified and TEMPO-oxidized CNCs.
A major relevant point is that CNC is a hydrophilic particle. Equivalently to the Bancroft rule for surfactant-stabilized emulsions, the continuous phase for Pickering emulsions is the one in which the particles are preferentially dispersed. This means that CNCs are able to stabilize direct o/w emulsions while predominantly hydrophobic particles should be used to stabilize reverse w/o emulsions allowing compatibility with apolar organic media. Several studies have developed such modifications [28–30] mainly solvent based or using harmful systems, which limit the extension of these finding to industrial scale-up. Efforts to reach an environmentally friendly procedure are needed. A surface modification performed in aqueous solution is more acceptable. For instance, modification using quaternary ammonium salts bearing long alkyl, phenyl, glycidyl and diallyl groups via adsorption was developed based on TEMPO-oxidized CNCs [31]. In this study, hydrophobically modified CNCs were prepared by exchanging the counterions of the Na carboxylate groups of TEMPO-oxidized CNCs with quaternary alkylammonium groups. The successful ionic exchange was proved (i) by the presence of a new peak at 1480 cm−1 in Fourier transform infrared spectra (not shown) corresponding to the trimethyl groups of the quaternary ammonium and (ii) by the dispersability of the modified CNCs in toluene and hexadecane. As illustrated in figure 4, stearyltrimethylammonium chloride was used to graft alkyl chains on CNCs bearing carboxylic acid groups on the surface. The w/o emulsions were prepared using Milli-Q water and 2–4 g l−1 modified CNC dispersed in hexadecane. It resulted in stable inverse emulsions (figure 6) showing that simple hydrophobic modification enables the formation of inverse w/o emulsions.
Figure 6.

Water-in-hexadecane emulsion stabilized hydrophobically by CNCs modified by quaternary alkylammonium groups. (Online version in colour.)
As a result, CNC proves to be a versatile nanorod with a large range of various surface modifications. The various modifications obtained here indifferently from native CNC with various surface charge densities, after TEMPO oxidation leading to a highly carboxylated CNC, or hydrophobically modified using acceptable processes, can be used to produce w/o or o/w emulsions and various kinds of functional droplets.
(c). Multilayer drop preparation
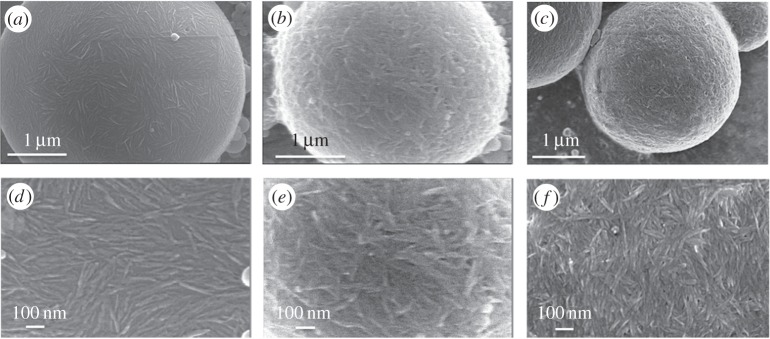
It appears then of interest to check if such emulsion would be preserved if modification is carried out directly on the droplet. Coming back to the pristine unmodified sulfated CNCs, negative charges are exposed to the surrounding area. Because these emulsions are highly stable, they can be dropped in a different solution and recovered by centrifugation. Another way of modifying the chemistry consists then of modulating the surface using electrostatic interactions as used in layer-by-layer systems. The change from a negative to a positive surface was carried out by mixing the emulsion with a positively charged polymer, in order to add an extra layer using PAH. The strong association between these two oppositely charged polymers results in the entropy gain resulting from the release of counterions and water molecules. As a result, it is possible to rinse the emulsion by simple repeated centrifugations and keep an isolated positively charged bead. Figure 7 shows SEM images of droplets with a negative monolayer of sulfated CNCs, before (figure 7a) and after coating with PAH (figure 7b). These images revealed a very different surface with jammed CNCs, confirming that PAH was deposited on the surface.
Figure 7.
SEM images of polymerized PS/W emulsions stabilized by sulfated CNC (a,d), after addition of a hydrosoluble positive polyelectrolyte (PAH) (b,e), and after a second deposition of CNC following a layer-by-layer process (c,f).
CNCs appear then of great interest as a rigid armour to stabilize droplets that might be functionalized at the surface on demand with non-surface-active agents. It is possible to change the surface chemistry in a second step with multi-layered systems. To check the modification of the surface chemistry and validate the ability to make multi-layered shells, an additional negatively charged layer of alginate previously stained with fluorescent die was used. As shown on the optical microscope with fluorescence detection (figure 8), when the sample is illuminated with a fluorescent light, stained alginate is revealed on the surface of the droplets.
Figure 8.
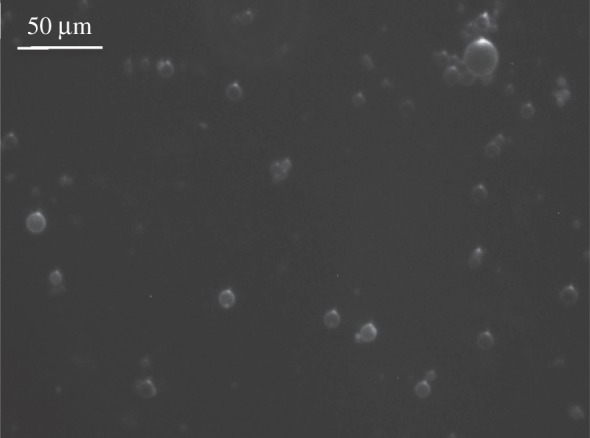
Optical fluorescence microscopy after deposition of a PAH/stained alginate bilayer evidencing a multi-layered shell.
The surface of the capsules was reinforced by adding another layer of CNC. Figure 7c shows that CNCs are again appearing clearly on SEM images and the absence of visible droplets by optical fluorescence microscopy showed that the negatively charged alginate did not associate anymore.
These results reveal that such Pickering emulsions constitute good substrates for capsule preparation. It means that taking into account the high stability of CNCs at the oil–water interface, not only modified CNCs can be used to produce functional emulsions, but also post-modifications can be carried out for surface adapted to the required formulation revealing the high versatility of these biobased Pickering emulsions. CNCs appear then as highly relevant particles to process a large range of highly stable surfactant-free functional emulsions.
4. Conclusion
CNCs are highly versatile nanorods able to stabilize a large range of long-term Pickering emulsions. They appear as platforms for preparation of biobased functional colloidal particles. It is shown that besides the surface charge density variation, a lot of different modifications such as carboxylation might be carried out maintaining the ability of CNCs to stabilize oil–water interfaces. Hydrophobically modified CNCs allow stabilizing w/o emulsions that might also improve the processability and performances of nanocellulose-based materials in apolar media. It is finally illustrated that the high stability of such CNC stabilized emulsion also allows modification of preformed emulsions with an extra layer leading to capsules according to a layer-by-layer process. The different routes described in the present article (figure 9) aim at paving the way for innovative complex formulations and materials in very different targeted applications.
Figure 9.
Schematic of the various emulsions based on pristine or modified CNC. (Online version in colour.)
Acknowledgements
The authors acknowledge Joelle Davy for sample preparation and visualization of SEM images. The BIBS and IMN platforms (Nantes) are also acknowledged for access to TEM and SEM facilities, respectively.
Competing interests
We declare we have no competing interests.
Funding
D.S. gratefully acknowledges the financial support of INRA and INRA Transfert (Verniscell project). This work has been also funded by the local council programme MATIERES and is a contribution to the Labex Serenade (no. ANR-11-LABX-0064) funded by the ‘Investissements d’Avenir’ French Government programme of the French National Research Agency (ANR) through the A*MIDEX project (no. ANR-11-IDEX-0001-02).
References
- 1.Pickering SU. 1907. Emulsions. J. Chem. Soc. 91, 2001–2021. ( 10.1039/CT9079102001) [DOI] [Google Scholar]
- 2.Binks BP. 2002. Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 7, 21–41. ( 10.1016/S1359-0294(02)00008-0) [DOI] [Google Scholar]
- 3.Lopetinsky R, Masliyah J, Xu Z.. 2006. Solid stabilized emulsion: a review. In Colloidal particles at liquid interfaces (eds BP Binks, TS Horozov), ch. 6, pp. 185–224. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Leal-Calderon F, Schmitt V. 2008. Solid-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 13, 217–227. ( 10.1016/j.cocis.2007.09.005) [DOI] [PubMed] [Google Scholar]
- 5.Arditty S, Schmitt V, Giermanska-Kahn J, Leal-Calderon F. 2004. Materials based on solid-stabilized emulsions. J. Colloid Interface Sci. 275, 659–664. ( 10.1016/j.jcis.2004.03.001) [DOI] [PubMed] [Google Scholar]
- 6.Blaker JJ, Lee KY, Li X, Menner A, Bismarck A. 2009. Renewable nanocomposite polymer foams synthesized from Pickering emulsion templates. Green Chem. 11, 1321–1326. ( 10.1039/B913740H) [DOI] [Google Scholar]
- 7.Dickinson E. 2010. Food emulsions and foams: stabilization by particles. Curr. Opin. Colloid Interface Sci. 15, 40–49. ( 10.1016/j.cocis.2009.11.001) [DOI] [Google Scholar]
- 8.Charreau H, Foresti ML, Vazquez A.. 2013. Nanocellulose patents trends: a comprehensive review on patents on cellulose nanocrystals, microfibrillated and bacterial cellulose. Recent Pat. Nanotechnol. 7, 56–80. ( 10.2174/187221013804484854) [DOI] [PubMed] [Google Scholar]
- 9.Revol JF, Bradford H, Giasson J, Marchessault RH, Gray DG.. 1992. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 14, 170–172. ( 10.1016/S0141-8130(05)80008-X) [DOI] [PubMed] [Google Scholar]
- 10.Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, Dorris A.. 2011. Nanocelluloses: a new family of nature-based materials. Angew. Chem. Int. Ed. 50, 5438–5466. ( 10.1002/anie.201001273) [DOI] [PubMed] [Google Scholar]
- 11.Elazzouzi-Hafraoui S, Nishiyama Y, Putaux J-L, Heux L, Dubreuil F, Rochas C.. 2008. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 9, 57–65. ( 10.1021/bm700769p) [DOI] [PubMed] [Google Scholar]
- 12.Habibi Y, Lucia LA, Rojas OJ. 2010. Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem. Rev. 110, 3479–3500. ( 10.1021/cr900339w) [DOI] [PubMed] [Google Scholar]
- 13.Kalashnikova I, Bizot H, Bertoncini P, Cathala B, Capron I.. 2013. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 9, 952–959. ( 10.1039/C2SM26472B) [DOI] [Google Scholar]
- 14.Cherhal F, Cathala B, Capron I.. 2015. Surface charge density variation to promote structural orientation of cellulose nanocrystals. Nordic Pulp Paper Res. J. 30, 126–131. ( 10.3183/NPPRJ-2015-30-01-p126-131) [DOI] [Google Scholar]
- 15.Cherhal F, Cousin F, Capron I.. 2015. Influence of charge density and ionic strength on the aggregation process of cellulose nanocrystals in aqueous suspension, as revealed by small-angle neutron scattering. Langmuir 31, 5596–5602. ( 10.1021/acs.langmuir.5b00851) [DOI] [PubMed] [Google Scholar]
- 16.Kalashnikova I, Bizot H, Cathala B, Capron I.. 2011. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 27, 7471–7479. ( 10.1021/la200971f) [DOI] [PubMed] [Google Scholar]
- 17.Kalashnikova I, Bizot H, Cathala B, Capron I.. 2012. Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface. Biomacromolecules 13, 267–275. ( 10.1021/bm201599j) [DOI] [PubMed] [Google Scholar]
- 18.Mazeau K. 2011. On the external morphology of native cellulose microfibrils. Carbohydr. Polym. 84, 524–532. ( 10.1016/j.carbpol.2010.12.016) [DOI] [Google Scholar]
- 19.Cherhal F, Cousin F, Capron I.. 2016. Structural description of the interface of Pickering emulsions stabilized by cellulose nanocrystals. Biomacromolecules 17, 496–502. ( 10.1021/acs.biomac.5b01413) [DOI] [PubMed] [Google Scholar]
- 20.Stenstad P, Andresen M, Tanem BS, Stenius P. 2008. Chemical surface modifications of microfibrillated cellulose. Cellulose 15, 35–45. ( 10.1007/s10570-007-9143-y) [DOI] [Google Scholar]
- 21.Habibi Y. 2014. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 43, 1519–1542. ( 10.1039/C3CS60204D) [DOI] [PubMed] [Google Scholar]
- 22.Saito T, Kimura S, Nishiyama Y, Isogai A.. 2007. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 8, 2485–2491. ( 10.1021/bm0703970) [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama J, Vuong R, Chanzy H.. 1991. Electron diffraction study on the two crystalline phases occuring in native cellulose from an algal cell wall. Macromolecules 24, 4168–4175. ( 10.1021/ma00014a033) [DOI] [Google Scholar]
- 24.Habibi Y, Chanzy H, Vignon MR.. 2006. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 13, 679–687. ( 10.1007/s10570-006-9075-y) [DOI] [Google Scholar]
- 25.Isogai A, Saito T, Fukuzumi H.. 2011. TEMPO-oxidized cellulose nanofibers. Nanoscale 3, 71–85. ( 10.1039/C0NR00583E) [DOI] [PubMed] [Google Scholar]
- 26.Araki J. 2013. Electrostatic or steric?—Preparations and characterizations of well-dispersed systems containing rod-like nanowhiskers of crystalline polysaccharides. Soft Matter 9, 4125–4141. ( 10.1039/c3sm27514k) [DOI] [Google Scholar]
- 27.Missoum K, Belgacem MN, Bras J.. 2013. Nanofibrillated cellulose surface modification: a review. Materials 6, 1745–1766. ( 10.3390/ma6051745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee K-Y, Quero F, Blaker JJ, Hill CAS, Eichhorn SJ, Bismarck A. 2011. Surface only modification of bacterial cellulose nanofibres with organic acids. Cellulose 18, 595–605. ( 10.1007/s10570-011-9525-z) [DOI] [Google Scholar]
- 29.Cunha AG, Mougel J-B, Cathala B, Berglund LA, Capron I.. 2014. Preparation of double Pickering emulsions stabilized by chemically tailored nanocelluloses. Langmuir 30, 9327–9335. ( 10.1021/la5017577) [DOI] [PubMed] [Google Scholar]
- 30.Lee K-Y, Blaker JJ, Heng JYY, Murakami R, Bismarck A.. 2014. pH-triggered phase inversion and separation of hydrophobised bacterial cellulose stabilised Pickering emulsions. React. Funct. Polym. 85, 208–213. ( 10.1016/j.reactfunctpolym.2014.09.016) [DOI] [Google Scholar]
- 31.Salajkova M, Berglund LA, Zhou Q.. 2012. Hydrophobic cellulose nanocrystals modified with quaternary ammonium salts. J. Mater. Chem. 22, 19 798–19 805. ( 10.1039/c2jm34355j) [DOI] [Google Scholar]