Abstract
Evolutionary problems are often considered in terms of ‘origins', and research in human evolution seen as a search for human origins. However, evolution, including human evolution, is a process of transitions from one state to another, and so questions are best put in terms of understanding the nature of those transitions. This paper discusses how the contributions to the themed issue ‘Major transitions in human evolution’ throw light on the pattern of change in hominin evolution. Four questions are addressed: (1) Is there a major divide between early (australopithecine) and later (Homo) evolution? (2) Does the pattern of change fit a model of short transformations, or gradual evolution? (3) Why is the role of Africa so prominent? (4) How are different aspects of adaptation—genes, phenotypes and behaviour—integrated across the transitions? The importance of developing technologies and approaches and the enduring role of fieldwork are emphasized.
This article is part of the themed issue ‘Major transitions in human evolution’.
Keywords: human evolution, major transitions in human evolution, early hominins, evolution of Homo
1. From origins to transitions
The word probably most associated with our evolutionary past is ‘origins'. The history of science is awash with books and papers in search of human origins, or the origins of the things that made us human—upright walking or language or culture. Seeking origins is looking for the beginnings of something, finding out why and when something that did not exist before did so afterwards. Origins research is at its most ultimate in cosmology, when, to the layman at least, the origin of the universe is when something (matter) is there when previously (if one can use that word given that time itself did not exist!) there had been nothing.
Origins research has often been criticized, on both theoretical and practical terms. Theoretically, the argument has been made that a focus on origins prioritizes particular periods and features, and creates essential traits and moments of significance in a continuity of process [1]. Pragmatically, the search for origins is a recipe for frustration. There may be a hypothetical point of origin for Homo sapiens, but to find one fossil closer to that elusive point than another is only to engender the search for another that is closer still, until the path leads inexorably to the origins of something else.
And yet we know that there was a time when something did not exist—humans—and then a time when they did. How do we discover the process, timing and causes of such a change? This, of course, is not exclusive to humans, but would apply equally to dinosaurs, mammals, primates and the most insignificant house louse. How do we square the circle of explaining something new, while accepting that there is nothing entirely new, and that the roots of novelty in evolution lie in existing forms? As Dawkins shows in The Ancestor's Tale [2], humanity can be tracked back seamlessly to the first replicating cells. Origins disappear in continuity.
The challenge of studying evolutionary change, for any lineage or characteristic, is to steer a course between the Scylla and Charybdis of monotonous continuity and elusive origin points. This holds true for human evolution as much as any other part of biodiversity. On the one hand, there is continuity in many aspects of hominins back to the last common ancestor with apes, and on the other hand, there are many novel traits that appear successively across the subsequent five or more million years. The solution to this difficulty that is explored in this themed issue is to focus on transitions. Evolution is about the change from one state (at whatever biological level) to another, which demands a focus on the comparison of states across time, or across organisms and their adaptations. Such transitions can be major or minor, can be multiple or single, and can be related to the appearance and disappearance of whole taxa, or of particular traits. Human evolution is the sum of those transitions, and the papers here present new evidence and review some of these across the 5 million years of our lineage's history. Although diverse and broad-ranging, 16 papers cannot do justice to the whole range of issues involved in the multiple transformations that have led from an ape-like ancestor to the modern world, but they do bring to the fore some central and common themes.
2. Hominin evolution: Is there a major divide?
Football, it is often said, is a game of two halves. It is the same with human evolution, albeit of rather unequal lengths. One half comprises the evolution of what are usually referred to as the early hominins, those taxa that are closer to humans than to living apes, but are generally placed in other genera than Homo (Sahelanthropus, Ardipithecus, Ororrin, Kenyanthropus, Australopithecus and Paranthropus). These are highly diverse creatures, but are linked by showing a variety of hominin-like traits (mostly related to dentition and inferred locomotion), and an absence of the major markers of Homo, an enlarged brain and more complex behaviours. This half of human evolution is often referred to as the ‘bipedal apes', and occurs between approximately 7.0 and 2.8 Ma, when the first Homo appears in the record (or, depending on one's view, approximately 1.5 Ma, when the last ‘bipedal apes' disappear). The second half, from between approximately 2.8 Ma to the present day, is concerned with our own genus, and includes the evolution of modern humans themselves. Here, the focus is on encephalization, changes in life-history strategy, developing technology, expansion of diet and geographical range and the emergence of cultural processes of evolution.
One of the relatively rare elements of this volume is that both halves are included, so that rather than being seen as two different events, overlapping patterns can be observed. Jungers et al. [3], for example, consider body size across all hominins and show that the early bipedal ape versus the human-like hominins divide does not exist; they show overlapping patterns, and certainly no contrast between the two at a point of ‘origins'. The idea that the emergence of Homo is associated with an increase in body size is not supported. Other aspects of hominin biology also do not show a clear distinction between Homo and earlier hominins; Dean [4] shows that all hominins share some form of shift in life-history relative to living apes, and the Homo-Australopithecus boundary exists but is not one that stands out strongly among a range of life-history changes. It is not just a matter of body size and maturation; Lewis and Harmand [5] discuss their recent discoveries at Lomekwi in West Turkana, showing that hominins were making stone tools at 3.3 Ma, before the appearance of Homo as currently understood. In this context it is worth remembering that, when Leakey et al. [6] described Homo habilis, they argued for abandoning the cerebral Rubicon so that the genus Homo could include a smaller brained stone tool maker. None of this undermines the adaptive significance of changes in life history or body size, or tool-making, but shows that clear watershed points do not occur. Kimbel et al. [7] look back at the history of the Australopithecus-Homo divide and also come to the conclusion that its significance has been exaggerated, and that it is better to think of small transitions accumulated over a longer period (figure 1).
Figure 1.
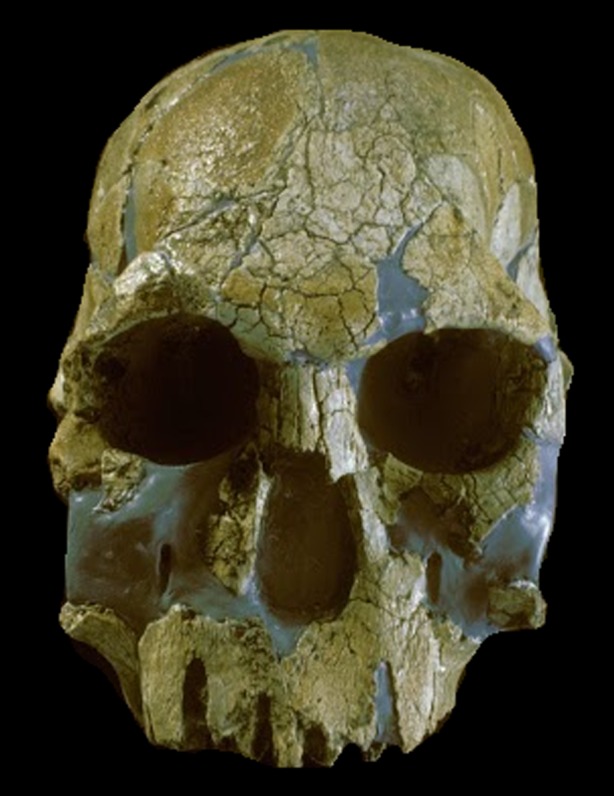
Early Homo. KNM-ER1470, from Koobi Fora, East Turkana. Discovered by Richard Leakey and his team in 1967. Photo credit: Turkana Basin Institute/Richard Leakey. (Online version in colour.)
One possibility is that the line is being drawn in the wrong place, and that, as has been argued before, the earliest members of Homo are indeed adaptively closer to the australopithecines, and that early H. erectus represents the major transition [8]. Or even closer to the present, that the divide lies between all archaic hominins and modern humans. Certainly there are grounds for seeing H. erectus (or in some taxonomic schemes H. ergaster; figure 2) as a grade shift relative to all earlier hominins, with biology and behaviour more similar to modern humans than to earlier hominins.
Figure 2.
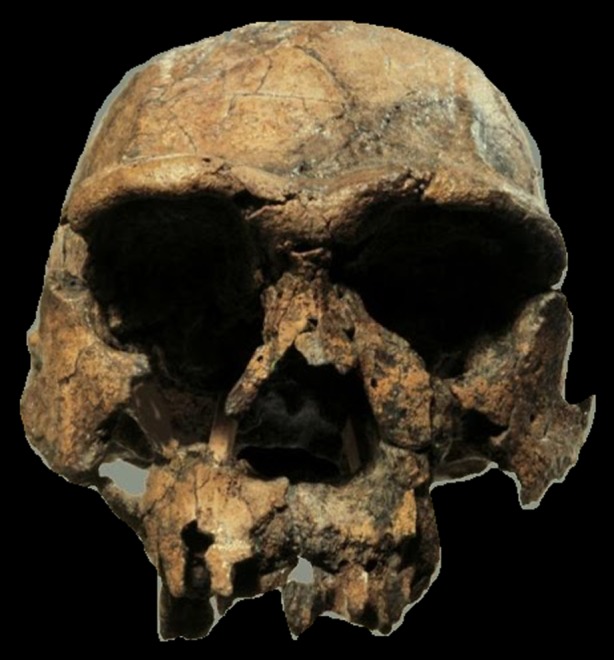
Homo erectus. Early representative (KNM-ER3733) of Homo erectus (sometimes referred to as Homo ergaster), from Koobi Fora, East Turkana, Kenya, discovered by Richard Leakey and his team in 1976. Photo credit: Turkana Basin Institute/Richard Leakey. (Online version in colour.)
A case can be made for each of these as a divide within hominin evolution, but a detailed examination of the evidence suggests a much more diverse and cumulative process. Antón et al. [9] show that H. erectus is highly variable, not just in body size (see [3]), but also in other aspects of its morphology. The appearance of modern humans is seen at about 195 Ka at Omo Kibish in Ethiopia (figure 3), but Stringer [10] argues that the lineage leading to it shows derived traits earlier than this, and so the transition is spread over several hundred thousand years, and that it is likely archaic populations persisted across the African continent, some until into the late Pleistocene [11].
Figure 3.
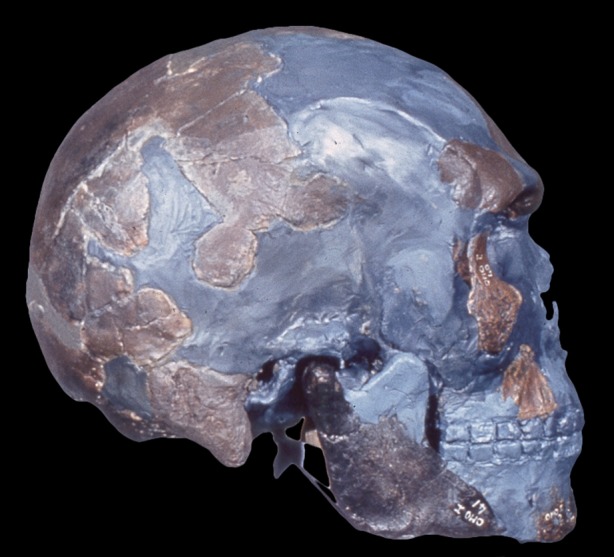
The earliest representative of Homo sapiens from Omo Kibish, Ethiopia. Discovered by Richard Leakey and his team in 1967. Reproduced with permission from C. Stringer and M. Day. (Online version in colour.)
Looking at the totality of hominin evolution, there is no broad division between the earlier and later phases, nor between archaic and modern humans. These transitions are significant, but the richer fossil record now in existence, and the multiple techniques available for studying it, show that the major transitions of human evolution are comprised of multiple smaller ones.
3. Is simple gradualism the best model?
If there are no big or single step transitions in human evolution, does this mean that the evolutionary history of our lineage is a straightforward case of unilineal, gradual change over millions of years? Is it a case of simple anagenetic change, with better adaptations replacing existing ones over time?
The evidence discussed in these papers cannot be said to support what was classically thought of as the punctuated equilibrium model of evolution, as proposed by Gould, Eldredge, Vrba and others [12,13]. This model predicted that human evolution should consist of prolonged periods of stasis, interspersed by sharp and rapid bursts of speciation and adaptive change. That is clearly not the case, but does it follow that what we see is simple gradualism?
Almost certainly not. Spoor et al. [14] looked at the fossil evidence in East Africa between about 3.5 and 2.5 Ma, focusing on Kenyanthropus platyops. Their morphometric study shows that K. platyops was different from both Australopithecus deyiremeda and A. afarensis, suggesting that there were three contemporary taxa in east Africa during the middle Pliocene. This mirrors the high level of diversity that is known from the succeeding Plio-Pleistocene [15]. Even within the Pleistocene, as Antón [9] discusses for H. erectus, and Stringer [10] and Mirazón Lahr [11] for H. sapiens and its contemporaries, there is considerable diversity, and clearly not a unilinear pattern.
This suggests that human evolution is not just one taxon evolving from another, but involves branching and speciation. This is not, of course, surprising, as it is how evolutionary change operates in other lineages, but it reinforces the similarity between patterns of human evolution and those seen in other groups of animals. That this may be part of the same process, subject to the same environmental pressures as other animals, is hinted at by Fortelius et al. [16] who analyse the Turkana Basin mammalian record, one of the richest repositories of hominin fossils, as a ‘species factory’ between 4 and 2 Ma—particular combinations of environmental conditions simultaneously creating refugia and novel selective pressures ahead of their occurrence over a wider area.
The branching pattern of hominin evolution, the diversity at most periods, the lack of linear change and the survival of archaic forms after their more derived descendants have evolved, all indicate something that is not simple phyletic gradualism, to use Gould and Eldredge's term [12]. Foley [15] looked at the overall patterns of hominin diversity over time and the appearance and disappearance of taxa (as proxies for speciation and extinction), and found that while such events are distributed broadly across the whole of the sequence, there are some phases—in the Pliocene, the Plio-Pleistocene and the later Pleistocene—when there were high rates of transition. This suggests that hominin evolution is neither a simple punctuated process, nor a constant gradual one, but a complex interaction between variable rates of change, environmental dynamics and the competitive interactions of the hominins and their sympatric fellow-travellers in evolution.
4. Is there a pattern to transitions? Geography and the role of Africa
If the overall evidence suggests that hominin evolution includes multiple transitions, the next question to be posed is whether there is any pattern to them. Perhaps the most striking of these is the centrality of Africa in evolutionary change—the first hominins, the earliest diversification of early hominins [14], the earliest stone tools [5], the earliest Homo [7], the earliest Acheulean [17], the first modern humans [10,11] and the beginnings of modern human behaviour and organization [18,19]. There might be a taphonomic bias in some of the dominance of Africa, but genetics, which is not subject to such a bias, certainly supports it for some of these events. While hominins were African, there is a recurrent pattern of an origin in East Africa [14]; when hominins became global, the recurrent pattern is of an African origin and dispersal beyond [9–11]. Thus the adaptive and contingent contexts for the transitions and early phases of diversification of several lineages are to be found in Africa, and perhaps East Africa more specifically. There may well be stochastic elements involved in setting the right initial conditions (the right sort of ape in the right sort of environment in Africa), but the repeated pattern merits attention. General biogeographical properties must certainly be involved (the tropics as areas of higher rates of speciation, for example, and the interaction between glacial cycles and the relationship between Eurasia and Africa). The dynamic nature of the African environment over the period between 3.5 and 2.0 Ma is discussed by Rose et al. [20], who show that the critical area in Africa, and its links to Eurasia (the north-eastern quadrant), generates a distinctive biogeographical pattern derived from the interaction between orbitally driven factors (such as eccentricity and precession) and local environmental conditions. Palaeoenvironmental research in recent years has built greatly on the marine isotope revolution of the second half of the twentieth century, by beginning to reveal the importance of local variation within broader patterns and trends. This goes some way to explaining the variation between Turkana and other lake basins in Africa, and even between East and West Turkana, and how this translates into evolutionary dynamics [16]. These palaeoclimatic and palaeoecological studies also allow us to explore the relationships between the environmental context and evolutionary change. Uno et al. [21] highlight evidence from Turkana showing marked changes around 1.9 Ma, which, as they note, more or less coincides with the appearance of the Acheulean, as described by de la Torre [17]. Fortelius [16] also notes marked changes between 1.87 and 1.5 Ma, and this similarly coincides with a period of evolutionary dynamism. These early phases suffer from a lack of precise chronological resolution, so that the links between specific events or transitions in hominin evolution and climate change cannot be made absolute, but this is more feasible for the last quarter of a million years, where Mirazón Lahr [11] is able to link events in the evolution of human diversity to a background of climate change.
There is no doubt that areas beyond Africa are also important in human evolution—the dispersals of hominins into Asia, the evolution of Neanderthals and Denisovans, the evolution of H. floresiensis—but the significance of Africa remains.
5. Is there a pattern to transitions? From genotype to extended phenotype
Changes in the course of hominin evolution take many forms—they can be the evolution of new species, and the extinction of existing ones; they can be changes in morphology, known mostly from the skeleton, especially the cranium and dentition; they can be changes in technology and other aspects of behaviour inferred from the archaeological record; and, increasingly, they can be changes in gene distributions and frequencies, and the inferred lineages and populations of ancient humans. How do these relate to each other? Does behaviour (as attested in technology, for example) precede morphological change? Or vice versa?
There is no simple answer to this question. On the one hand, the appearance of stone tools by approximately 3.3 Ma [5] predates the known appearance of Homo by about half a million years and so also predates the expected relationship between expanding brains and technology. On the other hand, the appearance of the Acheulean appears to be close to the first known H. erectus/ergaster [17], and so is suggestive of a relationship. But in both cases there remains room for doubt. The age of the earliest Homo was recently increased from 2.3 to 2.78 Ma [22], and Kimbel [7] outlines the difficulties of pinpointing significant change; the age of the first tools changed by nearly a million years with the discoveries at Lomekwi. The evidence we currently have is much richer than even at the beginning of the twenty-first century, but it has still not stabilized to the point where such associations (or lack of them) can be regarded uncritically; indeed, the Signor-Lipps effect [23] would suggest that it never could be.
The more recent parts of human evolution can offer insights. The broadly accepted time scale for the origins of the modern human lineage is thought to be between 0.4 and 0.7 Ma, but there are grounds for recognizing transitions around and after this date. The dating is such that we can resolve a number of important events within that time scale—a genetic divergence from the last common ancestors with Neanderthals about 0.5 ± 0.2 Ka, the appearance of prepared core technologies at approximately 0.3 Ka, the appearance of a modern morphology by at least 195 Ka, dispersals across Africa at approximately 120 Ka and beyond between 80–60 Ka or before, elements of modern human behaviour by at least 160 Ka, and various accelerations in the accumulation of such traits by 120 Ka, and especially after 100 Ka [10,11,18,19].
Mirazón Lahr's [11] detailed breakdown of the phases by which a small ancestral population in Africa was transformed into a global, high density one, shows that when it is possible to observe the evidence in fine detail, there was a complex micro-evolutionary interaction of population collapses and expansions that continuously reshuffled diversity along a lineage, and transformed the human species.
6. Is there a pattern to transitions? The causes of transformations in human evolution
Placing the transitions towards becoming human into a chronological pattern and determining their environmental contexts are the first steps in a broader scientific enterprise, namely to gain insights into what caused these transformations. ‘Cause’ is, of course, a strong word, and by and large studies of human evolution have not been short of theories and models that attempt to find a single cause for the whole of it—an aquatic phase, seed-eating, hunting, pair-bonding, hair-losing, tool-making and so on. As has already been discussed, there cannot be a single cause of human evolution, because it consists of many independent transitions. Indeed, any of the transitions is likely to involve multiple events that have many factors contributing to them, ranging from the changing climate, the immediate environment, local resource availability, and demographic, cultural and social context. Biological factors, ranging from genes to life-history strategies, will play a part, but so too will behaviour. Bringing these all together is a major challenge to the field.
Several of the papers address this, particularly for the later part of the evolutionary time scale, and emphasize something that is often invisible for earlier periods—that demography and social group size and organization are central to human evolution. Marean [18] makes the points that it is strong group structures and equally strong inter-group rivalry that are important derived human characteristics, and so the critical question is under what ecological conditions might these occur in a way that transforms archaic human behaviour. Marean's answer is that it is the rich and predictable aquatic resources of southern Africa that—uniquely—provide these conditions. Tryon and Faith [19] pursue a similar argument in their paper in relation to the origins of the Later Stone Age in East Africa and propose that human adaptations may have been a response to coping with increased population size or density. Key to both arguments is that it is the interaction between ecological conditions and the way in which human social groups can distribute themselves across the landscape that are at the heart of major changes, so providing a direct link between evolutionary transitions and the ecological conditions in which hominins would have lived on the landscape. Collard [24] extends this through the analysis of ethnographic data to suggest that what is most likely to influence human hunter–gatherer adaptation is environmental risk, and that many of the adaptations that develop in the course of human evolution, particularly technological complexity, may be selected to reduce risk.
These examples focus on the foraging and ecological aspects of human adaptations, but these are just part of our overall phenotypic set, the traits that have emerged as critical to survival during the course of human evolution. Dean's [4] presentation of maturation patterns shows that shifts in life-history are likely to be equally important, and underlie many of the behavioural and cultural adaptations, while Antón [9] reminds us that the gap between genetics and culture is filled by many other possibilities, of which phenotypic plasticity in response to variable environments is an important one.
We are a long way from understanding the nature of the various transitions in human evolution, but we have made considerable progress in recognizing that there is no single cause, as there is no single event, and there is no single cause because many levels of biology and behaviour come into play.
7. Is there a major transition?
Maynard Smith and Szathmary [25] proposed that across the whole of evolution there have been a small number of major transitions, ones where the rules of the biological world are changed (for example, when sexual reproduction evolved), and greater complexity emerged. Human evolution was on their list, partly because humans possess a new means of information transmission (language and culture more generally), and thus potentially change the rules of evolution, and partly because we are so distinct compared with other animals. Vinicius [26] added concepts of changing patterns of modularity in the generation of complexity, leading to new information systems and higher levels of biological organization. Foley [15] considers the question of whether humans represent a major transition or not, focusing on the cumulative and dispersed nature of the events, seeing a pattern that is consistent with many of the individual papers in this volume. In terms of impact, and uniqueness among extant animals, humans in the course of their evolution have undergone major changes, but the relatively good visibility of the evolutionary record for humans (compared to other manor transitions) shows that it consists of many smaller cumulative changes, not a single major transition.
8. Future directions
A volume such as this is a snapshot of current research about human evolution, and strongly reflects recent discoveries and ideas. We hope that it will also serve a purpose in signposting future directions for research.
(i) Multi-proxy approaches. Traditionally, research into human evolution has been compartmentalized, by approach, by time and by continent. Day-to-day this may be inevitable, but the growth of multi-disciplinary projects is encouraging. Theoretically, models of cultural evolution are becoming more and more important, thus bringing archaeology into a more evolutionary framework; practically, material from sites can be subject to analyses from many different technologies, bringing light to bear on human evolution as a whole. The answers to many of the questions raised in this volume will come from greater integration across the disciplines involved, and welcoming further disciplines and technologies into the field.
(ii) New technologies and analytical methods. One of the unifying threads across the papers in this volume is the enormous impact of scientific techniques—for dating, for environmental reconstruction, for analysing morphology, for modelling behaviour. New ventures may start with finding a fossil or site, but it is the application of new technologies that is transforming the field. Beyond the sense of awe that we must feel knowing the colour of the eyes of people at the end of the Upper Palaeolithic, there is the recognition that palaeogenomics can test many old ideas more thoroughly than ever before, and reach aspects of the past that were not accessible from observations on fossils and stone tools alone.
(iii) The limits of concepts. We may like to think that research answers questions and solidifies knowledge, but it also opens up areas of uncertainty. This is true across the whole range of palaeoanthropology—for example, both the early hominins and the close relatives of modern humans are exposing the difficulties of thinking in terms of species as entities, thus pushing us towards more fluid models of how diversity evolves. The same holds true of archaeological concepts, as entities such as the Oldowan come under scrutiny through the discovery of the even more ancient lithics at Lomekwi. Future research is not just a question of empirically filling in the gaps around transitions, but also developing new concepts and models to tackle a more resolved set of data.
(iv) The nature of hominin variation. In the relatively simple world of palaeoanthropology of 50 years ago, variation in morphology reflected biology and genetics, and variation in technology reflected culture. We are now aware that there is much greater overlap and complexity. Stone tools may effectively be under some level of genetic control (see Collard [27]), and as Antón et al. discussed [9], morphological variation can reflect various levels of plasticity. But this in turn raises questions about the genetic basis for plastic responses and reaction norms. Future research will have to grapple with a much closer and more complex interaction between the biological and the behavioural, the cultural and the genetic. The exciting prospect is that new technologies may provide the tools to do so.
9. Expanding the record
The papers in this volume are testimony to the growing strength of new scientific methods, the breadth and integration of approaches, and the ability to place human evolution into the context of broader evolutionary concepts. These approaches have demonstrated remarkable power and offer equal potential. However, in the end, what we know about human evolution depends upon its fundamental basis, the fossil and archaeological records. It might seem that modelling, ancient genomes and three-dimensional morphometrics are the keys to the future, but all these depend on the continuing accumulation of new fossils and the discovery of new archaeological and palaeontological sites.
Expanding the record is hard work, time consuming, frequently arduous and is often considered to be high-risk by research funding bodies. Better to keep re-analysing existing material than spend money on unknown areas of unknown potential. There is often a long fallow period before significant results come in—the case of Louis and Mary Leakey working for three decades at Olduvai Gorge before finding ‘Zinjanthropus' is a classic case in point. However, without that personal and financial investment, there can be no progress in the field. Since the 1960s, that progress has been remarkable, particularly in Africa. In 1958, there were five or six accepted hominin taxa; now there are probably more than 25 recognized species, and seven genera have been named. Many people have contributed to this expansion, but none more so than Richard Leakey, both directly and through the support and assistance he has given to others (figure 4). Richard Leakey's career and achievements go well beyond palaeoanthropology, but within the field they are unparalleled. In the 1960s, he was instrumental in establishing the Omo as a major palaeoanthropological field site, swiftly followed by setting up the East Rudolf and Koobi Fora Projects. This rapidly yielded hominin fossils that revolutionized the understanding of the early African radiations. In the 1980s, Richard Leakey extended work to the western side of Lake Turkana and produced spectacular fossils ranging from the early Miocene to the early Pleistocene. One of the key things about this palaeoanthropological progress was the way in which fieldwork was interwoven with building up institutions—first with the National Museums of Kenya, then the Louis Leakey Memorial Institute, and most recently the Turkana Basin Institute, with its field stations on each side of the lake. Leakey was the first to recognize the importance of providing an infrastructure in which not only his work, but the work of others, could thrive, and, for a newly independent nation such as Kenya, capacity building could begin. Such initiatives take vision, but they also take practical organization and fund-raising, and over the decades Leakey has brought tens of millions of dollars into the field. Part of the skill of getting things done is to work well with others, and this has been a signature of Richard's approach to the many paleoanthropologists and related scientists with whom he has worked—most notably Glynn Isaac, Alan Walker and Bernard Wood. However, it is not just a matter of Richard leading and others following, for there is also the crucial partnership with Meave Leakey, who continues the pattern of major discoveries that she and Richard began in the 1960s.
Figure 4.

Richard Leakey in the field, with Kamoya Kimeu. Photo credit: Turkana Basin Institute/Richard Leakey. (Online version in colour.)
This volume is devoted to exploring the major transitions in hominin evolution, and shows that it is a complex and cumulative process. As we developed the programme and decided to focus on the major transitions we realized that all of these were topics to which Richard Leakey and his colleagues have contributed critical new finds. That we now recognize this complexity is in no small part due to the discoveries that Richard and Meave Leakey have made. In any CV, it is not too bad to be able to list the revelation of the diversity of early Miocene African apes [28,29], the earliest known australopithecine [30], the diversity of early Homo [31], the earliest known African H. erectus [32], the most complete ancient hominin skeleton [33], the most complete early paranthropine or robust australopithecine [34] and the first known anatomically modern human [35].
The Discussion Meeting sponsored by the Royal Society and the British Academy on which these papers are based was in part an opportunity to celebrate Richard Leakey's major contributions to the field as well as tackle some of the ‘big’ issues in human evolution, and it is a great pleasure and honour to dedicate this volume to him. We hope that his achievements will continue to inspire new generations to go out and explore our past in all corners of the world.
Acknowledgements
We would like to thank the Royal Society and the British Academy for their generous support. Clive Gamble, Hélène Roche, Mark Thomas and Bernard Wood chaired the sessions at the Discussion Meeting with great skill. This paper was improved by two anonymous reviewers. We would like to thank Jenna Lane and the staff at the Royal Society for their help and very efficient organization, and Helen Eaton for all the guidance she has provided in putting this issue together.
Biographies
Editor biographies
 Robert Foley is the Leverhulme Professor of Human Evolution at the University of Cambridge, a Fellow of King's College and a Fellow of the British Academy. He is a co-founder, with Marta Mirazón Lahr, of the Leverhulme Centre of Human Evolutionary Studies at Cambridge, an inter-disciplinary research centre. His research has focused on the ecological basis of human evolution, and in particular the application of Darwinian models to patterns and processes of human evolution, ranging from the early hominins to recent human populations. He has worked extensively in East Africa.
Robert Foley is the Leverhulme Professor of Human Evolution at the University of Cambridge, a Fellow of King's College and a Fellow of the British Academy. He is a co-founder, with Marta Mirazón Lahr, of the Leverhulme Centre of Human Evolutionary Studies at Cambridge, an inter-disciplinary research centre. His research has focused on the ecological basis of human evolution, and in particular the application of Darwinian models to patterns and processes of human evolution, ranging from the early hominins to recent human populations. He has worked extensively in East Africa.
 Lawrence Martin is Director of the Turkana Basin Institute and Professor of Anthropology at the University of New York at Stony Brook. He is a palaeoprimatologist whose research focuses on the evolution of hominoid primates and the development, structure and thickness of dental enamel. He has conducted paleontological fieldwork at Miocene sites in Cameroon, Kenya, Pakistan and Turkey. His laboratory research on enamel structure involves the use of confocal microscopy, polarised light microscopy, and scanning electron microscopy. His research has addressed phylogeny reconstruction for extant and extinct hominoid primates, species recognition in the fossil record and enamel development and thickness.
Lawrence Martin is Director of the Turkana Basin Institute and Professor of Anthropology at the University of New York at Stony Brook. He is a palaeoprimatologist whose research focuses on the evolution of hominoid primates and the development, structure and thickness of dental enamel. He has conducted paleontological fieldwork at Miocene sites in Cameroon, Kenya, Pakistan and Turkey. His laboratory research on enamel structure involves the use of confocal microscopy, polarised light microscopy, and scanning electron microscopy. His research has addressed phylogeny reconstruction for extant and extinct hominoid primates, species recognition in the fossil record and enamel development and thickness.
 Marta Mirazón Lahr, a Fellow of Clare College, is a Reader in Human Evolutionary Biology and Director of the Duckworth Laboratory at the University of Cambridge, where she and Robert Foley founded the Leverhulme Centre for Human Evolutionary Studies. The focus of her research is the evolution and diversity of our species, Homo sapiens. Her work involves a range of disciplines - human palaeontology, evolutionary genetics, behavioural ecology, linguistics and prehistoric archaeology. She has carried out fieldwork in the Amazon, the South Pacific, India, Oman, Libya and Kenya. She is the director of the ERC-funded IN-AFRICA Project in Kenya, and a co-investigator in the Trans-Sahara Project, and the African Genomes Project with the GeoGenetics Centre of Copenhagen.
Marta Mirazón Lahr, a Fellow of Clare College, is a Reader in Human Evolutionary Biology and Director of the Duckworth Laboratory at the University of Cambridge, where she and Robert Foley founded the Leverhulme Centre for Human Evolutionary Studies. The focus of her research is the evolution and diversity of our species, Homo sapiens. Her work involves a range of disciplines - human palaeontology, evolutionary genetics, behavioural ecology, linguistics and prehistoric archaeology. She has carried out fieldwork in the Amazon, the South Pacific, India, Oman, Libya and Kenya. She is the director of the ERC-funded IN-AFRICA Project in Kenya, and a co-investigator in the Trans-Sahara Project, and the African Genomes Project with the GeoGenetics Centre of Copenhagen.
 Chris Stringer has worked at The Natural History Museum London since 1973 and is now Research Leader in Human Origins and a Fellow of the Royal Society. His early research was on the relationship of Neanderthals and early modern humans in Europe, but through his work on the ‘Recent African Origin’ theory of modern human origins, he now collaborates with archaeologists, dating specialists and geneticists in attempting to reconstruct the evolution of modern humans globally. He has excavated at sites in Britain and abroad, and he is currently co-directing the Pathways to Ancient Britain project, funded by the Calleva Foundation.
Chris Stringer has worked at The Natural History Museum London since 1973 and is now Research Leader in Human Origins and a Fellow of the Royal Society. His early research was on the relationship of Neanderthals and early modern humans in Europe, but through his work on the ‘Recent African Origin’ theory of modern human origins, he now collaborates with archaeologists, dating specialists and geneticists in attempting to reconstruct the evolution of modern humans globally. He has excavated at sites in Britain and abroad, and he is currently co-directing the Pathways to Ancient Britain project, funded by the Calleva Foundation.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Conkey MH. 1991. Original narratives: the political economy of gender in archaeology. In Gender at the crossroads of knowledge: feminist anthropology in the postmodern era (ed. di Leonardo M.), pp. 102–139. San Francisco, CA: University of California Press. [Google Scholar]
- 2.Dawkins R. 2004. The ancestors’ tale London, UK: Weidenfield & Nicholson. [Google Scholar]
- 3.Jungers WL, Grabowski M, Hatala KG, Richmond BG. 2016. The evolution of body size and shape in the human career. Phil. Trans. R. Soc. B 371, 20150247 ( 10.1098/rstb.2015.0247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean MC. 2016. Measures of maturation in early fossil hominins: events at the first transition from australopiths to early Homo. Phil. Trans. R. Soc. B 371, 20150234 ( 10.1098/rstb.2015.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis JE, Harmand S. 2016. An earlier origin for stone tool making: implications for cognitive evolution and the transition to Homo. Phil. Trans. R. Soc. B 371, 20150233 ( 10.1098/rstb.2015.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leakey LSB, Tobias PV, Napier JR. 1964. A new species of the genus Homo from Olduvai Gorge. Nature 202, 7–9. ( 10.1038/202007a0) [DOI] [PubMed] [Google Scholar]
- 7.Kimbel WH, Villmoare B. 2016. From Australopithecus to Homo: the transition that wasn't. Phil. Trans. R. Soc. B 371, 20150248 ( 10.1098/rstb.2015.0248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood B, Collard M. 1999. Anthropology—the human genus. Science 284, 65 ( 10.1126/science.284.5411.65) [DOI] [PubMed] [Google Scholar]
- 9.Antón SC, Taboada HG, Middleton ER, Rainwater CW, Taylor AB, Turner TR, Turnquist JE, Weinstein KJ, Williams SA. 2016. Morphological variation in Homo erectus and the origins of developmental plasticity. Phil. Trans. R. Soc. B 371, 20150236 ( 10.1098/rstb.2015.0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stringer C. 2016. The origin and evolution of Homo sapiens. Phil. Trans. R. Soc. B 371, 20150237 ( 10.1098/rstb.2015.0237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirazón Lahr M. 2016. The shaping of human diversity: filters, boundaries and transitions. Phil. Trans. R. Soc. B 371, 20150241 ( 10.1098/rstb.2015.0241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gould SJ. 1980. Is a new and general theory of evolution emerging? Paleobiology 6, 119–130. [Google Scholar]
- 13.Vrba ES. 1985. Testing models of macroevolution—examples from Miocene-Recent African mammals. S. Afr. J. Sci. 81, 300. [Google Scholar]
- 14.Spoor F, Leakey MG, O'Higgins P. 2016. Middle Pliocene hominin diversity: Australopithecus deyiremeda and Kenyanthropus platyops. Phil. Trans. R. Soc. B 371, 20150231 ( 10.1098/rstb.2015.0231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley RA. 2016. Mosaic evolution and the pattern of transitions in the hominin lineage. Phil. Trans. R. Soc. B 371, 20150244 ( 10.1098/rstb.2015.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortelius M, et al. 2016. An ecometric analysis of the fossil mammal record of the Turkana Basin. Phil. Trans. R. Soc. B 371, 20150232 ( 10.1098/rstb.2015.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Torre I. 2016. The origins of the Acheulean: past and present perspectives on a major transition in human evolution. Phil. Trans. R. Soc. B 371, 20150245 ( 10.1098/rstb.2015.0245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marean CW. 2016. The transition to foraging for dense and predictable resources and its impact on the evolution of modern humans. Phil. Trans. R. Soc. B 371, 20150239 ( 10.1098/rstb.2015.0239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tryon CA, Faith JT. 2016. A demographic perspective on the Middle to Later Stone Age transition from Nasera rockshelter, Tanzania. Phil. Trans. R. Soc. B 371, 20150238 ( 10.1098/rstb.2015.0238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose C, Polissar PJ, Tierney JE, Filley T, deMenocal PB. 2016. Changes in northeast African hydrology and vegetation associated with Pliocene–Pleistocene sapropel cycles. Phil. Trans. R. Soc. B 371, 20150243 ( 10.1098/rstb.2015.0243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uno KT, Polissar PJ, Kahle E, Feibel C, Harmand S, Roche H, deMenocal PB. 2016. A Pleistocene palaeovegetation record from plant wax biomarkers from the Nachukui Formation, West Turkana, Kenya. Phil. Trans. R. Soc. B 371, 20150235 ( 10.1098/rstb.2015.0235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villmoare B, Kimbel WH, Seyoum C, Campisano CJ, DiMaggio EN, Rowan J, Braun DR, Arrowsmith JR, Reed KE. 2015. Early Homo at 2.8 Ma from Ledi-Geraru, Afar, Ethiopia. Science 347, 1352–1355. ( 10.1126/science.aaa1343) [DOI] [PubMed] [Google Scholar]
- 23.Signor PPW, Lipps JJH. 1982. Sampling bias, gradual extinction patterns and catastrophes in the fossil record. Geol. Soc. Am. Spec. Papers190, 291–296. ( 10.1130/SPE190-p291) [DOI] [Google Scholar]
- 24.Collard M, Vaesen K, Cosgrove R, Roebroeks W. 2016. The empirical case against the ‘demographic turn’ in Palaeolithic archaeology. Phil. Trans. R. Soc. B 371, 20150242 ( 10.1098/rstb.2015.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maynard Smith J, Szathmáry E. 1997. Major transitions in evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Vinicius L. 2010. Modular evolution: how natural selection produces biological complexity. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 27.Corbey R, Jagich A, Vaesen K, Collard M. 2016. The Acheulean handaxe: more like a bird's song than a Beatles’ tune? Evol. Anthropol. Issues News Rev. 25, 6–19. ( 10.1002/evan.21467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leakey RE, Leakey MG, Walker AC. 1988. Morphology of Turkanapithecus kalakolensis from Kenya. Am. J. Phys. Anthropol. 76, 277–288. ( 10.1002/ajpa.1330760302) [DOI] [PubMed] [Google Scholar]
- 29.Leakey RE, Leakey MG, Walker AC. 1988. Morphology of Afropithecus turkanensis from Kenya. Am. J. Phys. Anthropol. 76, 289–307. ( 10.1002/ajpa.1330760303) [DOI] [PubMed] [Google Scholar]
- 30.Ward C, Leakey M, Walker A. 1999. The new hominid species Australopithecus anamensis. Evol. Anthropol. Issues News Rev. 7, 197–205. ( 10.1002/(SICI)1520-6505(1999)7:6%3C197::AID-EVAN4%3E3.0.CO;2-T) [DOI] [Google Scholar]
- 31.Leakey MG, Spoor F, Brown FH, Gathogo PN, Kiarie C, Leakey LN, McDougall I. 2001. New hominin genus from eastern Africa shows diverse middle Pliocene lineages. Nature 410, 433–440. ( 10.1038/35068500) [DOI] [PubMed] [Google Scholar]
- 32.Walker AC, Leakey RE. 1978. The East Turkana hominids. Sci. Am. 239, 54–66. ( 10.1038/scientificamerican0878-54) [DOI] [PubMed] [Google Scholar]
- 33.Walker AC, Leakey RE. 1985. The Nariokotome skeleton. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 34.Walker A, Leakey RE, Harris JM, Brown FH. 1986. 2.5 Myr Australopithecus boisei from west of Lake Turkana, Kenya. Nature 322, 517–522. ( 10.1038/322517a0) [DOI] [Google Scholar]
- 35.Leakey REF. 1969. Early Homo sapiens remains from the Omo River Region of south-west Ethiopia: faunal remains from the Omo Valley. Nature 222, 1132–1133. ( 10.1038/2221132a0) [DOI] [PubMed] [Google Scholar]


