Abstract
Scientists have identified a series of milestones in the evolution of the human food quest that are anticipated to have had far-reaching impacts on biological, behavioural and cultural evolution: the inclusion of substantial portions of meat, the broad spectrum revolution and the transition to food production. The foraging shift to dense and predictable resources is another key milestone that had consequential impacts on the later part of human evolution. The theory of economic defendability predicts that this shift had an important consequence—elevated levels of intergroup territoriality and conflict. In this paper, this theory is integrated with a well-established general theory of hunter–gatherer adaptations and is used to make predictions for the sequence of appearance of several evolved traits of modern humans. The distribution of dense and predictable resources in Africa is reviewed and found to occur only in aquatic contexts (coasts, rivers and lakes). The palaeoanthropological empirical record contains recurrent evidence for a shift to the exploitation of dense and predictable resources by 110 000 years ago, and the first known occurrence is in a marine coastal context in South Africa. Some theory predicts that this elevated conflict would have provided the conditions for selection for the hyperprosocial behaviours unique to modern humans.
This article is part of the themed issue ‘Major transitions in human evolution’.
Keywords: human origins, hunter–gatherer, theory of economic defendability, territoriality, Africa
1. Introduction
The scope and diversity of the food quest among modern humans (Homo sapiens) is uniquely broad among all known species. In the past few millennia, the majority of humans have transitioned from predominantly hunting and gathering to more intensive forms of agriculture. The transition to food production has many hypothesized explanations, but a consequence is that the food resources became denser and more predictable in time and space. I will argue here that the evolution of hunting and gathering economies has also, in some regions and rather late in human evolution, trended in the direction of an increasing focus on dense and predictable resources. This reached a crescendo in Holocene climatic stability in a variety of locations worldwide. In several areas of Africa during the origins of modern humans, this shift was made relatively early.
Shifting one's food supply to denser and more predictable resources has many consequences. The theory of economic defendability [1], a key theory in behavioural ecology (BE), posits that the denser and more predictable a resource is, the more profitable it becomes to engage in costly behaviours to defend it. Dense and predictable resources are also attractive to a competitor and thus can stimulate recurring conflict. The theory of economic defendability has proved to be a powerful explanation for understanding when species practise territorial patrols and active defence of their territories [2–4]. It has been shown to be useful for predicting territorial behaviour among small-scale human societies as well [5–7], and its general anthropological significance has been recently reviewed [8] and formally modelled [9].
My goal is to initiate a discussion of the evolutionary significance of the shift to dense and predictable resources in human evolution, integrate territoriality theory into a broader general theory of hunter–gatherer variation, and then use this integrated theory to make some predictions about the sequence of evolution of some key evolved features of modern humans. Scientists have long identified a series of trends and milestones in the evolution of the human food quest and diet that are understood to have had far-reaching impacts on biological, behavioural and cultural evolution. These include the shift to a diet with substantial portions of meat, the broad spectrum revolution and the transition to food production. I propose that the shift to dense and predictable resources is another key dietary change that had consequential impacts on the later part of modern human origins. When certain populations made this shift it altered the selection regime in some fundamental ways. I hypothesize that the origin population for modern humans made this shift to dense and predictable resources, and thus was subject to high levels of territoriality and intergroup conflict, which provided the selection regime for high levels of cooperation with unrelated individuals within one's group. The downstream effect was that all modern humans inherited these hyperprosocial proclivities that are unique to our species.
2. A macroecological hunter–gatherer adaptive system theory
A general informal theory for hunter–gatherer mobility, social structure and technology has developed over the past 40 years, primarily in the archaeological literature, and has become useful for understanding past and present hunter–gatherer behaviour. While ethnographic exceptions to the theory certainly exist, overall it works well and provides some causative understanding for the variability observed among hunter–gatherers. This theory allows us to make the first-order well-informed predictions of the expected range of variation of fully modern hunter–gatherer adaptations under certain environmental conditions. It is helpful in contextualizing the modern human origins palaeoanthropological record [10,11]. I will summarize the theory here by identifying several dichotomous states that in reality are extremes of a continuum.
The theory as currently constructed deals most explicitly with three components of the hunter–gatherer adaptive system: mobility, technology and sociality. Territoriality has not been integrated, but I will do so in this paper. The theory began with the writings of Oswalt [12,13] and Binford [14–17], and was elaborated by others [18–23]. It developed as a consequence of hypotheses about the relationship between aspects of hunter–gatherer adaptations and environmental characteristics which were then explored with quantitative analyses, comparing these adaptations to each other and to environmental variables such as latitude, primary productivity and seasonality. These studies share some characteristics with macroecological theory and analysis [24–26].
Mobility refers to the way hunter–gatherer bands (i.e. local groups) move about the landscape. Movement types have been commonly classified as residential or logistical [14,16]. Residential moves are when the band moves all their members from one residential camp to a new location. Logistical moves are when a specialized task group, often targeting food that is clumped, moves away from a residential site and uses a separate camp and overnight stay (e.g. field camps). The collected food is then typically returned to a residential camp or stored somewhere (caches), for example where it can be intercepted on an annual round, for future use. War parties normally are not included in this classification, but there are examples of logistical moves to collect raw materials, such as the long and sometimes risky forays to collect ochre and special stone tool raw materials by some Australian Aborigines [27]. So, if a group of young men and women leave camp for 5 days to hunt and process caribou and return with it to the residential camp, as documented with the Nunamiut Inuit [28] that is a logistical move. The annual mobility of a hunter–gatherer group can be composed of varying combinations of logistical and residential moves, and a formal model has been offered [29]. In situations where residential mobility is much reduced, groups are often considered more ‘sedentary’.
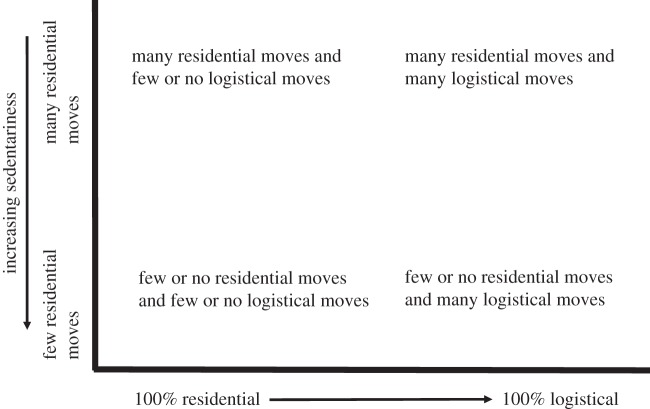
Generally, the more logistical moves a group makes, the fewer residential moves they make, though this is not always the case. Some groups, such as the Hadza [30,31], !Ko San [32] and Seriono [33] practised residential mobility almost exclusively. Most Inuit such as Central Inuit [34] and Northern Athapaskans such as the Kutchin [35] commonly have logistical moves embedded in a system of low residential mobility, though there is a great deal of specific variation between the groups. It is possible for groups to be highly sedentary (few residential moves) yet still practise little logistical mobility. For example, coastal hunter–gatherers residing in small territories that lack clumped and widely spaced terrestrial resources can be highly sedentary yet practise little or no logistical mobility, such as was typical of the descriptions of many California Indians [36]. While mobility studies typically consider only variation along an axis of relative amount of logistical versus residential mobility, they also need to simultaneously consider these relative to degree of sedentariness (figure 1).
Figure 1.
A schematic of the relative representation of residential versus logistical moves relative to the amount of sedentariness on an annual scale. Current mobility theory tends to classify groups only on the x-axis, but in reality the y-axis needs to be considered simultaneously.
Technological organization and complexity have received similar attention beginning with the work of Oswalt [12,13]. Some hunter–gatherers have very light technologies composed of few separate parts where the emphasis is on its suitability for regular movement while still being effective. Other hunter–gatherers have highly complex technologies with many separate parts, often composed of multiple raw materials, and these can sometimes be rather heavy to carry. Following our example above, the Hadza and !Kung San have light technologies with few separate parts, while the Central Inuit [34], Kwakiutl of the Pacific northwest coast [37] and Kutchin [35] have elaborate, multi-raw material and heavy technologies. There have been many functional reasons postulated for this variation. Oswalt originally proposed that the relative amount of hunting in the diet was a driver of technological complexity, while Osborn [38] showed that aquatic hunting tended to produce more complex tools than terrestrial hunting. There is an emerging consensus that resource risk drives much of this variation among hunter–gatherers [23,39–41], while high levels of residential mobility constrain the complexity of toolkits [42].
Hunter–gatherer social structure has been a focus of this research and most researchers recognize a spectrum of variation from egalitarian to non-egalitarian (hierarchical, ranked or stratified) systems [43–45]. In egalitarian systems, differential power and authority held by individuals are reduced or even non-existent. Sometimes, there are strong social mechanisms in place to ‘level’ people [46] of differing abilities and inclinations, and there can be an ethos of egalitarianism. The Hadza [44] and the !Kung San [47] are strongly egalitarian. In non-egalitarian systems, power and authority differ between people, social and economic stratification can occur, and some hunter–gatherer groups even reach levels of ranking and social complexity near to that of food-producing societies. The Aleuts of the Aleutian Islands [48], the Chumash Indians of California [49], and some Northwest Alaskan Inuit [50] had non-egalitarian societies and even kept slaves.
There are systematic co-associations of mobility, technology and social structure across these systems driven by similar adaptations to structural aspects of the environment. This has been probed and recognized by a wide range of studies that have essentially followed what would be recognized as a macroecology approach [25,26]. In macroecology, the various characteristics of species (body mass, size of range, etc.) are statistically analysed relative to each other and environmental variables. In this ‘human macroecology’, ethnolinguistic groups typically replace species [17,24,51]. Many interesting relationships have been uncovered that have revealed potential causative relations that have important implications for human origins.
Binford [14–17] recognized that at the level of mobility and technology, hunter–gatherers tend to adapt differently to environments where resources occur sequentially and clumped versus those where they occur more homogeneously in both time and space. He argued that in environments with high levels of temperature seasonality, hunter–gatherers have short periods of time during the year when they must collect large quantities of prey and store the food they provide. In such environments, most of the available food is in the form of animal prey, with plants often absent between late autumn through to spring. These animals are often clumped in time and space, and include prey like salmon, whales, migrating caribou, etc. This often requires complex technology that has a high performance quality, resulting in complicated technological systems. In such situations, logistical mobility of specialized task groups makes sense to create these surpluses, and the surplus is moved to people and stored for future use. The stored food and logistical moves lessen the need for regular residential moves, so residential mobility is reduced and logistical moves increased. Overall, these contexts often result in sedentary behaviour. Woodburn [44] argued that in such situations the storage of food offers the opportunity for individuals to accumulate differential surplus, and this then stimulates in these ‘delayed-return’ systems a breakdown in egalitarianism and can lead to the non-egalitarian societies typical of ‘complex hunter–gatherers'. Mattison et al. [52] argue that the optimal conditions for the emergence of ‘persistent institutionalized inequality’ are when hunter–gatherers increasingly rely on dense and predictable resources.
Environments with more muted temperature seasonality provide conditions less dependent on the production of stored food and often have more plant foods available for longer periods of the year than highly seasonal environments. This reduced reliance, or even non-existence of storage, results in little need for logistical moves and complex technologies to create surplus, and the overall pattern is to forage across the landscape, moving people to the food. Woodburn [44] recognized that such ‘immediate-return’ societies tend to be egalitarian. Tropical terrestrial environments tend to have muted temperature seasonality, and thus the African environment of human origins would have primarily been one where we would expect, with perhaps some exceptions, a modern human hunter–gatherer adaptive system typified by high residential mobility, little to only short-term storage, light technology with relatively few separate parts and egalitarian social systems. Our closest living relatives the chimpanzees lack egalitarian social structures and have significant hierarchy, so the egalitarian system is derived [45].
3. Integrating intergroup conflict and the general hunter–gatherer adaptive system theory
The theory of economic defendability rests on the differing characteristics of resource availability, as does the basic hunter–gatherer theory outlined in §2, and thus it is appropriate to synthesize the two. Territoriality and intergroup conflict are part of the broader hunter–gatherer adaptive system and need to be considered in the context of the resource base, mobility and sociality. Violence is an integrated part of the hunter–gatherer adaptive system, being a ways and means to dominate control of food and raw materials and mates, obtain all three through a risky act, and weaken competition over the short and long terms. Zefferman & Mathew [53] define warfare as requiring three dozen warriors. I will relax the ‘number of warriors' as there is excellent ethnographic evidence for significant conflicts among hunter–gatherers that are clearly group cooperative yet smaller in scale, and I will use the term ‘intergroup conflict’. It is important to recognize that intergroup conflict, at any scale, normally requires solving collective action dilemmas [53]. I simply accept here that these dilemmas were solved [8], as my focus is on the stimuli underlying conflict among African hunter–gatherers.
A territory is ‘an area occupied more or less exclusively by an animal or group of animals by means of repulsion through overt defense or advertisement’ [54, p. 256]. There has yet to be a systematic assessment of the societal scale at which hunter–gatherer warfare occurs, despite the widespread interest in the topic. There is clear evidence that intergroup conflict occurs regularly between ethnolinguistic groups, and ethnographers have described very high levels of distrust and fear between ethnolinguistic groups (e.g. Australian Aborigines [55] and Inuit and Northern Athapaskans [56]). Intergroup conflict is documented within ethnolinguistic groups (such as among villages of Chumash [49] and clans of Murngin Aborigines [57]). Modern human territoriality is then the overt defence of a geographical space by a group, and the societal scale can vary.
What then is the relationship between territoriality, its theory and intergroup conflict? Territoriality as a strategy for resource control is often the trigger for intergroup conflict when that conflict has as its goal defending incursions from other groups, attacking other groups to take their resources or land, or an overall posture of aggressive spatial defence and control. Often this aggressive posture is about food resources, raw materials of importance or women. Defence may not be of the entire home range, but rather specific resources and their habitat. Warner describes how Murngin defended specific ‘land along the sea, the bays, inlets and tidal rivers' and myths and folklore documented the case for exclusive access. The dry lands in between had no known ownership [58, p. 18].
(a). The theory of economic defendability
The theory of economic defendability, originally proposed by Brown [1], has risen to the level of a ‘unifying principle of BE’ [2], and is considered one of the most well-known and powerful theories in BE [59]. The principle is that resource defence entails costs along with the benefits that accrue from exclusive access. When fitness benefits of defence surpass costs, then natural selection will favour organisms who defend access to those resources by delimiting a space around them and patrolling and defending that space. Costs are higher when the temporal and spatial predictability of the resource is low, and when density is so low that a large area is required for patrol. In sum, boundary defence is favoured where high-ranked resources are dense and predictable. This is a cost–benefit theory where ‘territorial behavior is expected when the costs of exclusive use and defense of an area are outweighed by the benefits gained from this pattern of resource use’ [5, p. 23].
The theory has clear and important implications that have been demonstrated among numerous animals. Because larger areas cost more to defend [60], they are less likely to be defended. Resource defence leads to more extreme resource monopolization. Highly clumped resources of this type can be privatized, and privatization has many benefits. Privatized resources can be held for future use by the owner or others chosen by the owner, such as offspring, kin or an ethnolinguistic group [61]. Thus, territorial defence of dense and predictable resources can be expected to stand at the origin point of land tenure systems. Resources are best defended cooperatively, and thus modern humans with language and highly cooperative proclivities have the tool-sets to organize territorial behaviours at great complexity. For example, hunter–gatherers do not always practise active boundary defence—they can employ a more subtle ‘passive’ or social boundary defence [7,62,63].
(b). Resource density, predictability and the general hunter–gatherer adaptive system theory
Defended resources can include anything of fitness value within a geographical space, but normally include food, raw materials and mates. Most BE applications examine defence of space within which are found food and mates [64]; modern humans defend these same things. The general hunter–gatherer adaptive system theory described above rests strongly on resource seasonality as a causative mechanism. Many resources that are dense and predictable are also highly seasonal: salmon runs on the northwest coast, caribou migrations in the Brooks Range and fur animals clustered about streams in Canada are all seasonal, dense and predictable resources that led to territoriality. It is important to note that all stored resources are, once stored, dense and predictable. Settlements of sedentary hunter–gatherers, with their stocks of valuable material culture and stored food, warrant defence. Alternatively, some resources that are dense and predictable are not always seasonal. Intertidal molluscs on the South African coast are dense and predictable, but their abundance is not a function of season, though some do undergo changes in quality that are related to seasonal breeding cycles. They are easily exploited every new and full moon during these low spring tides, and a forager who understands this can exploit them at high returns during these times [65,66] at levels that can exceed the return-rate of hunting [67]. Owing to this dense and predictable nature of coastal resources, coastal hunter–gatherers are some of the most territorial and conflict-ridden among all known hunter–gatherers [50,68–71].
In summary, the highly seasonal resources that typically lead to low residential mobility, storage, complex technologies and reduction of egalitarianism are often dense and predictable, but not always. We can use economic defendability theory to propose the following: when humans expanded their foraging niche to include dense and predictable resources as a regular food item, they became more territorial to protect the space surrounding those resources, and used intergroup conflict as a strategy against competing groups. Hunter–gatherers exploiting dense and predictable resources tend to be more sedentary, and settlements themselves take on value due to the presence of stored food, stocks of material culture, and mates and offspring. Economic defendability theory would treat these settlements as a hotspot of value to be defended. Elsewhere I have summarized other ethnographically and archaeologically documented, and theoretically sound, impacts of a shift to coastal resources (table 1). Such societies have the pre-conditions for a more sedentary mobility strategy, reduced egalitarian ethos and more complex technologies, as predicted by the general hunter–gatherer adaptive system theory. Such hunter–gatherer societies are pre-adapted to food production and thus we might expect that the transition to food production may have sometimes occurred with them.
Table 1.
The impacts and consequences of a hunter–gatherer foraging shift to regular use of coastal resources. See [72] for discussion.
| consequences | |
|---|---|
| diet | |
| constant year-long access to protein, fatty acids, omega-3 | reduced child mortality |
| overall improved health | |
| population growth | |
| mobility | |
| residential mobility reduced, more sedentary | stored resources, material culture, mates, and children concentrated in a ‘village’ |
| more permanent dwellings and formation of ‘villages’ | |
| defence of territory | |
| technology | |
| sometimes increased investment in complex technologies | increased craft specialization |
| more intensive storage | |
| permanent dwellings | |
| sociality | |
| larger group (band/local group) sizes | increased population density |
| easier to defend resources and land around them | |
| easier to assemble large war parties | |
| sometimes a reduction in egalitarian social ethos | hierarchical or ranked societies |
| elevated levels of intergroup conflict | high injury and mortality rates |
| group extinction | |
| general societal discord | |
| slavery | |
| long range social networks | information flow and trade |
| long range gifting and trade | |
| reciprocal exogamy cycles widely across geography |
4. Which African environments have dense and predictable food resources?
The African ethnographic record for hunter–gatherers is a limited sample of the variation that was originally present prior to the introduction of food production, but we do have excellent records of hunter–gatherers in arid bushlands and savannahs, (Khoi-San and Hadza), montane forests (Okiek) and tropical forests (Pygmies). We lack ethnographic records of hunter–gatherers in what we might think of as the environmental sweet-spot of Africa—rich well-watered (600–1200 mm yr−1) tropical savannahs (see discussions in [73,74]). Hunter–gatherers were driven out of these areas during the expansion of pastoralists. I will use a combination of environmental and ethnographic records to conduct an assessment of the potential availability of dense and predictable resources, making a basic distinction between the African Paleotropics and the Greater Cape Floristic Region (GCFR), since these differ so widely in basic botanical characteristics.
In African Paleotropical arid lands and savannahs, the plant foods are highly seasonal. This is well illustrated in the ethnographic reports [30,75,76]. Plants with underground storage organs are present but require substantial effort to harvest and generally have low return rates and are fibrous [77–81]. Above-ground plant foods including fruits, seeds, pods and other edible parts are found mostly on bushes and trees [82–85]. With some exceptions, the ethnographic records emphasize a strategy of collection shaped by a highly diverse and sparse plant food base. Only the mongongo nut [47,86] approaches a dense and predictable plant food, but fails to approach the density and predictability of acorns in California [87] and pinyon pine nuts in the Great Basin [88], both of which drove moderate levels of logistical mobility and variable levels of territorial behaviour. Honey is highly valued and is dense upon encounter, but is unpredictable in location, and is depleted after one foraging bout [89].
In the GCFR, there is no systematic study of the plant foods that could be exploited by people. The diversity of plants with underground storage organs surpasses all known environments, with approximately 2300 species [90,91], and there are archaeological records and historical observations of some of these being consumed [92,93]. Recent studies are just beginning to document their abundance and foraging potential, but so far the data suggest that these would not rise to the level of a dense and predictable food resource that warranted territorial behaviour [94,95].
The animal foods in Paleotropical African grasslands include both residential and migratory animals. The GCFR lacks the large grasslands that support migratory prey, though in the Pleistocene these were available on the now submerged Paleo-Agulhas Plain [93]. The African residential prey (such as duikers, dik-diks and bushbuck) tend to occur as solitary or paired animals in bush and woodlands habitats [96,97], never reach the densities required to be classified as a dense and predictable resource, and are easily depleted in their patches in one foraging bout. Migratory animals tend to be gregarious and occur in open environments. I have reviewed the characteristics of tropical grassland ungulates as a prey item and the manner in which ethnographically documented hunter–gatherers exploited them [74]. Overall, one would not expect African hunter–gatherers to practise territorial behaviour in defence of the ranges of these migratory prey, except perhaps in some rare locations where bottlenecks in their migration would occur. Great Plains Indians were known to be territorial [98], but the costs of patrol were lower due to the use of horses.
African tropical and montane forests have a good ethnographic record from which to infer the character of the resource base. The abundance and richness of plant foods in tropical forests has been the subject of debate, but it is safe to say that there is nothing that would be considered dense and predictable and high ranked [99,100]. Similarly, in the montane forests, plant foods are rare and unpredictable [101,102]. Honey is a dense and high-ranked food resource, but unless one is controlling the location of the hives, it is unpredictable. Okiek hunter–gatherers increased honey harvests by seeding territories with hollowed out tree trunks [102–104]. It is unclear from the published record if the Okiek defended these territories, but we certainly would expect that. There are no prey animals in these forest environments of a dense and predictable nature.
Lake and riverine environments in Africa can in some cases have substantial dense and predictable resources including fish, crocodiles and hippopotamus. The one hunter–gatherer record I know of documenting intensive fishing is the El Molo of Lake Turkana, Kenya [105,106]. All of these lake and riverine resources require substantially complex material culture to exploit. Some coastlines in Africa offer dense and predictable intertidal zones that require no complex technology to exploit, but do require a sophisticated knowledge of the relationship between lunar-driven tides and intertidal foraging [65,66]. The richest coastlines for intertidal foraging are in colder waters that are often enriched with upwelling systems. These are present across southern Africa [107,108] and the Atlantic coast of North Africa [109]. The Mediterranean has relatively low nutrient availability and thus is not a highly productive environment for intertidal foraging [109].
In summary, the evidence suggests that for the most part African terrestrial ecosystems lack the type of dense and predictable food resources that would trigger territoriality. Aquatic resources in Africa, including the rich intertidal zones off the coasts of northern and southern Africa, and certain riverine and lacustrine ecosystems, have resources worth defending.
5. Discussion
(a). What does this theory predict for the evolution of modern humans?
One of the primary goals of palaeoanthropology is to describe when, where and under what contexts various important anatomical, behavioural and cultural characters evolved. We also seek to explain the evolution of these characters. A primary step in this endeavour is to put the key features on a temporal sequence (e.g. [45,110])—this not only functions to create a beginning informal model that can be tested, but it also helps begin the process of placing them on a chronometric timeline so that it can be compared to the empirical palaeoanthropological record. I recently proposed such a sequence [72,111] for the later stages of human origins. Here, I integrate that sequence with the general hunter–gatherer adaptive system theory outlined above and compare its predictions to the empirical evidence. I start with the propositions that modern humans have a unique capacity for culture, the primary mode of human adaptation, and that this capacity rests on three key evolved features that are shared by all modern humans [111]: (i) a uniquely advanced cognition, (ii) a proclivity to cooperate with non-kin that exists at a level well above any other animal (hyperprosociality resulting in ultra-sociality [112]) and (iii) an extreme reliance on special types of social learning.
Given the above discussion, we can conclude that with the exception of eutrophic coastlines and technologically advanced exploitation of rivers and lakes, we would expect that a modern human in Africa exploiting terrestrial resources would generally not have access to dense and predictable food resources. Given this, the dominant adaptation of early modern humans in Africa prior to a shift to dense and predictable resources would be highly mobile and use a large territory, practise high residential mobility with little logistical mobility, rely on light technologies with relatively few differing parts and raw materials, and have relatively low levels of territoriality. Only when modern humans broke into either the coastal or lake and riverine foraging niche did this system move substantially in the direction of persistent territorial behaviour and intergroup conflict. This means that much of the variation in mobility through human evolution would be on the far left side of mobility in figure 1, with increasing sedentariness with shifts to dense and predictable resources.
I have argued elsewhere that the archaeological evidence suggests that the shared common ancestor of modern humans and our other close relatives such as Neanderthals likely had a cognition and social learning machinery that was close to that of modern humans, but that the hyperprosociality characteristic of modern humans was not in place and thus was the last key addition to the modern human suite of unique features [111]. Its appearance requires an evolutionary explanation. In this model, which shares some features with that proposed by Tomasello [113,114], this shared common ancestor (perhaps Homo heidelbergensis), in Africa lived primarily off these terrestrial resources that were generally sparse and unpredictable. This species was reliant on technology, and as a result of this experienced a slow dual ratcheting up of technology and cognition. It used stabbing and hand-cast stone-tipped spears as its primary weapons [115], but lacked a complex technological kit. It hunted cooperatively in a way made possible by an evolved ‘joint intentionality’ [113,114]. But lacking ‘collective intentionality’ and the modern human hyperprosociality, it had essentially a chimpanzee-like social structure, no inter-band multi-scale structure (no tribes), and thus no ethnolinguistic group structure. These groups would have reasonably low levels of territoriality due to the sparse and unpredictable resource base, and would have been highly mobile with only residential mobility. It is possible that egalitarian social structure characterized this hominin, and this would have been possible if joint intentionality had evolved on top of at least moderate levels of prosocial behaviour and language capacity [113]. Technology evolved slowly because populations were small and networks ineffective at information transfer, as predicted by theory [116–118] and documented by the long relatively unchanging nature of technology from 800 to 300 ka [119]. The ‘cultural niche’ [120] was not at this time the predominant mode of hominin adaptation, because both technology and sociality were relatively simple, though hominins had become reliant on technology.
Over hundreds of thousands of years of slow gene–culture coevolution, the cognition of this hominin evolved to the point where it was able to recognize novel associations, use symbols, communicate via language and thus start to do complex analytical tasks such as construct symbolic systems of time and space. Elsewhere I have suggested that once this happened, early modern humans could recognize the relationship between lunar cycles, tidal systems and their productivity, and thus design symbolic calendar systems that allowed them to time their visits to the coast so as to make productive use of the coastal zone [65,66,72]. I made a distinction between ‘systematic coastal foraging’ and a true ‘coastal adaptation’: ‘systematic use of coastal resources is when coastal resources are part of a plan, occur regularly and recurrently, but the use of these resources is not transforming’ [72]. A ‘coastal adaptation’ is when the adaptation has been transformed to revolve around the sea. Marine intertidal resource use does not require complex technology, while systematic fishing of African lakes and rivers does. In the case of the latter, harpoons, often finely made in bone [121], and/or woven nets are required [122]. Fishing the intertidal zone can be done with stone fish traps, which are not particularly complicated, but also with hook and line tackle, which is a more complicated technology. I think it likely that the development and retention of fishing technologies likely requires large inter-connected populations. For this reason, I suggest that the first entry into the dense and predictable foraging niche involved the ocean intertidal, not the lakes and rivers. This is of course directly testable with sufficient archaeological data.
In my model, this entry into the coastal foraging niche triggers a cascade of new adaptations, so well documented in the ethnographic literature, including reductions in residential mobility with consequent increased sedentariness, increasing population size and territoriality in defence of those intertidal food sources. This sets up the conditions identified as optimal for the multi-level selection for highly cooperative behaviours (hyperprosociality) [123,124], and also provides the selective drive for the ‘group-mindedness' made possible by an evolved ‘collective intentionality’ [113,114]. Human sedentariness creates a resource of unusual density and predictability—a ‘village’ replete with stored food, costly material culture, and a concentration of females and offspring, which to an antagonistic group can be viewed as potential mates, slaves or food. Wilson [125] observes that every known case of insect eusociality involves a ‘nest’, and he suggests that the trigger for the evolution of insect eusociality was the defence of these nests. He draws a parallel between insect nests and early human campsites. Campsites among hunter–gatherers with high levels of residential mobility, of the type we would expect before the shift to dense and predictable resources, lack the energetic investment of insect nests. But when hunter–gatherers shift to higher levels of sedentariness with the transition to dense and predictable resources, campsites now become more like villages, and at this stage we may have the parallelism to insect nests suggested by Wilson.
(b). The palaeoanthropological record for a shift to dense and predictable resources
Table 2 is a summary of the evidence for the location and timing of systematic use of dense and predictable resources in Africa. Marine intertidal foraging is separated from lake and river fishing. For the former, I include both systematic coastal resource use and a true coastal adaptation. Elsewhere I have argued that, with the evidence at hand, the North African record does not document a coastal adaptation, but it certainly shows consistent use of the intertidal zone [72].
Table 2.
The archaeological evidence in Africa for the exploitation of dense and predictable resources. NR, no record; ka, thousands of years; MSA, Middle Stone Age; LSA, Later Stone Age; GCFR, Greater Cape Floristic Region.
| time interval ka | marine isotope stage | industrial complex | systematic lake and river fishing |
systematic marine intertidal foraging |
other | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| North Africa | East Africa | Central Africa | southern Africa | North Africa | East Africa | South Africa | ||||
| <14 | 1 | LSA | Nile river | rift lakes | river and lake | Kalahari lakes | NR | NR | GCFR coast | land snails |
| Saharan lake [122,126] | [127] | [128] | [93] | Maghreb [129] | ||||||
| ∼29–14 | 2 | LSA | Nile river [122] | NR | river and lake [130] | NR | Atlantic coast [131] | NR | NR, but probable [93] | land snails Maghreb [129] |
| ∼60–29 | 3 | LSA/MSA | NR | NR | NR | NR | Atlantic coast [131] | NR | GCFR coast [93] | NR |
| ∼74–60 | 4 | MSA | NR | NR | NR | NR | Atlantic coast [131] | NR | GCFR coast [93] | |
| ∼130–74 | 5 | MSA | NR | NR | river [132] | NR | Atlantic coast [131] | NR | GCFR coast [93] | |
| ∼195–130 | 6 | MSA | NR | NR | NR | NR | NR | NR | GCFR coast [93] | |
| ∼240–195 | 7 | MSA | NR | NR | NR | NR | NR | NR | NR | |
The earliest evidence for the exploitation of dense and predictable resources is in the intertidal zones of the GCFR, with Pinnacle Point (PP) 13B dated to approximately 162 ka, and then many sites dating between 110 and 50 ka. We can confidently conclude that the coastal intertidal zone in the GCFR was consistently used from MIS5 to MIS4. Owing to the dramatically lower sea levels during MIS6, and the far distance to the coast during that time, it will be a challenge to find sites dating to this time in the GCFR [133] though the attempt is underway where the continental shelf drops off abruptly [134].
The Pleistocene fossil ungulates in the MSA deposits at PP are dominated by gregarious species of the type we would expect to migrate [135]. The western portion of the GCFR is dominated by winter rains and the east by summer. It has been hypothesized that during glacials and lower sea levels when the Paleo-Agulhas Plain was exposed, a large ungulate migratory ecosystem evolved that passed between the PP sites and the coast [66]. At glacial maximum, the width of the plain between the PP sites and the coast was as much as 95 km, but during most of MIS5 the plain was rather narrow, fluctuating 1–5 km in width. A strontium isotope study of the fossil migratory ungulates found in the PP sites shows that these animals lived exclusively on this now submerged Paleo-Agulhas Plain [136]. Thus, there existed a rather unique situation where on a regular seasonal basis, a large ungulate migration passed across a plain in front of the caves. During glacial phases such as MIS4, this plain was wider, and during MIS5 it was narrower. Where the caves and rockshelters are today, coastal cliffs normally rise abruptly.
During MIS5, it is thus plausible that during times when sea level was moderately high, recurrent bottlenecks were created where migratory prey were in direct proximity to the coastal intertidal zone and the caves and rockshelters. The narrowness of this plain and the concentrated nature of the migration could very well have made possible territorial defence during the seasons of migration, and certainly the rich intertidal zones in close juxtaposition would be predicted to have been defended. The archaeological records at PP5–6 and PP13B document the continued importance of shellfish throughout MIS5, and during periods of very high sea level in MIS5 when the plain was just several kilometres wide, very intense use of the intertidal zone [66,137] combined with large ungulate hunting at PP13B [138].
By contrast, during MIS4 the width of the plain would have made the migration more dispersed and less predictable and removed the bottleneck between the coast and the cliffs. While the intertidal zone was more distant, the record at PP5-6 shows clearly that during MIS5 through MIS4 the coastal zone remained a significant target for foraging. At this time, there would be no local migration bottleneck, but it is likely that, due to the significantly larger plain, the migration ecosystem included substantially larger ungulate populations. With the coastal zone further removed, and the ungulate populations large, it seems likely that large mammal hunting would have taken on a greater role in the adaptive system. There is sedimentological evidence that occupation intensities at PP5-6 increased dramatically from MIS5 to 4, with the MIS4 sediments displaying intense occupation and on-site burning, and the older MIS5 sediments showing short sporadic occupation [139]. This suggests that the combined abundance of large plains animals and rich intertidal beds made the PP locality extremely attractive for lengthy hunter–gatherer occupation.
The Atlantic North African coast records consistent use of the intertidal zone in MIS5. The density of shell, as currently reported, does not meet the definition of a shell midden, so at this time, the intensity of coastal resource use does not meet the level displayed in South Africa, and does not appear to be a focused coastal adaptation [72]. However, we need further research on these important localities, and more detailed publication of the mollusc assemblages. There is one isolated MIS5 example of riverine resource use, complete with complex bone harpoon technology in association with fish fauna, at the Katanda locality on the shore of Lake Rutanzige [132,140]. Validating this observation should be a field research priority.
By MIS2, there is a focused commitment to the use of dense and predictable resources throughout the African continent where rivers, lakes and rich coastlines were available. It has long been recognized that there is a widespread pattern of intensive fishing with harpoon technology centred on the Early Holocene lakes of the Sahara and spread south and east to the Nile river and East and Central African Rift lakes [141]. Holocene harpoons and fishing in the Kalahari attest to the continental scale of this tradition [128], documenting a continentally connected network. The sites at Ishango show clearly that this harpoon-based fishing tradition has an origin at least as early as 18 ka [121]. On the marine shores of the GCFR in the Holocene, there was also a shift to intensive fishing, though here it was done with hook and line tackle [93].
(c). The palaeoanthropological evidence for intergroup conflict
One prediction of the model set forth here is that systematic intergroup conflict, occurring between ethnolinguistic groups and/or other lower level social units (such as clans), should increase substantially and become a societal fixture after a shift to significant use of dense and predictable resources. This will be difficult to evaluate archaeologically because such an evaluation requires large skeletal populations where the analyst can confidently assign levels of violence to intergroup conflict versus interpersonal violence.
There is an increasingly rich record of butchery of hominins, but this does not necessarily document violence—it is possible that some of this includes ritual defleshing or cannibalism of deceased group members. For example, cannibalism has been argued for the group of Neanderthals at El Sidrón [142] and ritual defleshing for Herto [143]. In many Palaeolithic archaeological cave and rockshelter sites, isolated human skeletal material is sometimes found co-mingled with artefactual and non-human faunal material. When this is the context the hominin remains are often butchered, e.g. Plio-Pleistocene Sterkfontein Member 5 [144], Middle Pleistocene Gran Dolina TD6 [145] and Upper Paleolithic Eshkaft E-Gavi [146]. At this stage, despite the clear evidence for butchery of hominins, we cannot assess levels of intergroup conflict in the Paleolithic.
The Late Pleistocene and Holocene record of hunter–gatherer skeletal material may indicate a change. In Africa, one of the best-known cemeteries of Early Holocene hunter–gatherers is Jebel Sahaba in a riverine context where about 40% of the people died violently [147]. An intergroup violence incident is recorded in West Turkana, also in the context of an aquatic adaptation [148]. During the Holocene in South Africa, there is a large population of skeletal material derived primarily from individual burials. Signs of violence are rare, though it is important to note that the poison arrows that were one of the main weapons in this region are unlikely to have left skeletal trauma. There is an increase in the evidence for violence during a period in the Late Holocene when there are signs of more sedentariness and strong territorial marking [149]. While this evidence may suggest an increase in violence with the shift to dense and predictable resources, a problem is that the needed skeletal populations normally are only present in cemeteries, and cemeteries typically are produced by hunter–gatherers with more sedentary lifestyles [150] often supported by a subsistence regime based on dense and predictable resources. So while there are intriguing signs of inter-group conflict in Africa associated with the use of dense and predictable resources, the record is insufficient to allow a confident inference that there is an increase in intergroup conflict when the shift to dense and predictable resources occurs. More research and data are needed.
6. Conclusion
There is broad agreement that three major dietary transitions occurred in human evolution: a commitment to meat eating as a regular and substantial portion of the diet, the broad spectrum revolution and the transition to food production. A fourth is offered here—the transition to hunting and gathering of dense and predictable resources. This transition involved numerous substantial impacts on the overall hunter–gatherer adaptive system and created a new selection regime that may have brought about hyperprosociality.
I have shown how the macroecological hunter–gatherer adaptive system theory can be integrated with the theory of economic defendability. When combined with a review of the main terrestrial resource types in Africa, this predicts that the majority of human evolution in Africa was characterized by high residential mobility, little or no logistical mobility, large undefended territories and very light technology with few separate parts. By the middle Middle Pleistocene with Homo heidelbergensis, the larger evolved brain provided the capacity for an advanced cognition, but this hominin did not yet have the cultural niche as its primary adaptation, because it lacked the social learning and hyperprosociality of modern humans, which evolved later under special circumstances uniquely with H. sapiens, as outlined in §5a.
Eventually, this hominin reached a cognitive level where it could make novel connections, create symbolic systems and develop basic analytical systems such as calendars. When this occurred it broke into the coastal foraging niche on the coasts of South Africa and North Africa. The theory of economic defendability predicts that at this time, when these new dense and predictable resources were exploited, territoriality and intergroup conflict would have occurred, and this provided the selective pressures for the evolution of high levels of cooperation with unrelated kin. The marine coastal zones of South and North Africa provide the first recurrent evidence anywhere in the world for a commitment to the systematic exploitation of dense and predictable resources.
Acknowledgements
I thank the organizers of the conference where this paper was presented, and Kim Hill, Bob Kelley, John Shea and Eric Alden Smith for helpful comments. This paper benefitted enormously from discussions with Michael Tomasello.
Competing interests
I have no competing interests.
Funding
I recognize the support of the National Science Foundation (BCS-1138073), the Hyde Family Foundations, the John Templeton Foundation, and the Institute of Human Origins (IHO) at Arizona State University.
Disclaimer
The opinions expressed in this publication are those of C.W. Marean and do not necessarily reflect the views of these funding organizations.
References
- 1.Brown JL. 1964. The evolution of diversity in avian territorial systems. Wilson Bull. 76, 160–169. [Google Scholar]
- 2.Grant JWA. 1993. Whether or not to defend? The influence of resource distribution. Mar. Freshwater Behav. Physiol. 23, 137–153. ( 10.1080/10236249309378862) [DOI] [Google Scholar]
- 3.Dubois F, Giraldeau L-A, Grant JWA. 2003. Resource defense in a group-foraging context. Behav. Ecol. 14, 2–9. ( 10.1093/beheco/14.1.2) [DOI] [Google Scholar]
- 4.Dubois F, Giraldeau L-A. 2005. Fighting for resources: the economics of defense and appropriation. Ecology 86, 3–11. ( 10.1890/04-0566) [DOI] [Google Scholar]
- 5.Dyson-Hudson R, Smith EA. 1978. Human territoriality: an ecological reassessment. Am. Anthropol. 80, 21–41. ( 10.1525/aa.1978.80.1.02a00020) [DOI] [Google Scholar]
- 6.Durham WH. 1976. Resource competition and human aggression, part I: a review of primitive war. Q. Rev. Biol. 51, 385–415. ( 10.1086/409471) [DOI] [Google Scholar]
- 7.Cashdan EA. 1983. Territorality among human foragers: ecological models and an application to four Bushmen groups. Curr. Anthropol. 24, 47–66. ( 10.1086/202934) [DOI] [Google Scholar]
- 8.Chabot-Hanowell B, Smith EA. 2012. Territorial and nonterritorial routes to power: reconciling evolutionary ecological, social agency, and historicist approaches. Archeol. Pap. Am. Anthropol. Assoc. 22, 72–86. ( 10.1111/apaa.12004) [DOI] [Google Scholar]
- 9.Baker MJ. 2003. An equilibrium conflict model of land tenure in hunter–gatherer societies. J. Polit. Econ. 111, 124–173. ( 10.1086/344800) [DOI] [Google Scholar]
- 10.Marean CW. 2005. From the tropics to the colder climates: contrasting faunal exploitation adaptations of modern humans and Neanderthals. In From tools to symbols. From hominids to modern humans (eds d'Errico F, Backwell LR), pp. 333–371. Johannesburg, South Africa: Witwatersrand University Press. [Google Scholar]
- 11.Marean CW. 2007. Heading north: an Africanist perspective on the replacement of Neanderthals by modern humans. In Rethinking the human revolution (eds Mellars P, Stringer C, Bar-Yosef O, Boyle K), pp. 367–379. Cambridge, UK: MacDonald Institute for Archaeological Research. [Google Scholar]
- 12.Oswalt WH. 1976. An anthropological analysis of food-getting technology. New York, NY: John Wiley and Sons. [Google Scholar]
- 13.Oswalt WH. 1973. Habitat and technology: the evolution of hunting. New York, NY: Reinhart and Winston. [Google Scholar]
- 14.Binford LR. 1980. Willow smoke and dogs tails: hunter–gatherer settlement systems and archaeological site formation. Am. Antiq. 45, 4–20. ( 10.2307/279653) [DOI] [Google Scholar]
- 15.Binford LR. 1982. The archaeology of place. J. Anthropol. Arch. 1, 5–31. ( 10.1016/0278-4165(82)90006-X) [DOI] [Google Scholar]
- 16.Binford LR. 1983. In pursuit of the past. New York, NY: Thames and Hudson. [Google Scholar]
- 17.Binford LR. 2001. Constructing frames of reference: an analytical method for archaeological theory building using ethnographic and environmental data sets. Berkeley, CA: University of California Press. [Google Scholar]
- 18.Kelly RL. 1983. Hunter–gatherer mobility strategies. J. Anthropol. Res. 39, 277–306. ( 10.1086/jar.39.3.3629672) [DOI] [Google Scholar]
- 19.Kelly R. 1992. Mobility/sedentism: concepts, archaeological measures, and effects. Annu. Rev. Anthropol. 21, 43–66. ( 10.1146/annurev.an.21.100192.000355) [DOI] [Google Scholar]
- 20.Kelly RL. 1995. The foraging spectrum. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 21.Kelly RL. 2013. The lifeways of hunter–gatherers: the foraging spectrum. New York, NY: Cambridge University Press. [Google Scholar]
- 22.Torrence R. 1983. Time budgeting and hunter–gather technology. In Hunter–gatherer economy in prehistory: a European perspective (ed. Bailey G.), pp. 11–22. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Torrence R. 1989. Retooling: towards a behavioral theory of stone tools. In Time, energy, and stone tools (ed. Torrence R.), pp. 57–66. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 24.Burnside WR, Brown JH, Burger O, Hamilton MJ, Moses M, Bettencourt LMA. 2012. Human macroecology: linking pattern and process in big-picture human ecology. Biol. Rev. 87, 194–208. ( 10.1111/j.1469-185X.2011.00192.x) [DOI] [PubMed] [Google Scholar]
- 25.Brown JH, Maurer BA. 1989. Macroecology: the division of food and space among species on continents. Science 243, 1145 ( 10.1126/science.243.4895.1145) [DOI] [PubMed] [Google Scholar]
- 26.Brown JH. 1995. Macroecology. Chicago, IL: University of Chicago Press. [Google Scholar]
- 27.Mulvaney J, Kamminga J. 1999. Prehistory of Australia. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 28.Binford LR. 1978. Nunamiut ethnoarchaeology. New York, NY: Academic Press. [Google Scholar]
- 29.Perreault C, Brantingham PJ. 2011. Mobility-driven cultural transmission along the forager–collector continuum. J. Anthropol. Archaeol. 30, 62–68. ( 10.1016/j.jaa.2010.10.003) [DOI] [Google Scholar]
- 30.Marlowe F. 2010. The Hadza: hunter–gatherers of Tanzania. Berkeley, CA: Univeristy of California Press. [Google Scholar]
- 31.Woodburn J. 1972. Ecology, nomadic movement and the composition of the local group among hunters and gatherers: an East African example and its implications. In Man, settlement and urbanism (eds P Ucko, R Tringham, GW Dimbleby), pp. 193–206. London, UK: Duckworth. [Google Scholar]
- 32.Heinz HJ. 1972. Territoriality among the Bushmen in general and the !ko in particular. Anthropos 67, 405–416. [Google Scholar]
- 33.Holmberg A. 1950. Nomads of the long bow. Washington, DC: US Government Office. [Google Scholar]
- 34.Boas F. 1888. The Central Eskimo. Lincoln, NE: University of Nebraska Press. [Google Scholar]
- 35.Osgood C. 1936. Contributions to the ethnography of the Kutchin. Yale Univ. Publ. Anthropol. 14, 1–189. [Google Scholar]
- 36.Kroeber AL. 1925. Handbook of the Indians of California. Washington, DC: Smithsonian Institution, Bureau of American Ethnology. [Google Scholar]
- 37.Boas F. 1909. The Kwakiutl of Vancouver Island. New York, NY: GE Stechert. [Google Scholar]
- 38.Osborn AJ. 1999. From global models to regional patterns: possible determinants of Folsom hunting weapon design diversity and complexity. In Folsom lithic technology: explorations in structure and variation (ed. Amick DS.), pp. 188–213. Ann Arbor, MI: International Monographs in Prehistory. [Google Scholar]
- 39.Collard M, Buchanan B, Morin J, Costopoulos A. 2011. What drives the evolution of hunter-gatherer subsistence technology? A reanalysis of the risk hypothesis with data from the Pacific Northwest. Phil. Trans. R. Soc. B 366, 1129–1138. ( 10.1098/rstb.2010.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collard M, Buchanan B, O'Brien MJ, Scholnick J. 2013. Risk, mobility or population size? Drivers of technological richness among contact-period western North American hunter–gatherers. Phil. Trans. R. Soc. B 368, 20120412 ( 10.1098/rstb.2012.0412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torrence R. 2001. Hunter-gatherer technology: macro-and microscale approaches. In Hunter-gatherers: an interdisciplinary perspective (eds Panter-Brick C, Layton RH, Rowley-Conwy P), pp. 73–98. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 42.Shott M. 1986. Technological organization and settlement mobility: an ethnographic examination. J. Anthropol. Res. 42, 15–51. ( 10.1086/jar.42.1.3630378) [DOI] [Google Scholar]
- 43.Freid MH. 1967. The evolution of political society: an essay in political anthropology. New York, NY: Ramden House. [Google Scholar]
- 44.Woodburn J. 1982. Egalitarian societies. Man 17, 431–451. ( 10.2307/2801707) [DOI] [Google Scholar]
- 45.Boehm C. 1999. Hierarchy in the forest: the evolution of egalitarian behavior. Cambridge, MA: Harvard University Press. [Google Scholar]
- 46.Wiessner P. 1996. Leveling the hunter: constraints on the status quest in foraging societies. In Food and the status quest (eds Wiessner P, Schiefenhovel W), pp. 171–191. Oxford, UK: Berghahn Books. [Google Scholar]
- 47.Lee RB. 1979. The !Kung San: men, women, and work in a foraging society. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 48.Townsend JB. 1983. Precontact political organization and slavery in Aleut societies. In The development of political organization in native North America (ed. Tooker E.), pp. 120–132. Washington, DC: American Ethnological Society. [Google Scholar]
- 49.Johnson JR. 2007. Ethnohistoric descriptions of Chumash warfare. In North American indigenous warfare and ritual violence (eds Chacon RJ, Mendoza RG), pp. 74–113. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 50.Burch ES. 1980. Traditional Eskimo societies in northwest Alaska. In Alaska native culture and history (eds Y Kotani, WB Workman), pp. 253–304. Senri Ethnology Studies, no. 4. Osaka, Japan: National Museum of Ethnology. [Google Scholar]
- 51.Hamilton MJ, Milne BT, Walker RS, Burger O, Brown JH. 2007. The complex structure of hunter–gatherer social networks. Proc. R. Soc. B 274, 2195–2203. ( 10.1098/rspb.2007.0564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mattison SM, Smith EA, Shenk MK, Cochrane EE. In press The evolution of inequality. Evol. Anthropol. [DOI] [PubMed] [Google Scholar]
- 53.Zefferman MR, Mathew S. 2015. An evolutionary theory of large-scale human warfare: group-structured cultural selection. Evol. Anthropol. Issues News Rev. 24, 50–61. ( 10.1002/evan.21439) [DOI] [PubMed] [Google Scholar]
- 54.Wilson EO. 1975. Sociobiology: the new synthesis. Cambridge, MA: Harvard University Press. [Google Scholar]
- 55.Tindale NB. 1974. Aboriginal tribes of Australia, their terrain, environmental controls, distribution, limits, and proper names. Berkeley, CA: University of California Press. [Google Scholar]
- 56.Burch ES. 1979. Indians and Eskimos in North Alaska, 1816–1977: a study in changing ethnic relations. Arct. Anthropol. 16, 123–151. [Google Scholar]
- 57.Warner WL. 1931. Murngin warfare. Oceania 1, 457–494. ( 10.1002/j.1834-4461.1931.tb00016.x) [DOI] [Google Scholar]
- 58.Warner WL. 1937. A black civilization: a social study of an Australian tribe. New York, NY: Harper.
- 59.Davies NB, Krebs JR, West SA. 2012. An introduction to behavioural ecology. New York, NY: John Wiley & Sons. [Google Scholar]
- 60.Schoener TW. 1983. Simple models of optimal feeding-territory size: a reconciliation. Am. Nat. 121, 608–629. ( 10.1086/284090) [DOI] [Google Scholar]
- 61.Strassmann JE, Queller DC. 2014. Privatization and property in biology. Anim. Behav. 92, 305–311. ( 10.1016/j.anbehav.2014.02.011) [DOI] [Google Scholar]
- 62.Peterson N. 1975. Hunter-gatherer territoriality: the perspective from Australia. Am. Anthropol. 77, 53–68. ( 10.1525/aa.1975.77.1.02a00040) [DOI] [Google Scholar]
- 63.Peterson N. 1979. Territorial adaptations among desert hunter–gatherers: the !Kung and Australians compared. In Social and ecological systems (eds Burnham P, Ellen R), pp. 112–129. New York, NY: Academic Press. [Google Scholar]
- 64.Maher CR, Lott DF. 2000. A review of ecological determinants of territoriality within vertebrate species. Am. Midland Nat. 143, 1–29. ( 10.1674/0003-0031(2000)143%5B0001:AROEDO%5D2.0.CO;2) [DOI] [Google Scholar]
- 65.Marean CW. 2011. Coastal South Africa and the co-evolution of the modern human lineage and coastal adaptations. In Trekking the shore: changing coastlines and the antiquity of coastal settlement (eds Bicho N, Haws JA, Davis LG), pp. 421–440. New York, NY: Springer. [Google Scholar]
- 66.Marean CW. 2010. Pinnacle Point Cave 13B (Western Cape Province, South Africa) in context: the Cape floral kingdom, shellfish, and modern human origins. J. Hum. Evol. 59, 425–443. ( 10.1016/j.jhevol.2010.07.011) [DOI] [PubMed] [Google Scholar]
- 67.Vynck JCD, Anderson R, Atwater C, Cowling RM, Fisher EC, Marean CW, Walker RS. 2016. Return rates from intertidal foraging from Blombos Cave to Pinnacle Point: understanding early human economies. J. Hum. Evol. 93, 101–115. [DOI] [PubMed] [Google Scholar]
- 68.Lambert PM. 1997. Patterns of violence in prehistoric hunter-gatherer societies of coastal southern California. In Troubled times: violence and warfare in the past (eds Martin DL, Frayer DW), pp. 77–110. Amsterdam, The Netherlands: Amsteldijk. [Google Scholar]
- 69.Lambert PM. 2002. The archaeology of war: a North American perspective. J. Archaeol. Res. 10, 207–241. ( 10.1023/A:1016063710831) [DOI] [Google Scholar]
- 70.Moss ML, Jon ME. 1992. Forts, refuge rocks, and defensive sites: the antiquity of warfare along the North Pacific Coast of North America. Arct. Anthropol. 29, 73–90. [Google Scholar]
- 71.Maschner HDG, Reedy-Maschner KL. 1998. Raid, retreat, defend (repeat): the archaeology and ethnohistory of warfare on the North Pacific Rim. J. Anthropol. Archaeol. 17, 19–51. ( 10.1006/jaar.1997.0315) [DOI] [Google Scholar]
- 72.Marean CW. 2014. The origins and significance of coastal resource use in Africa and western Eurasia. J. Hum. Evol. 77, 17–40. ( 10.1016/j.jhevol.2014.02.025) [DOI] [PubMed] [Google Scholar]
- 73.Foley R. 1982. A reconsideration of the role of predation on large mammals in tropical hunter-gatherer adaptation. Man 17, 393–402. ( 10.2307/2801704) [DOI] [Google Scholar]
- 74.Marean CW. 1997. Hunter-gatherer foraging strategies in tropical grasslands: model building and testing in the East African Middle and Later Stone Age. J. Anthropol. Archaeol. 16, 189–225. ( 10.1006/jaar.1997.0309) [DOI] [Google Scholar]
- 75.Tanaka J. 1976. Subsistence ecology of central Kalahari San. In Kalahari hunter–gatherers (eds Lee RB, DeVore I), pp. 98–119. Cambridge, MA: Harvard University Press. [Google Scholar]
- 76.Tanaka J. 1980. The San hunter–gatherers of the Kalahari: a study in ecological anthropology. Tokyo, Japan: University of Tokyo Press. [Google Scholar]
- 77.Vincent AS. 1985. Plant foods in savanna environments: a preliminary report of tubers eaten by the Hadza of northern Tanzania. World Archaeol. 17, 131–148. ( 10.1080/00438243.1985.9979958) [DOI] [PubMed] [Google Scholar]
- 78.Vincent AS. 1985. Underground plant foods and subsistence in human evolution. Berkeley, CA: University of California at Berkeley. [Google Scholar]
- 79.Laden G, Wrangham R. 2005. The rise of the hominids as an adaptive shift in fallback foods: plant underground storage organs (USOs) and Australopith origins. J. Hum. Evol. 49, 482–498. ( 10.1016/j.jhevol.2005.05.007) [DOI] [PubMed] [Google Scholar]
- 80.Schoeninger MJ, Bunn HT, Murray SS, Marlett JA. 2001. Composition of tubers used by Hadza foragers of Tanzania. J. Food Compos. Anal. 14, 15–25. ( 10.1006/jfca.2000.0961) [DOI] [Google Scholar]
- 81.Marlowe FM, Berbesque JC. 2009. Tubers as fallback foods and their impact on Hadza hunter–gatherers. Am. J. Phys. Anthropol. 140, 751–758. ( 10.1002/ajpa.21040) [DOI] [PubMed] [Google Scholar]
- 82.Murray SS, Schoeninger MJ, Bunn HT, Pickering TR, Marlett JA. 2001. Nutritional composition of some wild plant foods and honey used by Hadza foragers of Tanzania. J. Food Compos. Anal. 14, 3–13. ( 10.1006/jfca.2000.0960) [DOI] [Google Scholar]
- 83.Sept JM. 1986. Plant foods and early hominids at site FxJj 50, Koobi Fora, Kenya. J. Hum. Evol. 15, 751–770. ( 10.1016/S0047-2484(86)80008-2) [DOI] [Google Scholar]
- 84.Sept J. 1992. Was there no place like home? A new perspective on early hominid archaeological sites from the mapping of chimpanzee nests. Curr. Anthropol. 33, 187–207. ( 10.1086/204050) [DOI] [Google Scholar]
- 85.Sept JM. 1994. Beyond bones: archaeological sites, early hominid subsistence, and the costs and benefits of exploiting wild plant foods in East African riverine landscapes. J. Hum. Evol. 27, 295–320. ( 10.1006/jhev.1994.1047) [DOI] [Google Scholar]
- 86.Lee RB. 1973. Mongongo: the ethnography of a major wild food resource. Ecol. Food Nutr. 2, 307–321. ( 10.1080/03670244.1973.9990351) [DOI] [Google Scholar]
- 87.Arnold JE, Walsh MR. 2010. California's ancient past: from the pacific to the range of light. Washington, DC: SAA Press. [Google Scholar]
- 88.Steward JH. 1938. Basin-plateau aboriginal socio-political groups. Smithsonian Inst. Bureau Am. Ethnol. Bull. 120, 1–346. [Google Scholar]
- 89.Marlowe FW, Berbesque JC, Wood B, Crittenden A, Porter C, Mabulla A. 2014. Honey, Hadza, hunter-gatherers, and human evolution. J. Hum. Evol. 71, 119–128. ( 10.1016/j.jhevol.2014.03.006) [DOI] [PubMed] [Google Scholar]
- 90.Goldblatt P, Manning J, Snijman D. 2002. The color encyclopedia of Cape Bulbs. Portland, OR: Timber Press. [Google Scholar]
- 91.Proches S, Cowling RM, Goldblatt P, Manning JC, Snijman DA. 2006. An overview of the Cape geophytes. Biol. J. Linnean Soc. 87, 27–43. ( 10.1111/j.1095-8312.2006.00557.x) [DOI] [Google Scholar]
- 92.Deacon J. 1984. Later Stone Age people and their descendants in southern Africa. In Southern African prehistory and paleoenvironments (ed. Klein RG.), pp. 221–328. Rotterdam, The Netherlands: Balkema. [Google Scholar]
- 93.Marean CW, Cawthra HC, Cowling RM, Esler KJ, Fisher E, Milewski A, Potts AJ, Singels E, De Vynck J. 2014. Stone Age people in a changing South African Greater Cape floristic region. In Fynbos: ecology, evolution, and conservation of a megadiverse region (eds Allsopp N, Colville JF, Verboom T), pp. 164–199. Oxford, UK: Oxford University Press. [Google Scholar]
- 94.De Vynck JC, Cowling RM, Potts AJ, Marean CW. 2015. Seasonal availability of edible underground and aboveground carbohydrate resources to human foragers on the Cape south coast, South Africa. PeerJ PrePrints 3, e1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singels E, Esler KJ, Cowling RM, Potts AJ, Marean CW, Vynck JD. In press. Foraging potential of underground storage organ plants in the Southern Cape, South Africa. J. Hum. Evol. [DOI] [PubMed] [Google Scholar]
- 96.Spinage CA. The natural history of antelopes. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 97.Kingdon J. 1982. East African mammals IIIC: bovids. Chicago, IL: University of Chicago Press. [Google Scholar]
- 98.Lowie RH. 1963. Indians of the plains. New York, NY: Natural History Press. [Google Scholar]
- 99.Hart TB, Hart JA. 1986. The ecological basis of hunter-gatherer subsistence in African rain forests: the Mbuti of eastern Zaire. Hum. Ecol. 14, 29–55. ( 10.1007/BF00889209) [DOI] [Google Scholar]
- 100.Bailey RC, Head G, Jenike M. 1989. Hunting and gathering in a tropical rainforest: is it possible? Am. Anthropol. 91, 82 ( 10.1525/aa.1989.91.1.02a00040) [DOI] [Google Scholar]
- 101.Blackburn RH. 1974. The Okiek and their history. Azania 9, 139–157. ( 10.1080/00672707409511720) [DOI] [Google Scholar]
- 102.Blackburn RH. 1982. In the land of milk and honey: Okiek adaptations to their forests and neighbours. In Politics and history in band societies (eds Leacock E, Lee R), pp. 283–305. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 103.Huntingford GWB. 1929. Modern hunters: some account of the Kamelilo-Kapchepkendi Dorobo (Okiek) of Kenya colony. J. R. Anthropol. Inst. 59, 333–378. [Google Scholar]
- 104.Huntingford GWB. 1955. The economic life of the Dorobo. Anthropos 50, 602–634. [Google Scholar]
- 105.Dyson WS, Fuchs VE. 1937. The Elmolo. J. R. Anthropol. Inst. Great Britain Ireland 67, 327–338. ( 10.2307/2844144) [DOI] [Google Scholar]
- 106.Kiura P. 2005. El-molo: The forgotten people of Lake Turkana. Kenya Past Present 35, 11–16. [Google Scholar]
- 107.Branch GM, Menge BA. 2001. Rocky intertidal communities. In Marine community ecology (eds Bertness MD, Gaines SD, Hay ME), pp. 221–251. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 108.Branch G, Griffiths CL, Branch ML, Beckley LE. 2008. Two oceans: a guide to the marine life of southern Africa. Cape Town, South Africa: Struik. [Google Scholar]
- 109.Colonese AC, Mannino MA, Bar-Yosef Mayer DE, Fa DA, Finlayson JC, Lubell D, Stiner MC. 2011. Marine mollusc exploitation in Mediterranean prehistory: an overview. Quat. Int. 239, 86–103. ( 10.1016/j.quaint.2010.09.001) [DOI] [Google Scholar]
- 110.Chapais B. 2008. Primeval kinship. Cambridge, MA: Harvard University Press. [Google Scholar]
- 111.Marean CW. 2015. An evolutionary anthropological perspective on modern human origins. Annu. Rev. Anthropol. 44, 533–556. ( 10.1146/annurev-anthro-102313-025954) [DOI] [Google Scholar]
- 112.Richerson PJ, Boyd R. 1998. The evolution of human ultra-sociality. In Indoctrinability, ideology, and warfare: evolutionary perspectives (eds I Eibl-Eibesfeldt, FK Salter), pp. 71–95. New York, NY: Berghahn Books. [Google Scholar]
- 113.Tomasello M, Melis AP, Tennie C, Wyman E, Herrmann E. 2012. Two key steps in the evolution of human cooperation: the interdependence hypothesis. Curr. Anthropol. 53, 673–692. ( 10.1086/668207) [DOI] [Google Scholar]
- 114.Tomasello M, Vaish A. 2013. Origins of human cooperation and morality. Annu. Rev. Psychol. 64, 231–255. ( 10.1146/annurev-psych-113011-143812) [DOI] [PubMed] [Google Scholar]
- 115.Wilkins J, Schoville BJ, Brown KS, Chazan M. 2012. Evidence for early hafted hunting technology. Science 338, 942–946. ( 10.1126/science.1227608) [DOI] [PubMed] [Google Scholar]
- 116.Henrich J. 2004. Demography and cultural evolution: how adaptive cultural processes can produce maladaptive losses: the Tasmanian case. Am. Antiq. 69, 197–214. ( 10.2307/4128416) [DOI] [Google Scholar]
- 117.Powell A, Shennan S, Thomas MG. 2009. Late Pleistocene demography and the appearance of modern human behavior. Science 324, 1298–1301. ( 10.1126/science.1170165) [DOI] [PubMed] [Google Scholar]
- 118.Shennan S. 2001. Demography and cultural innovation: a model and its implications for the emergence of modern human culture. Camb. Archaeol. J. 11, 5–16. ( 10.1017/S0959774301000014) [DOI] [Google Scholar]
- 119.Marean CW, Assefa Z. 2005. The Middle and Upper Pleistocene African record for the biological and behavioral origins of modern humans. In African archaeology (ed. Stahl AB.), pp. 93–129. New York, NY: Blackwell. [Google Scholar]
- 120.Boyd R, Richerson PJ, Henrich J. 2011. The cultural niche: why social learning is essential for human adaptation. Proc. Natl Acad. Sci. USA 108, 10 918–10 925. ( 10.1073/pnas.1100290108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yellen JE. 1998. Barbed bone points: tradition and continuity in Saharan and sub-Saharan Africa. Afr. Archaeol. Rev. 15, 173–198. ( 10.1023/A:1021659928822) [DOI] [Google Scholar]
- 122.Garcea EAA. 2006. Semi-permanent foragers in semi-arid environments of North Africa. World Archaeol. 38, 197–219. ( 10.1080/00438240600693968) [DOI] [Google Scholar]
- 123.Bowles S, Gintis H. 2011. A cooperative species: human reciprocity and its evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 124.Bowles S. 2009. Did warfare among ancestral hunter-gatherers affect the evolution of human social behaviors? Science 324, 1293–1298. ( 10.1126/science.1168112) [DOI] [PubMed] [Google Scholar]
- 125.Wilson EO. 2012. The social conquest of Earth. New York, NY: WW Norton. [Google Scholar]
- 126.Holl A. 2005. Holocene ‘aquatic’ adaptations in north tropical Africa. In African archaeology (ed. Stahl AB.), pp. 174–186. New York, NY: Blackwell. [Google Scholar]
- 127.Prendergast ME, Lane PJ. 2010. Middle Holocene fishing strategies in East Africa: zooarchaeological analysis of Pundo, a Kansyore shell midden in northern Nyanza (Kenya). Int. J. Osteoarchaeol. 20, 88–112. [Google Scholar]
- 128.Robbins LH, Murphy ML, Stewart KM, Campbell AC, Brook GA. 1994. Barbed bone points, paleoenvironment, and the antiquity of fish exploitation in the Kalahari Desert, Botswana. J. Field Archaeol. 21, 257–264. ( 10.2307/529874) [DOI] [Google Scholar]
- 129.Lubell D. 2001. Late Pleistocene-Early Holocene Maghreb. In Encyclopedia of prehistory (eds Peregrine P, Ember M), pp. 129–149. New York, NY: Springer. [Google Scholar]
- 130.Brooks A, Smith C. 1987. Ishango revisited: new age determinations and cultural interpretations. Afr. Archaeol. Rev. 5, 65–78. ( 10.1007/BF01117083) [DOI] [Google Scholar]
- 131.Steele TE. 2013. Late Pleistocene human subsistence in northern Africa: the state of our knowledge and placement in a continental context. In Modern origins: a North African perspective (eds Hublin J-J, McPherron SP), pp. 107–125. New York, NY: Springer. [Google Scholar]
- 132.Brooks AS, et al. 1995. Dating and context of three Middle Stone Age sites with bone points in the upper Semliki Valley, Zaire. Science 268, 548–553. ( 10.1126/science.7725099) [DOI] [PubMed] [Google Scholar]
- 133.Fisher EC, Bar-Matthews M, Jerardino A, Marean CW. 2010. Middle and Late Pleistocene paleoscape modeling along the southern coast of South Africa. Q. Sci. Rev. 29, 1382–1398. ( 10.1016/j.quascirev.2010.01.015) [DOI] [Google Scholar]
- 134.Fisher EC, et al. 2013. Archaeological reconnaissance for Middle Stone Age sites along the Pondoland 1 coast, South Africa. PaleoAnthropology 2013, 104–137. [Google Scholar]
- 135.Rector AL, Reed KE. 2010. Middle and late Pleistocene faunas of Pinnacle Point and their paleoecological implications. J. Hum. Evol. 59, 340–357. ( 10.1016/j.jhevol.2010.07.002) [DOI] [PubMed] [Google Scholar]
- 136.Copeland SR, Cawthra HC, Fisher EC, Lee-Thorp JA, Cowling RM, Roux PJL, Hodgkins J, Marean CW. 2016. Strontium isotope investigation of ungulate movement patterns on the Pleistocene Paleo-Agulhas plain of the Greater Cape floristic region, South Africa. Q. Sci. Rev. 141, 65–84. [Google Scholar]
- 137.Jerardino A, Marean CW. 2010. Shellfish gathering, marine paleoecology and modern human behavior: perspectives from cave PP13B, Pinnacle Point, South Africa. J. Hum. Evol. 59, 412–424. ( 10.1016/j.jhevol.2010.07.003) [DOI] [PubMed] [Google Scholar]
- 138.Thompson JC. 2010. Taphonomic analysis of the Middle Stone Age faunal assemblage from Pinnacle Point Cave 13B, Western Cape, South Africa. J. Hum. Evol. 59, 321–339. ( 10.1016/j.jhevol.2010.07.004) [DOI] [PubMed] [Google Scholar]
- 139.Karkanas P, Brown KS, Fisher EC, Jacobs Z, Marean CW. 2015. Interpreting human behavior from depositional rates and combustion features through the study of sedimentary microfacies at site Pinnacle Point 5-6, South Africa. J. Hum. Evol. 85, 1–21. ( 10.1016/j.jhevol.2015.04.006) [DOI] [PubMed] [Google Scholar]
- 140.Yellen JE, Brooks AS, Cornelissen E, Mehlman MJ, Stewart K. 1995. A Middle Stone Age worked bone industry from Katanda, Upper Semliki Valley, Zaire. Science 268, 553–556. ( 10.1126/science.7725100) [DOI] [PubMed] [Google Scholar]
- 141.Sutton JEG. 1977. The African aqualithic. Antiquity 51, 25–34. ( 10.1017/S0003598X00100559) [DOI] [Google Scholar]
- 142.Rosas A, et al. 2006. Paleobiology and comparative morphology of a late Neandertal sample from El Sidrón, Asturias, Spain. Proc. Natl Acad. Sci. USA 103, 19 266–19 271. ( 10.1073/pnas.0609662104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Clark JD, et al. 2003. Stratigraphic, chronological and behavioural contexts of Pleistocene Homo sapiens from Middle Awash, Ethiopia. Nature 423, 747–752. ( 10.1038/nature01670) [DOI] [PubMed] [Google Scholar]
- 144.Pickering TR, White TD, Toth N. 2000. Brief communication: cutmarks on a Plio-Pleistocene hominid from Sterkfontein, South Africa. Am. J. Phys. Anthropol. 111, 579–584. ( 10.1002/(SICI)1096-8644(200004)111:4%3C579::AID-AJPA12%3E3.0.CO;2-Y) [DOI] [PubMed] [Google Scholar]
- 145.Fernández-Jalvo Y, Carlos Díez J, Cáceres I, Rosell J. 1999. Human cannibalism in the Early Pleistocene of Europe (Gran Dolina, Sierra de Atapuerca, Burgos, Spain). J. Hum. Evol. 37, 591–622. ( 10.1006/jhev.1999.0324) [DOI] [PubMed] [Google Scholar]
- 146.Scott JE, Marean CW. 2009. Paleolithic hominin remains from Eshkaft-e Gavi (southern Zagros Mountains, Iran): description, affinities, and evidence for butchery. J. Hum. Evol. 57, 248–259. ( 10.1016/j.jhevol.2009.05.012) [DOI] [PubMed] [Google Scholar]
- 147.Wendorf F. 1968. Site 117: a Nubian final paleolithic graveyard near Jebel Sahaba, Sudan. In The prehistory of Nubia (ed. Wendorf F.), pp. 954–1040. Dallas, TX: Southern Methodist University Dallas. [Google Scholar]
- 148.Lahr MM, et al. 2016. Inter-group violence among early Holocene hunter-gatherers of West Turkana, Kenya. Nature 529, 394–398. ( 10.1038/nature16477) [DOI] [PubMed] [Google Scholar]
- 149.Pfeiffer S. 2016. An exploration of interpersonal violence among Holocene foragers of southern Africa. Int. J. Paleopathol. 13, 27–38. ( 10.1016/j.ijpp.2016.01.001) [DOI] [PubMed] [Google Scholar]
- 150.Binford LR. 2004. Beliefs about death, behaviour, and mortuary practices among hunter–gatherers: a search for causal structure? In Explaining social change: studies in honour of Colin Renfrew (eds JF Cherry, C Scarre, S Shennan), pp. 1–15. McDonald Institute for Monographs. Cambridge, UK: McDonald Institute for Archaeological Research. [Google Scholar]