Abstract
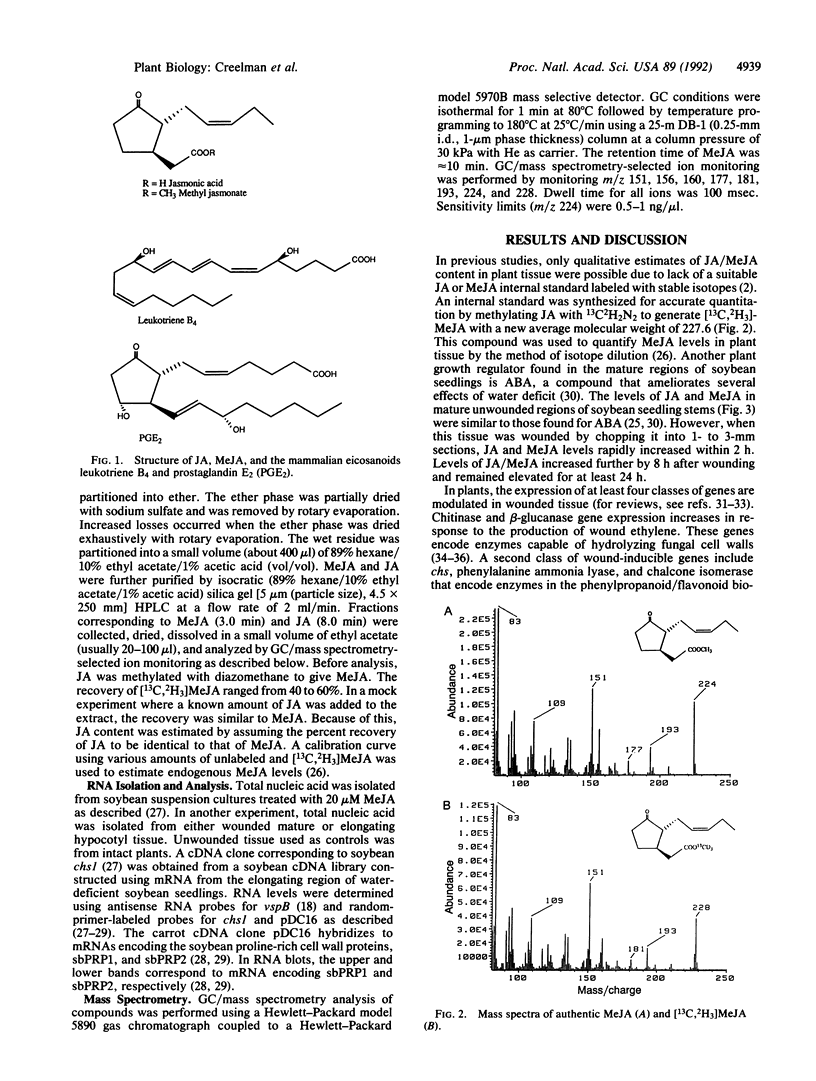
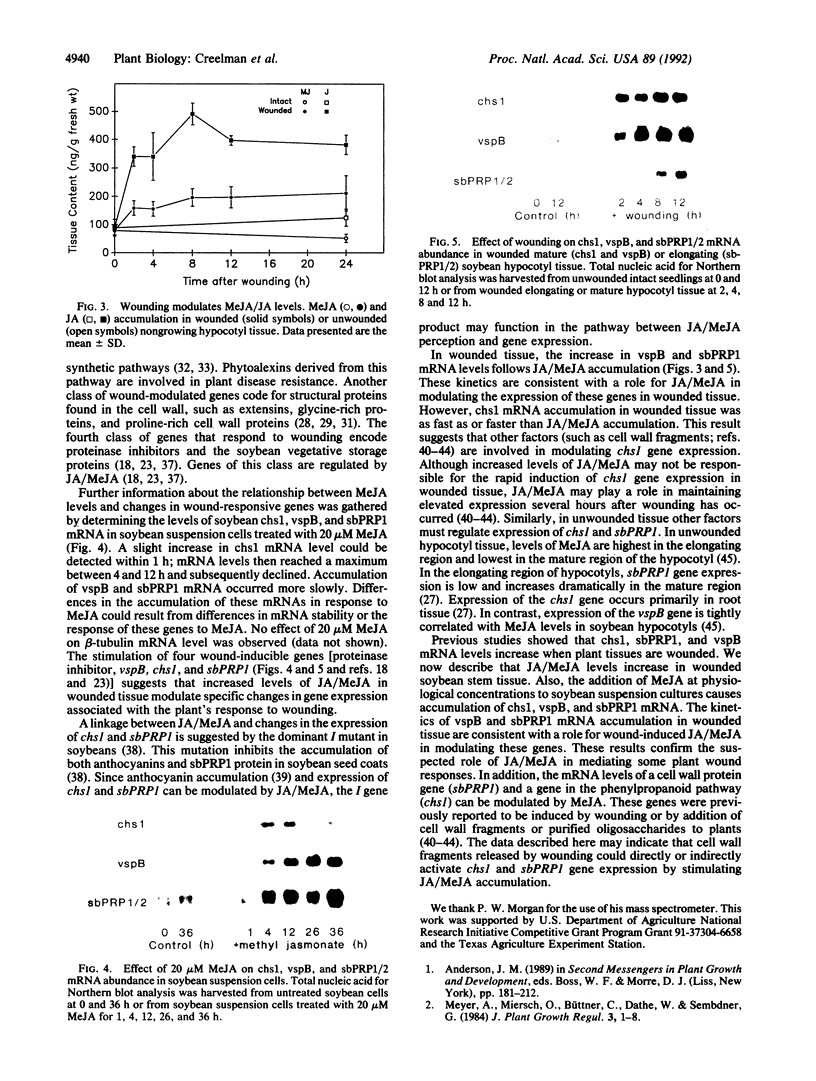
Jasmonic acid (JA) and its methyl ester, methyl jasmonate (MeJA), are plant lipid derivatives that resemble mammalian eicosanoids in structure and biosynthesis. These compounds are proposed to play a role in plant wound and pathogen responses. Here we report the quantitative determination of JA/MeJA in planta by a procedure based on the use of [13C,2H3]MeJA as an internal standard. Wounded soybean (Glycine max [L] Merr. cv. Williams) stems rapidly accumulated MeJA and JA. Addition of MeJA to soybean suspension cultures also increased mRNA levels for three wound-responsive genes (chalcone synthase, vegetative storage protein, and proline-rich cell wall protein) suggesting a role for MeJA/JA in the mediation of several changes in gene expression associated with the plants' response to wounding.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Bosshart R. P., Forrence L. E., Habig W. H. Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol. 1971 Jan;47(1):129–134. doi: 10.1104/pp.47.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Broglie K. E., Gaynor J. J., Broglie R. M. Ethylene-regulated gene expression: molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6820–6824. doi: 10.1073/pnas.83.18.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Mason H. S., Bensen R. J., Boyer J. S., Mullet J. E. Water Deficit and Abscisic Acid Cause Differential Inhibition of Shoot versus Root Growth in Soybean Seedlings : Analysis of Growth, Sugar Accumulation, and Gene Expression. Plant Physiol. 1990 Jan;92(1):205–214. doi: 10.1104/pp.92.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Mullet J. E. Abscisic Acid accumulates at positive turgor potential in excised soybean seedling growing zones. Plant Physiol. 1991 Apr;95(4):1209–1213. doi: 10.1104/pp.95.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Mullet J. E. Water deficit modulates gene expression in growing zones of soybean seedlings. Analysis of differentially expressed cDNAs, a new beta-tubulin gene, and expression of genes encoding cell wall proteins. Plant Mol Biol. 1991 Oct;17(4):591–608. doi: 10.1007/BF00037046. [DOI] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Grimes H. D. Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6745–6749. doi: 10.1073/pnas.88.15.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco S. M., Tierney M. L. Isolation and characterization of a proline-rich cell wall protein from soybean seedlings. Plant Physiol. 1990 Dec;94(4):1897–1902. doi: 10.1104/pp.94.4.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grab D., Loyal R., Ebel J. Elicitor-induced phytoalexin synthesis in soybean cells: changes in the activity of chalcone synthase mRNA and the total population of translatable mRNA. Arch Biochem Biophys. 1985 Dec;243(2):523–529. doi: 10.1016/0003-9861(85)90529-6. [DOI] [PubMed] [Google Scholar]
- Hammarström S. Leukotrienes. Annu Rev Biochem. 1983;52:355–377. doi: 10.1146/annurev.bi.52.070183.002035. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Hahlbrock K., Lamb C. J. Elicitor induction of mRNA activity. Rapid effects of elicitor on phenylalanine ammonia-lyase and chalcone synthase mRNA activities in bean cells. Eur J Biochem. 1983 Jan 17;130(1):131–139. [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J. T., Vodkin L. O. A soybean cell wall protein is affected by seed color genotype. Plant Cell. 1991 Jun;3(6):561–571. doi: 10.1105/tpc.3.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., Dewald D. B., Creelman R. A., Mullet J. E. Coregulation of soybean vegetative storage protein gene expression by methyl jasmonate and soluble sugars. Plant Physiol. 1992 Mar;98(3):859–867. doi: 10.1104/pp.98.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E. Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell. 1990 Jun;2(6):569–579. doi: 10.1105/tpc.2.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Preisig C. L., Kuć J. A. Inhibition by Salicylhydroxamic Acid, BW755C, Eicosatetraynoic Acid, and Disulfiram of Hypersensitive Resistance Elicited by Arachidonic Acid or Poly-l-Lysine in Potato Tuber. Plant Physiol. 1987 Jul;84(3):891–894. doi: 10.1104/pp.84.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pēna-Cortés H., Sánchez-Serrano J. J., Mertens R., Willmitzer L., Prat S. Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder T. B., Cramer C. L., Bell J. N., Robbins M. P., Dixon R. A., Lamb C. J. Elicitor rapidly induces chalcone synthase mRNA in Phaseolus vulgaris cells at the onset of the phytoalexin defense response. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5724–5728. doi: 10.1073/pnas.81.18.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzig D. A., Allen R. D., Bhatia S. K. Inhibition of phytoalexin synthesis in arachidonic Acid-stressed potato tissue by inhibitors of lipoxygenase and cyanide-resistant respiration. Plant Physiol. 1983 Jul;72(3):746–749. doi: 10.1104/pp.72.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranbarger T. J., Franceschi V. R., Hildebrand D. F., Grimes H. D. The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell. 1991 Sep;3(9):973–987. doi: 10.1105/tpc.3.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda J., Kato J. Isolation and Identification of a Senescence-promoting Substance from Wormwood (Artemisia absinthium L.). Plant Physiol. 1980 Aug;66(2):246–249. doi: 10.1104/pp.66.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender R., Röhrig H., Höricke C., Wing D., Schell J. Differential regulation of soybean chalcone synthase genes in plant defence, symbiosis and upon environmental stimuli. Mol Gen Genet. 1989 Aug;218(2):315–322. doi: 10.1007/BF00331284. [DOI] [PubMed] [Google Scholar]





