Abstract
Humans are uniquely unique, in terms of the extreme differences between them and other living organisms, and the impact they are having on the biosphere. The evolution of humans can be seen, as has been proposed, as one of the major transitions in evolution, on a par with the origins of multicellular organisms or the eukaryotic cell (Maynard Smith & Szathmáry 1997 Major transitions in evolution). Major transitions require the evolution of greater complexity and the emergence of new evolutionary levels or processes. Does human evolution meet these conditions? I explore the diversity of evidence on the nature of transitions in human evolution. Four levels of transition are proposed—baseline, novel taxa, novel adaptive zones and major transitions—and the pattern of human evolution considered in the light of these. The primary conclusions are that changes in human evolution occur continuously and cumulatively; that novel taxa and the appearance of new adaptations are not clustered very tightly in particular periods, although there are three broad transitional phases (Pliocene, Plio-Pleistocene and later Quaternary). Each phase is distinctive, with the first based on ranging and energetics, the second on technology and niche expansion, and the third on cognition and cultural processes. I discuss whether this constitutes a ‘major transition’ in the context of the evolutionary processes more broadly; the role of behaviour in evolution; and the opportunity provided by the rich genetic, phenotypic (fossil morphology) and behavioural (archaeological) record to examine in detail major transitions and the microevolutionary patterns underlying macroevolutionary change. It is suggested that the evolution of the hominin lineage is consistent with a mosaic pattern of change.
This article is part of the themed issue ‘Major transitions in human evolution’.
Keywords: human evolution, mosaic evolution, major transitions, human evolutionary ecology, hominins, tempo and mode of evolution
1. Introduction
Evolution—that is evolution simply as change through time—can be broken down into two elements. One element is the incremental, persistent change, from ancestor to descendant, from parent to offspring, which gives the continuity to life. It was this that Darwin was at such pains to emphasize in much of his work—the continuous and cumulative process of descent with modification. This element can be referred to as gradualism, but because this has become so tied up with the punctuated equilibrium debate [1–4] is probably best thought of as normal evolution, as it is so pervasive and ubiquitous, and is common to all evolutionary changes. It occurs all the time because variation, mutation, isolation, gene flow, drift and selection are inevitably present.
The second element is a more fundamental and radical side to evolutionary change. The origin of a species is more than just one more mutation, but signifies a step change in an evolving lineage. And it does not stop there, of course. The evolution of some species is more significant than that of others. There is a difference between just one more beetle, and the first land vertebrate, or the first warm-blooded creature. However, even looking beyond that, there is a difference between the evolution of major new adaptations, and the evolution of entirely new biological systems, such as multicellularity. These elements can be referred to as transitional evolution.
The tension between these two elements—normal and transitional evolution—has manifested itself in numerous debates and controversies [4,5]. The most well-known of these was the so-called punctuated equilibrium debate [3,6,7], but that was one major battle in what has been a prolonged skirmishing war. There are precursors in the works of Simpson [8,9] in developing the modern synthesis, or going back further to Goldschmidt [10] and Rensch [11], and the nineteenth-century founders [12]. There are modern echoes in molecular biology [13,14], and the debates can move across whole arenas of evolutionary biology [4,5,15].
We can sum this up—to misquote George Orwell in Animal Farm—as ‘all evolutionary change is equal, but is some more equal than others?’ This is a major question when it comes to human evolution. On the one hand, there is little doubt that humans represent a significantly different sort of species from other primates, and that their impact on the biosphere has been massive, and not only continues to be so, but is likely to increase [16]. But, if this is a major evolutionary outcome, is it a ‘major transition’ in terms of the processes that created it, for, on the other hand, human biological organization is not that much different from that of a chimpanzee [17]. Does human evolution constitute a major transition, and if so, when and how did it occur?
The purpose of this paper is to explore these issues. It should be made clear at the outset that the aim in doing so is not to label human evolution one way or the other. Major transitions, of whatever sort, are not biological processes, but descriptive or analytical categories. One person's major transition is another person's new adaptation. Rather, the purpose is to use the concept of evolutionary transitions to explore the tempo and mode of the changes that led to humans as a uniquely unique species.
In the first part, I will discuss different levels of evolutionary change, and introduce a four-part classification. The distinction between normal and transitional evolution is an oversimplification, and there are in fact a scaled series of types of change in evolution that will be described. In the second, I consider the evidence for these in human evolution, and when they may have occurred. Finally, I will look at the overall evidence in terms of the tempo and mode of human evolution, and the nature of its causes. The main theme is that evolutionary change occurs persistently throughout the five or more million years of our lineage, but that it is more significant in some periods than others, with cascades of change that may be inter-related. In moving from the specifics of human evolution to the general processes of evolution, I will argue that the advantage of human evolution as a model for evolutionary change is that we have a detailed and rich record, one that includes behaviour, and that this shows how macroevolutionary change—whether a major transition or not—is embedded in microevolutionary patterns and processes.
2. Evolutionary transitions
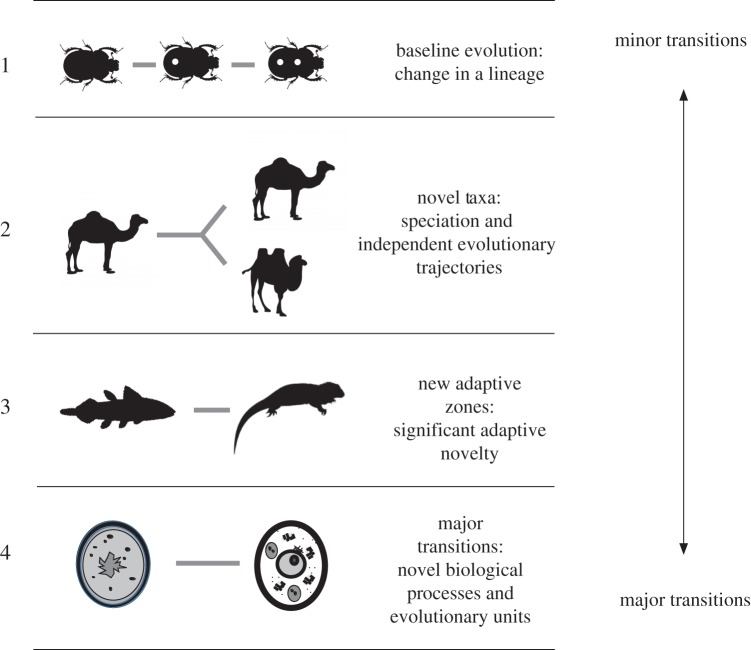
Evolutionary change can be as small as a minimal change in the number of hairs on a Drosophila, to an entirely new means of reproduction. Although each of these can be hierarchically nested, four fundamental types of evolutionary change can be described (figure 1).
Figure 1.
Transitions in evolution. Evolution is change through time in biological organisms, and it can be categorized in four levels: (1) baseline evolution, or normal evolutionary changes in characters over time within a lineage; (2) novel taxa, or the appearance of new lineages, usually through cladogenesis and speciation; (3) novel adaptive zones, or significant new adaptations which open up new ecological structures and opportunities; and (4) major transitions, or transitions where new biological processes emerge, or new units of selection, and there is increased complexity. (Online version in colour.)
(a). Baseline evolution
Baseline evolution is used here to refer to evolutionary change which is the acquisition of new traits, through mutation, so that the species phenotype shifts in some incremental way. This is basically the classic gradualist process of evolution that Darwin described, where small changes would accumulate to produce a trajectory of evolution and new adaptations. Baseline evolution is the quintessential Darwinian gradualism—the number of spots on a beetle's carapace, the different shades of coloration on cercopithecine monkeys. Baseline evolution can be produced, as Darwin and Wallace argued, through selection [18], or as we would now recognize, also through processes of genetic drift. Where new species occur, it is through anagenesis in a lineage accumulating small changes, although in practice this is likely to be rare.
(b). Novel taxa
The gradual accumulation of traits comprises the most minor of evolutionary change; at the next level is the formation of new species. They key difference between the appearance of new taxa and baseline evolution is that independent evolutionary trajectories occur, and there are two lineages where there had been one, and difference where there had been similarity. This cladogenesis is the fundamental basis of biodiversity, and the core mechanism is speciation. While this may occur through the accumulation of baseline changes, in the end it also requires further mechanisms, such as character displacement [19], allopatry [20] or genetic incompatibility [21], for it to become long-lasting. The appearance of new taxa is a more ‘major’ transition in evolution.
(c) New adaptive zones: significant novel traits and adaptations
Small changes such as the evolution of minor phenotypic differences (four spots on a beetle instead of two), or even the appearance of new taxa (red squirrels and grey squirrels) are still very much the small change of evolutionary biology. Speciation is extraordinarily common, hence the 3–8 million known species [22]. However, in most cases, sister species are not that different from each other. Different species of hartebeest vary mostly in minor elements of coloration and horn morphology [23]. The differences between Cercopithecus ascanius and C. cephus are very minor [24]. The fundamental adaptation of each is essentially the same. In some cases, though, the scale of evolutionarily change is such that an entirely new adaptive zone is achieved. This can be part of changes that open up entirely new opportunities and types of life, such as the colonization of land by amphibians about 370 Ma [25], or homeothermy independently among mammals and birds about 250 Ma [26]. These adaptations transformed the range of evolutionary diversity and ecosystem structures [27]. However, such adaptive novelty does not have to be at such a substantial scale—the ruminant stomach among ungulates, bat echolocation or cetacean marine physiology—would all be examples of new adaptive zones. Such is the nature of adaptive evolution that many such step changes occurred several times, also revealing major convergence in evolution [28].
(d) Major evolutionary transitions: additional evolutionary processes
Maynard Smith & Szathmáry [29] provided a definition and list of major transformations in evolution. Their perspective was distinctive and restrictive; while there are in evolution many transformations, few meet the criteria of a major change. For them the key element is increased complexity and changed systems of information transmission. A eukaryotic cell is more complex than a prokaryotic cell, sexual reproduction is more complex than asexual reproduction, etc. Major transitions are ones where there is a change in the level of organization, the consequences for which are capable of changing the rules of life. In major transitions, entities that previously reproduced independently subsequently reproduced as part of a larger unit, which can result in a change in the units and levels of selection. Such a change can lead to specialization (and so diversity of functions in an organism) and to a change in the way in which information is transmitted between generations (table 1). Maynard Smith & Szathmáry [29] suggested that there were certain common underlying genetic mechanisms (duplication, symbiosis or combination, and expression), and that these transitions impose such a major reproductive reorganization that they are, in effect, irreversible. Szathmáry [30] has recently provided a critical review of progress in transition theory, narrowing down the number of transitions, and recognizing that there may be distinct evolutionary phases involved—origin, maintenance and transformation or further evolution.
Table 1.
Major evolutionary transitions. (a) Proposed major transitions by Maynard Smith and Szathmáry. (b) Markers and conditions of the major transitions in evolution, showing possible candidates of traits that would make human evolution a major transition. Adapted from [29] and [30].
| (a) the major transitions | ||
|---|---|---|
| ancestral condition | derived condition | |
| replicating molecules | → | populations of molecules |
| independent replicators | → | chromosomes |
| RNA | → | DNA |
| prokaryotes | → | eukaryotes |
| asexual clones | → | sexual populations |
| protists | → | fungi, plants, animals |
| solitary individuals | → | social colonies |
| primate societies | → | language and human societies |
| (b) markers of major transitions | ||
|---|---|---|
| characteristic | human candidates | |
| emergence of larger entities from smaller entities | social and cultural groups with demic selection | |
| division/specialization of roles | sexual division of labour, specialist foraging activities, social roles | |
| loss of independent replication | successful reproduction dependent upon high levels of cooperation among individuals | |
| increased inter-dependency can cause fragility | breakdown of social systems can lead to population collapse | |
| novel ways of transmitting evolution | language, symbols, material culture | |
Categorizing and understanding different types of evolution has been the focus of much work, but that of Simpson [8] effectively sets the main themes that have been discussed. Simpson recognized that not all evolution was the same, and that rates varied. His main contribution was to establish that evolutionary rates could be measured, and then assessed in terms of process. This was built on by Haldane [31] who produced a unit of change (the Darwin), Kurtén [32] and Stanley [33]. All recognized that much hinged on how evolutionary rates were measured—as simple trait change, as initiation and survivorship of lineages and taxa—in other words, the units over which it was measured. The types of evolutionary change referred to above can be thought of as moving from measuring phenotypic change over time in a quantitative way to assessing the scale of the biological patterns and processes involved.
3. Transitions in human evolution: at what level do they occur?
Given these four levels of evolutionary change, it is reasonable to ask whether humans are candidates for a level 4 transition (figure 1). Does the evolution of humans constitute one of the major transitions?
At one level this is perhaps not a very interesting question; evolutionary transitions are not, in practice clearly labelled as such, and the distinctions are analytical and interpretative rather than a reflection of actual biological processes. However, the question opens up the possibility of looking at how and when humans underwent the transitions to their current condition.
In broad outline, there are certainly reasons for seeing humans as being the product of a major transition. Szathmáry [30, p 10110] states ‘biology gives room to technological and communal cultural evolution. Due to social care (including medicine) and agriculture, the biology of humans has become gradually de-Darwinized. It is culture where the main action is going on’. For him, the transition is basically a case where culture replaces biology as the principal domain of change and selection. The evidence for this lies in the significance of language as a means of communication, hyper-cooperation being made possible by this, cumulative culture occurring as a result. The key element is perhaps the significance of groups of tightly bound individuals, maximizing benefits via cooperation, which in turn affects the levels and nature of selection—more group selection and more non-genetic adaptation. He argues this falls short of the complete inter-dependence of social insects, but is significant nonetheless. In terms of factors promoting these new systems, in addition to language, Szathmáry cites confrontational scavenging and grand-mothering, the first being a candidate for an ecological trigger, the second one relating to parenting and social behaviour [30].
Beyond Maynard Smith & Szathmáry's [29] assessment, two other aspects of the human species can be cited as reasons for seeing its evolution as a major transition. The first is that the gap between humans and their nearest relatives is vast—chimpanzees may show many elements of complex behaviour and cognition, but the gap between special ways of folding a leaf and the works of Shakespeare is not trivial; humans, by any objective reckoning, are not just different, but uniquely and qualitatively different. This would underscore the hypothesis of their evolution involving a major transition [29]. The second is that humans are, without doubt, the globally dominant species. This hardly needs further elaboration—the size of the human population, and its impact on the planetary ecosystem is unparalleled, and now extends to changing the climate itself [16]. The case here would be that even if the causes of human evolution do not involve any particularly novel processes, the consequences are massively different.
There are, however, arguments that can be made against this claim. Three can be briefly mentioned. One, that the extent of biological difference between humans and other primates, especially apes, is relatively little. Much has been made of the ‘98% like a chimpanzee’ genetic perspective [17], and that is important in contextualizing human differences. Even in terms of the approximately 30 000 genes that humans have, differences are modified variants of ones shared with other primates and different interactions between regulatory genes. There has been nothing like the major biological reorganization that characterizes, for example, sexually reproducing organisms from asexual ones. Second, there are no sharp breaks between humans and other animals. The fossil record shows a remarkably continuous pattern of variation, with overlap in time and morphology between taxa [34,35], so that in biological terms it is not easy to define the distinct threshold that might represent a major transition. Certainly, the endpoint is very different from the beginning (taken as the divergence from the last common ancestor with Pan), but the intermediate steps belie the continuity of process. And third, humans do not represent anything like a major new evolutionary lineage—they are one very small twig on the tree of life [36]. Were humans to persist, of course, and more and more closely related species become extinct, then the twig would become a branch, and so on, and a more radical evolutionary position would come about through differential extinction. A major transition is as much about what is missing as what is there.
It is not profitable to enter into a discussion of what is essentially a matter of scientific classification. However, in order to understand how and when humans evolved into their modern form—biologically and behaviourally—it is worth examining the evidence for different types of evolutionary change, and in particular, whether there are phases in our evolution when particularly significant change occurred, and whether there is a pattern to the sequence of change.
4. Evidence for the different levels of transitions in human evolution
(a) Baseline evolution
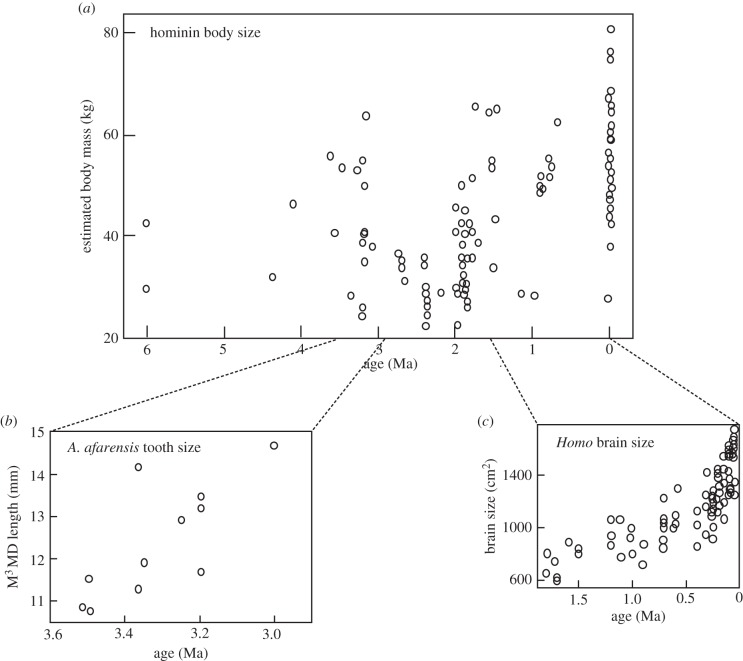
There is ample evidence for simple, baseline evolution across the span of human evolution. Indeed, it would be impossible for that not to be the case. Changes in brain size, body size, dental size and shape have all been attested over time (figure 2). We can see this at various levels. One example is the pattern of brain size increase across time, from the australopithecines and their precursors to Upper Pleistocene Homo. While there is an acceleration of the rate of increase over time, there is nonetheless an incremental change, an additive process (http://www.genetic-inference.co.uk/blog/2010/04/crunching-the-data-on-human-brain-evolution/). The shift from an approximate basal brain size of 400 cm3 to one of about 1400 cm3 by 100 000 years ago represents an increase of about 20 cm3 per 100 000 years; even looking at the last half-million of years, and a conservative basal starting point of about 900 cm3, only yields an incremental rate of about 1 cm3 per 103 years. No matter how great an impact such a brain size increase is, it is still a small rate of change, and would qualify as baseline evolution. Grabowski et al.'s [38] recent presentation of body size changes across the hominin range also illustrates what must be simple baseline—but not unidirectional—change in body size [39].
Figure 2.
Baseline changes in hominin evolution. Much of the changes seen across time in the lineage are small incremental metrical changes, or character shifts. (a) Body mass among hominins [36] over 6 Myr; (b) dental length within A. afarensis over 0.7 Myr [37]; and (c) brain size expansion within the genus Homo since 2.0 Ma (http://www.genetic-inference.co.uk/blog/2010/04/crunching-the-data-on-human-brain-evolution/).
The problem with most examinations of changes in broad parameters such as brain size and body size is that they are often not lineage specific (e.g. [37]), and in the case of human evolution, not unidirectional (e.g. reduced brain and body size of Homo floresiensis in the recent past). It might be argued that a better framework for exploring baseline evolution among hominins would be to look at changes within a single evolving lineage, where continuity can be demonstrated. Sadly, the fossil record is seldom good enough to look at lineages or within-species change. An exception to this is the observed pattern of increased molar size in Australopithecus afarensis between 3.5 and 3.0 Ma [40].
Molecular approaches have brought other dimensions to the discussion of baseline evolution among hominins, with debates about mutation rates [41–43], or whether, as has been argued for modern humans, there has been a recent acceleration in the rate of change [44]. However, regardless of whether the change is constant or not, there is consensus about the cumulative nature of small-scale change at all levels in human evolution. Baseline evolution is the raw material on which other changes depend.
(b) Novel taxa
A distinction is often made between macroevolution and microevolution, with the former being patterns above the level of the species [33]. This means that the appearance (and disappearance) of taxa represents a step change in the evolutionary process. The appearance of new taxa represents significant transitions in evolution, above and beyond baseline anagenetic change. Speciation is essentially a cladogenetic process, where two lineages exist where one did formerly—even if one of these is the ancestral species. Speciation is a significant transition as it implies at least isolation and populational structure, and most likely adaptive and phenotypic change.
Identifying species in human evolution—or indeed in any palaeontological record—is notoriously difficult [45] and controversial [34,46]. To some extent this arises from the desire to apply the biological species concept (the formation of reproductive barriers between gene pools), which is clearly impossible to observe directly. Various approaches can be used as proxies for the recognition of biological species, but a simpler approach is to adopt one of the alternative species concepts—in this case, Simpson's evolutionary species [47]. Simpson argued that a species was a lineage that showed evidence for an independent evolutionary trajectory, independent of whether reproduction could or could not occur. This is a concept that both recognizes the importance of isolation and independence as a marker of an evolutionary transition, and also is practical in terms of the fossil record.
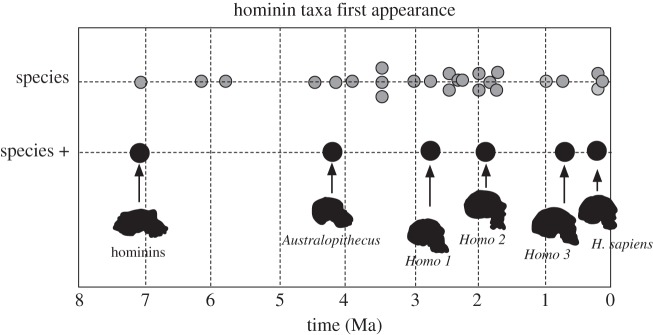
Figure 3 shows the pattern of the appearance of novel taxa in hominin evolution. It is based on dates of first appearances (FADs) in the fossil record [34,35]. One hypothesis would be that these first appearances would mark transitions in human evolution, and as such they might be unevenly distributed. It can be seen, however, that at this resolution, the first appearance data suggest a relatively dispersed pattern, with little overall clumping. Of course, not all species are equally distinctive; some of the proposed taxa are likely to be minor geographical or chronological variants, rather than major adaptive shifts—for example, the difference between Paranthropus robustus and P. boisei. Figure 3 also highlights (larger circles) those taxa that are likely to represent a significantly different creature—the first hominin (possibly Sahelanthropus) [48], the first australopithecine (Australopithecus anamensis) [49], the first Homo [50], and the first Homo that is fully aligned to modern humans in body and facial proportions [51], Homo heidelbergensis [52] and H. sapiens [53]. These points are, of course, dispersed across the time range of hominin evolution. The first three million years are thinly represented, but this is most probably a matter of paucity of fossils. Across the remainder of the period the appearances of new taxa occur frequently, and are certainly not clumped. The appearance of the ‘major taxa’ occurs at (approximately) 7, 4.2, 2.8, 1.8, 0.7 and 0.2 Ma.
Figure 3.
Chronological distribution of the appearance of taxa in hominin evolution. Dates of first appearance (FADs) are seen to be widely dispersed. ‘Species' (small grey circles) are the full range of recognized taxa; ‘species +’ are those for which it may be claimed there is a significant adaptive change. Homo 1 is the appearance of the genus (H. habilis); Homo 2 is the appearance of H. erectus/ergaster; Homo 3 is the appearance of H. heidelbergensis [33,34,47–52].
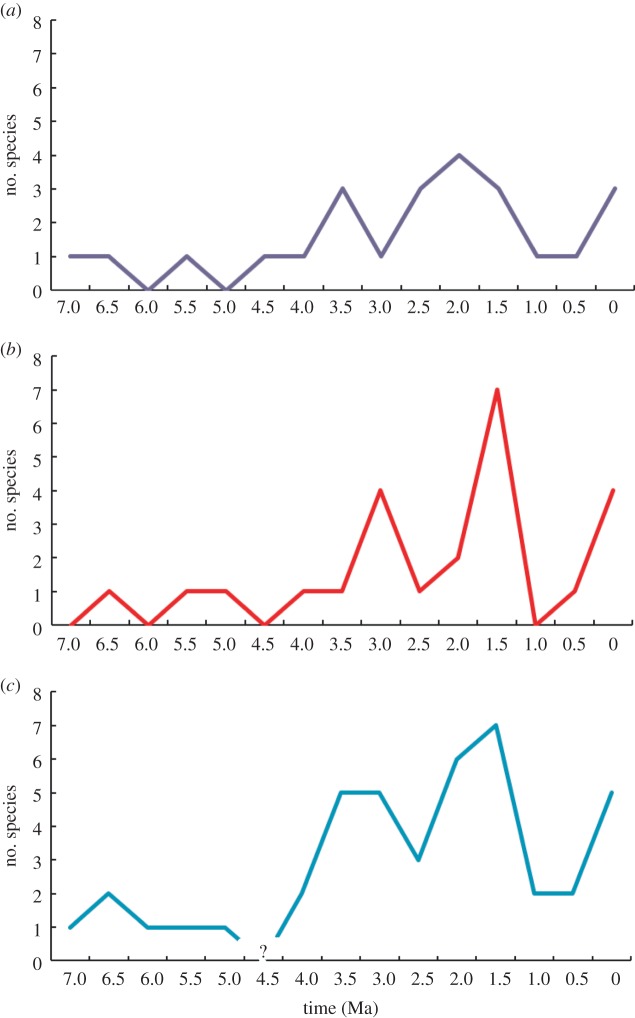
These data can be more easily assessed by examining the frequency of events in temporal bins, especially given the dating resolution. Figure 4 shows the frequency of first appearances (FAD), last appearances (LAD) and number of taxa present (Diversity) across the range of hominin evolution [34,54]. These measures can be treated as proxies for speciation, extinction and species richness in the palaeontological record, although obviously sampling and taphonomic factors would always inhibit an exact relationship between the two. Figure 4 shows that there are a number of peaks in each of these metrics. The highest level of ‘speciation’ (FADs) occurs at around 2–2.5 Ma, with other peaks at 3–3.5 and 0–0.5 Ma. For ‘extinction’ (LADs) the peak occurs at 2.0–1.5 Ma, with lesser peaks at 3.0–3.5 Ma and 0–0.5 Ma.
Figure 4.
Chronological distribution of (a) first appearance (FAD), (b) last appearance (LAD) and (c) taxonomic diversity over time. FADs are used as proxies for speciation, and LADs as proxies for extinction. Diversity is a record of evolutionary change and turnover. Sources as in figure 2. (Online version in colour.)
Do these data indicate clear periods of transitions? There is not an unequivocal answer. On the one hand, only 2 of the 14 periods have no new species being formed; novel species are spread throughout the course of hominin evolution when measured at this scale (an important caveat, as the probability of finding a new taxon will increase with larger bins, and reduce with smaller ones). On the other hand, some periods have more novelties than others; in other words, there are periods of more frequent ‘speciation’ (FADs). If we compare the peaks with the distribution of what were referred to above as more significant appearances (figure 3), then only one of these (H. sapiens) coincides with FAD peaks.
The conclusion must be that looking at human evolution as a macroevolutionary pattern certainly does not support a model of short periods of intense change. This level of transformation occurs throughout the course of our evolutionary history, and fits a pattern of cumulative change. That this is not simply gradual, anagenetic change, but a more interesting pattern, however, is seen when we compare the FAD data with the LAD and Diversity data (figure 4). The peak period for LADs (extinction) is 2.0–1.5 Ma, and this is the period immediately following the peak in FADs (speciation), and this may reflect the impact of the evolution and spread of the genus Homo on other forms of hominins. In addition, the patterns of diversity observed would fit a model of an adaptive radiation (albeit short-lived) among the hominins at this time.
The other period worthy of note is the last 0.5 Myr, when there is a high level of diversity, and first and last appearances. This is when modern humans evolve, along with a number of other lineages of Homo, suggesting a complex pattern of speciation and biogeographical patterning (Eurasian H. neanderthalensis and Denisovans versus African H. sapiens), and rapid evolutionary turnover, as by 30 ka, only H. sapiens remained. Again, this points to a complex pattern of interaction between the appearance and disappearance of new taxa [54–57].
The complexity and ubiquity of the macroevolutionary patterns seen among hominins is certainly evidence that in this way human evolution, like that of any other lineage, comprises transitions involving the appearance of new taxa. The rate of speciation is difficult to assess as there is so little consensus about the nature of the species concerned, but it is not out of line with that of other mammals across the same period. In terms of the drivers of these patterns, the time-lagged relationship between first and last appearances around 2 Ma suggests hypotheses about the competitive interactions between hominin lineages [58], and this may be the case. However, it is also worth considering evidence for this relationship more broadly. The appearance of novel species in human evolution has been linked to climate change [54,58], and also to variability in climate [57]. Others have suggested that the biotic interactions between competing lineages provide a better explanation, more in line with the Red Queen hypothesis [59]. A comparative approach shows that we can expect a much more complex set of interactions. Ezard et al. [60] looked at what drove speciation (FAD) and extinction (LAD) among marine invertebrates during the Cenozoic. They considered the effects of age, species diversity, climate, local ecology of the organisms and geology, as well as the interactive effects of each. They showed that the probability of speciation was most strongly influenced by diversity, followed equally by ecology and climate. The probability of extinction was most strongly affected by ecology, followed by climate. In short, the higher the level of species richness, the greater the number of species likely to evolve, influenced by local and more global conditions, while extinction tended to be more influenced by local ecological factors. These broader studies and the emerging complexity of human evolution point the way to interactions between local and global influences, with variable outcomes, something that can be seen in greater detail in relation to Neanderthal extinction [61,62].
The appearance of new taxa—speciation—and the extinction of existing ones are all significant transitions in human evolution, ones where microevolutionary processes accumulate sufficiently across geographically structured groups for independent lineages to evolve and die out. Recent findings through ancient DNA approaches have shown that there may, at least in the recent past, have been reproductive interactions between such lineages [63,64], but these are not the primary drivers of phenotypes and behaviours—indeed, they are identifiable because they are such brief events. The key finding is that speciation occurs throughout human evolution, and is not confined to specific periods, suggesting a complex and cumulative pattern of change.
As a final caveat, it should be noted that FADs and LADs are not entirely robust measures. Not only can they be strongly influenced by taphonomy and research intensity, but they are vulnerable to new discoveries. The FAD for the genus Homo, for example, was extended by approximately 0.5 Myr following the discoveries of early Homo at 2.8 Ma at Ledi-Geraru (Afar, Ethiopia) [50]. However, given the already dispersed nature of the speciation evidence, it is unlikely that further discoveries will result in greater compression to a few time horizons.
(c). A new adaptive zone
The third level of transition is where a new adaptive zone or a significant adaptive change occurs. For example, the difference between P. robustus and P. boisei is likely to have been adaptively trivial, reflecting more geographical variants than evolutionary novelty [65,66], but taken as a whole, however, the genus Paranthropus does represent a novel set of adaptations, with megadonty and associated morphological changes as a distinctive trait [67], arguably related to a particular niche inaccessible to other hominin species. However, given the ubiquity of larger teeth across hominin evolution, even this may not really be a significantly new adaptive zone. There is, though, little doubt that compared with the assumed last common ancestor with Pan, humans, as the endpoint of the hominin lineage, have definitely entered a new adaptive zone. Characterizing it may be complicated, but there is little dispute over that.
There are many candidates for the nature of the new adaptive zone that humans occupy. In one sense, the human adaptive niche is a single whole—for example, large brains are associated with most of the other phenotypic traits that form the basis for human behaviour—but that is not analytically helpful as it may be the case that across evolutionary time there may have been different associations. In fact, the timing and processes by which the human adaptive zone evolved, whether as a single transition or several, or as continuous and gradual process, or in bursts, is a major research issue. Evolutionary genetics is beginning to throw some light on these questions; for example, the discovery that humans and Neanderthals share the derived form of the FOXP2 gene [68], which may be an indicator of modern speech capacities, would indicate that the transition to spoken forms of communication had taken place at the time of their last common ancestor (about 0.45 Ma) [69]. However, such inferences are rare, and the primary source of information about phenotypic (morphology and behaviour) changes comes from the palaeoanthropological record.
We can divide derived human traits into a series of broad categories—terrestriality and ranging behaviour; life-history strategy; foraging, diet and technology; reproductive and social behaviour; cognitive and cultural. Each of these may also consist of a series of different elements—for example, terrestriality and ranging can be associated with changes in posture and locomotion, energetics and thermoregulation.
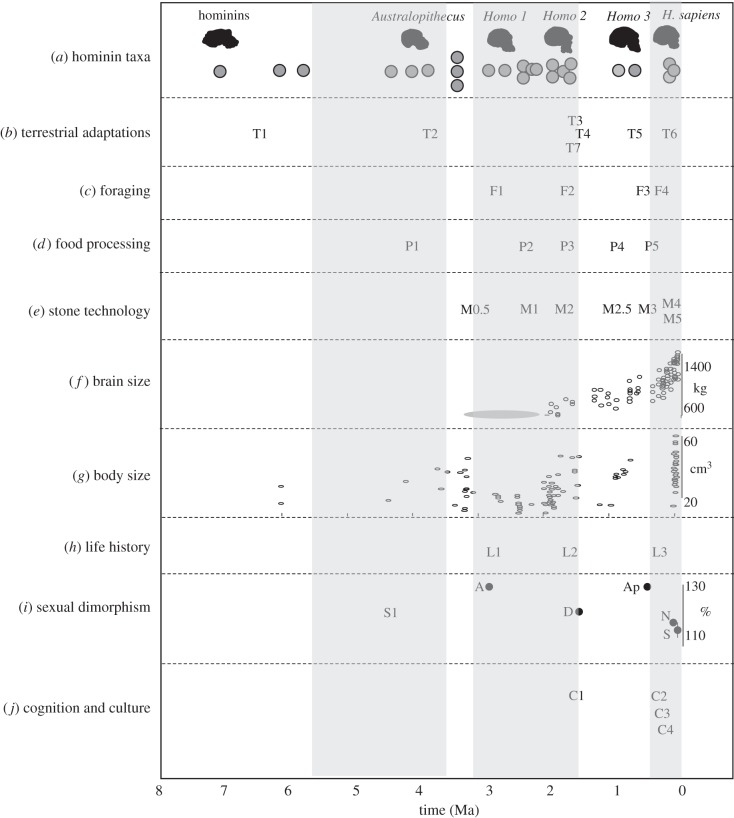
The problem to solve is to find a match between what is significant in the human adaptive zone and what is observable in the fossil or archaeological records. Figure 5 sets out the main characteristics, and possible associations with the palaeoanthropological record, and so provides a basis for a chronology of how humans achieved their novel adaptive zone. The data on which this is based are variable, with different degrees of resolution and reliability of inference, but provide a reasonable guide to the tempo of change (see the electronic supplementary material, Supplementary Evidence).
Figure 5.
Chronological distribution of the first appearances of major derived traits in human evolution. Data points indicate earliest proposed evidence for the diverse traits, some of which are disputed or open to different interpretations. Sources of evidence are listed in references in the electronic supplementary material. The shaded areas indicate the three potential phases of transitions to novel adaptive zones. See text for discussion. (a) Hominin taxa: first appearance (FADs) for major groups (skull icons) and for species (grey circles). Same data as in figure 3. (b) Terrestrial adaptations: T1, suggestive evidence of some level of terrestrial adaptation through a greater level of bipedalism; T2, habitual bidpedalism as seen in A. anamensis and later australopithecines; T3, striding bipedalism as seen in H. ergaster/erectus, similar to modern human locomotion; T4, disputed evidence for a ground nest/shelter (DK1 at Olduvai Gorge); T5, some evidence for base camp usage; T6, full residential mobility patterns; T7, endurance running. (c) Foraging behaviour: F1, ephemeral evidence for processing of meat/animals; F2, substantial evidence for meat processing/butchery/scavenging/hunting, and possible use of some aquatic resources; F3, projectile hunting; F4, complex and specialized foraging such as specialist hunting, plant resource modification, systematic use of aquatic resources and foraging similar to living hunter–gatherers. (d) Food processing: P1, evidence for posterior dental enlargement in hominins; P2, posterior megadonty; P3, dental reduction in Homo; P4, fire and possible cooking. P5, substantial evidence for cooking and processing. (e) Stone technology: M0.5, earliest evidence for fracturing of stone (Lomekwian); M1, mode 1 technologies (Oldowan); M2, mode 2 technologies (large cutting tools, bifaces); M2.5, more regular and refined production of bifaces; M3, mode 3 technologies (prepared core); M4, mode 4 technologies (blades); M5, mode 5 technologies (microliths). (f) Brain size: data (in cubic centimetres) from figure 2, for Homo; range for earlier hominins indicated by grey ellipse. (g) Body size: data from figure 2 (in kilograms). (h) Life history: L1, early hominins show evidence of differences in life-history strategy from extant apes; L2, first evidence of a shift towards the life-history strategies of modern humans; L3, modern human life-history patterns shown in early modern humans, but distinctive patterns observed in Neanderthals. (i) Sexual dimorphism: S1, reduced canines observed in Ardipithecus ramidus; sexual dimporphism of hominin taxa shown in percentage of female body weight. Only those samples for which there are grounds for thinking they are a population are used. A, A. afarensis; D, Denisovans; Ap, Atapuerca; N, Neanderthal; S, H. sapiens. (j) Cognition and culture: C1, KNM-WT15000 does not show language-based adaptations in its thoracic vertebrae; C2, evidence for regional population behaviours in African Middle Stone Age, and for language related adaptations in both Neanderthals and modern humans; C3, diverse evidence for cumulative cultural processes and complex behaviours; C4, evidence for symbolic thought, communication and representations.
Three general observations can be made. The first is that the changes are widely dispersed across the range of hominin evolution, as would be expected. This emphasizes that the transition to human adaptive traits is a cumulative one, not a single transitional phase. The second is that within that dispersed distribution there are three relatively distinct periods of transition when (i) there is a relatively high rate of change across a number of traits; and (ii) each of these has a distinctive evolutionary character. Broadly speaking, these can be considered to be in the Pliocene, during the Plio-Pleistocene and in the later Quaternary. It should be noted, however, that these represent very different scales—the first two covering more than a million years, the last less than half a million years. The resolution with which we can see changes is thus very different, and to refer to them as if they represent the same mode and tempo is probably misleading. Several ‘later Quaternary transitions' could occur within the time frames of the earlier ones [70].
The third observation is that each of the three periods of transition is distinctive in its character, relating to different aspects of hominin and human adaptation. The Pliocene transition, in as much as the evidence can show it, appears to be related to patterns of locomotion and ranging behaviour, suggesting a novel habitat and ecological niche, arguably as the environment became more dominated by woodland and grassland. Inevitably, there would have been shifts in diet, behaviour and socioecology as the populations responded to the new environments, but the absence of archaeological evidence makes this hard to detect. Some indication of these is provided by the possible change in the reduction of canines and canine/premolar honing relationship (as seen Ardipithecus ramidus), and the change in isotope signature from C3 to mixed C3/C4 in Australopithecus afarensis at the end of this phase [71,72]. The evidence suggests that the degree of committed terrestrial and arid specialization and adaptation was unique among apes. In other aspects—cultural transmission and cognition, for example—it is likely that the adaptive zone of the earliest hominins would have been not substantially different in scale from that among other ape species. This is an ‘energetics and ranging ecology’ transition, with consequences for social organization and group size.
The Plio-Pleistocene transitions are complex, and far better documented. These would be said to occur across the period from about 3.5 Ma to 1.5 Ma, an enormous span of time. The earliest elements of this transition would be the appearance of stone tools at Lomekwi dated to 3.3 Ma [73]; others would include the first evidence for processing of animals using tools (3.4 Ma) [74,75]; the appearance of the genus Homo [50], or more precisely, phenotypes associated with the human lineage, namely larger brains, reduced post-canine dentition, less prognathic face and the development of distinctive supra-orbital tori. The early part of this transition (2.8–1.9 Ma) is variable [76], with different fossil groups displaying different elements of the traits that defined the new adaptive zone—very much a mosaic of trends rather than a simple trajectory. This becomes more unified after 2.0 Ma, with the appearance of a more integrated suite of traits—a body shape and locomotor style similar to that of modern humans (KNM-WT 15000, 1.6 Ma), significantly larger brain size (KNM-ER 3733, 850 cm3), a shift towards a more modern life-history strategy (KNM-WT 15000, 1.6 Ma) [51,77]. The evidence for technology for the early part of the period is very limited, but from about 1.8 Ma there is a substantial increase in the number of sites and the size of assemblages, suggesting a shift to a more habitual pattern of tool use [78]. At about the same time, evidence for butchery of animals, possibly as a result of hunting, increases markedly [79]. The end of this period is also associated with the extinction of the australopithecines, the evolution of transitional and early members of the genus Homo, and the paranthropines, suggesting a substantial shift in niche structure, and overall a new adaptive zone for hominins. It also appears to be the basis for the first dispersals into northern Africa and Eurasia [80,81]. However, perhaps the major point to emphasize for this complex behavioural and life-history transition is that it is not a single compressed event, but spread over more than a million years, and likely to be the product of multiple smaller microevolutionary shifts.
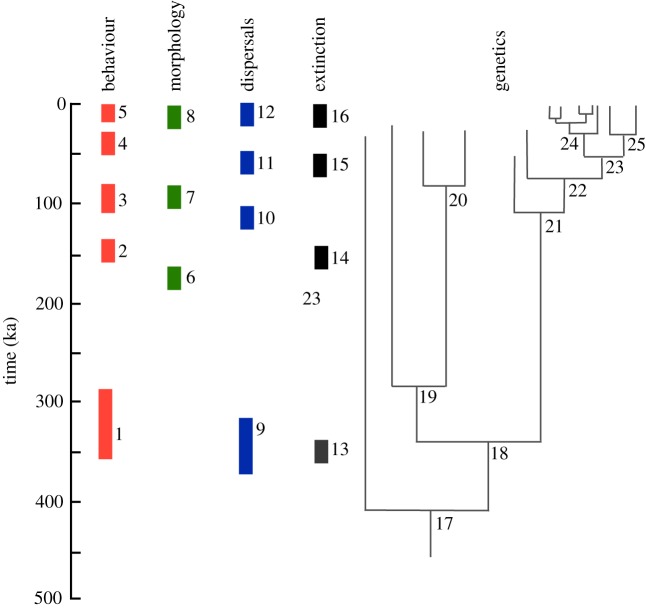
That this is not entirely the fully novel adaptive zone of humans can be seen by the extent of change that occurs one million years later. From about 0.5 Ma there is another phase of substantial change. This could be summed up as the evolution of H. sapiens, but as some of the traits are shared by the Neanderthal lineage, then it may be a phase that covers both the shift to an ancestor of all larger brained Homo, and uniquely to modern humans, depending on the traits [82]. This Late Quaternary transition is centred on major behavioural, cognitive and cultural changes [83,84] (and references therein). There is a substantial increase in brain size across the period, and changes in cranial morphology and overall robusticity, but compared with the physical changes taking place in the earlier transitions, these are relatively minor. However, in behavioural and cultural aspects there is a major change, both in the development of new traits, and also in the rate of change. The key elements of this phase of human evolution have been well-rehearsed—a ratcheting of rates of change and increased complexity in technology [85], the emergence of regional entities and identities [86], greater population densities [87], evidence for enhanced cultural processes [88], symbolic thought and representation [89]. The rate is significant too. The period of time involved, less than 0.5 Ma, is much shorter than the several million years of the other two transitions. Here is a transition that is firmly within the scale of microevolutionary change, and the details with which we can see it allows us to recognize that patterns of change are spread across the whole period, often in an asynchronous or discontinuous manner (figure 6).
Figure 6.
The multiple events of the evolution of modern humans. The evolution of modern humans is a very rapid event in the context of evolution as a whole, but is nonetheless composed of many dispersed events or transitions. Each of these (and others not yet discovered) contributed to the totality of the modern human transformation. Behaviour: 1, the development of mode 3 technologies (the African Middle Stone Age), common to all later hominins; 2, the appearance of novel behaviours in the African Middle Stone Age; 3, the appearance of symbolic use of material culture in the African Middle Stone Age; 4, the Eurasian Upper Palaeolithic; 5, Later Pleistocene cultural and technological intensifications; Morphology: 6, earliest appearance of anatomically modern humans (Omo Kibbish, Ethiopia); 7, widespread distribution of modern human phenotypes in Africa and the Levant; 8, establishment of extant human population distributions; Dispersals: 9, dispersals of ancestors of Neanderthals into Eurasia; 10, dispersals across Africa, and to a limited extent into Eurasia; 11, major Eurasian dispersals out of Africa; 12, post Last Glacial Maximum dispersals; Extinction: 13 and 14, extinction of H. heidelbergensis populations in parts of Africa and Eurasia; 15, extinction of modern humans in the Levant; 16, extinctions of Neanderthals, other archaic populations (?), and some modern human populations before or during the Last Glacial Maximum; Genetics: 17, divergence of ancestors of later larger brained hominins from ancestral H. heidelbergensis populations; 18, divergence of ancestors of Eurasian archaics (Neanderthals and Denisovans) and African modern human lineages; 19, divergence of Neanderthals and Denisovan lineages; 20, diversification of Eurasian (eastern and western) Neanderthal populations; 21, divergence of early African population and Levantine populations; 22, out of Africa/into Eurasia, Sunda, Sahul divergences; 23, divergence of Eurasian and Sunda/Sahul populations; 24, diversification of Eurasian populations; 25, divergence of Sunda and Sahul populations. Admixture events between populations not shown. (Online version in colour.)
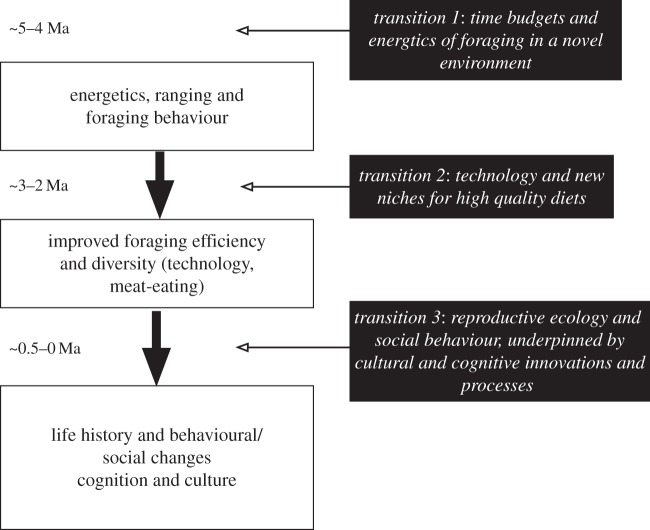
There is little doubt that humans occupy a novel adaptive zone, unexplored before. In this context, it can be safely argued that human evolution comprises to a large extent the third level of evolutionary change, comparable with the first land creatures. However, the wealth of archaeological and fossil evidence indicates strongly that the change occurs across the whole of the seven or less million years since the divergence from the last common ancestor with chimpanzees, and actually consists of three separate phases of substantial adaptive change. The first of these is related to locomotion, foraging and habitat adaptations; the second to a suite of behavioural changes that are linked to a change in diet, means of acquisition of resources (technology) and life-history strategy; and the final one is strongly based on cognitive and behavioural changes. The adaptive zone occupied by humans is one that was the product of cumulative, mosaic-based, transitions rather than a single shift (figure 7).
Figure 7.
Major transitional phases in human evolution.
(d). A major evolutionary transition?
The final question is whether the sum of all these levels of evolutionary change constitutes a major transition in the sense used by Maynard Smith & Szathmáry [29]. The key criteria are the emergence of larger entities of replication, a division of roles, the loss of independent replication, resulting in evolutionary fragility. The transition results in novel ways of transmitting information.
There are several obvious candidates that could lead to such a transformation—technological dependence, language, cumulative culture, high levels of reproductive cooperation and cooperation beyond kin-related individuals. To some extent they are all inter-related, such that it is probably impossible to untangle which is the key element. Language, for example, could be the driving force, as Maynard Smith & Szathmáry [29] originally argued, as it is an entirely novel means of communication, and so of transmitting information. However, it is likely that the underlying extreme levels of social cooperation, both for breeding and for constructing social tolerance, are as much at the centre of the process as language itself. Equally, it is unlikely that the high levels of communication and cooperation, which form the basis for modern society, would be possible without technological abilities. So the ‘key element’ remains elusive. Furthermore, the evidence we have explored at a lower level of evolutionary transition shows that the evolution of humans is not a single event, but a process of combination and accumulation. It is not one phase of becoming human that represents a major transition, but the cumulative effect of them, the processes of mosaic evolution, and the very recent extinction of all other hominins that enhances the distinctiveness of humans. The outcome is a fundamentally different species; whether, as Maynard Smith and Szathmáry originally argued [29], this is one of the major transitions, or, as Szathmáry later preferred [30], that it is, in comparison to other major changes, incomplete, is less important than being able to see in detail how major changes come about through microevolutionary changes. Only the extraordinarily detailed resolution of the recent fossil and archaeological records provides that insight into major evolutionary change.
While there may be some doubt about human evolution as a genuine radical transformation in evolution, there can be none about its consequences. In terms of rates of environmental change caused by humans, the impact on rates of extinction, and the consequences for life on the Earth, there can be no doubt. Lyons et al. [90] have recently shown that, since the beginning of the Holocene 10 000 years ago, the rate at which patterns of covariation between species, some of which have been stable for as long as 300 Myr, have been broken has greatly increased. It has also been argued that human impact in the Holocene has resulted in the first major restructuring of trophic systems since the establishment of terrestrial herbivory in the late Permian [91]. In that context, the evolution of humans is a major and irreversible transition.
5. Discussion
In posing the question of whether humans represent a major evolutionary transition, it was never the intention to provide a categorical answer. Such terms are analytical concepts, not biologically meaningful units. However, in asking the question, we can explore the processes by which humans did develop a unique and un-controversially different evolutionary profile.
Several points emerge. First, if unsurprisingly, that human evolution is a gradual and cumulative process, best described as mosaic evolution [92]. It is worth considering briefly what is meant by mosaic evolution. At the most local level it simply means that within a lineage, different traits evolve independently and at different times; this is the basis of Hublin's accretion model of Neanderthal evolution [93]. It is likely that within any lineage mosaic evolution at this level will occur, although due to pleiotropic effects there may also be degrees of coevolution, producing a more correlated evolutionary pattern. Thus, different traits appear and change at different times, and the rates of evolution vary not just between periods but also between elements of the hominin phenotype and extended phenotype. At a higher level, though, mosaic evolution is when different domains of evolution change at different times. Thus, one part of a lineage's history might see rapid changes in dental patterns, while during another phase it is body size that changes. The pattern of hominin evolution described here fits this higher level form of mosaic evolution. The transitions described relate to the different elements of human evolution—ranging behaviour and energetics, foraging and diet, reproduction and life history, and cognition and behavioural transmission (figure 7).
There is no ‘breakthrough moment’, but a series of different transitions. This is not just the case leading to the origin of modern humans (the last transition), as it is clear that since the appearance of H. sapiens about 200 ka, there has been substantial evolutionary change [94], and it could be argued that the ‘breakthrough’ to a dominant species transforming the planet did not occur until the last 10 000 years.
Second, the three transitions identified within a broader pattern of change are different elements of the mosaic; at its broadest level, the first is about the changes in how hominins ranged across the landscape; the second is about the nature of the resources they acquired, and how they acquired them; and the third is about changes in reproduction and sociality. Only when this last was in place do we observe the full impact of cultural evolution as a rapidly accumulating process. This sequence—ranging, diet breadth and resource extraction, and socioecology—can be seen as the necessary building blocks for being a modern human. What would be interesting is to explore further whether this is a sequence replicated in the evolution of other lineages.
Third, following on from this, it can be argued that these building blocks depend upon ecological foundations. There has been considerable discussion in studies of human evolution about the social brain and social factors driving hominin evolution, but such a view can only hold if a relatively short period of time in the evolution of our lineage is considered. The totality shows a strong ecological foundation.
Fourth, it is clear that behaviour—defined broadly, and including the later cultural mechanisms of behavioural innovation and transmission—plays a central role in the process. Approaches to human evolution have traditionally focused on morphology, as fossils have been the source of information, and more recently genes, as these provide excellent markers of evolutionary history, but in each of the major transitions behavioural changes can be seen not just as important, but also chronologically earlier. This would lead to further incorporation of behavioural processes in models of evolutionary transitions (e.g. Baldwin effect), and in evolutionary theory more generally [95].
Finally, it is worth stepping back and returning in a different way to the questions posed at the beginning about major transitions. Whether formally a major transition or not, humans are the product of major changes since the last common ancestor with apes, and this takes place over a period of 5–7 Myr. Parts of that evolutionary sequence can be observed on a millennial scale, and all within a resolution of tens of thousands of years. Had this been an evolutionary event occurring tens or hundreds of millions of years ago, such resolution and visibility would not be possible. Furthermore, the hominin habit of making and discarding stone tools provides a unique record of behaviour. It is that extension of the fossil record and the high level of palaeobiological visibility that allows us to see how major, macroevolutionary transitions are embedded in a sequence of microevolutionary ones. Human evolution, it turns out, is not just interesting in its own right, but for the insights it provides into evolutionary processes in general.
Supplementary Material
Acknowledgements
I am grateful to Susana Carvalho for advice on the early archaeological record, and Marta Mirazón Lahr for discussion of many of the ideas in this paper.
Competing interests
I declare I have no competing interests.
Funding
I thank the Leverhulme Trust for support in the form of a Major Research Fellowship.
References
- 1.Gould SJ. 1980. Is a new and general theory of evolution emerging. Paleobiology 6 119–130. [Google Scholar]
- 2.Hecht MK, Eldredge N, Gould SJ. 1975. Morphological transformation, the fossil record, and the mechanisms of evolution: a debate. In Evolutionary biology (eds Dobzhansky TG et al.), pp. 295–308. Boston, MA: Springer. [Google Scholar]
- 3.Maynard Smith J. 1983. The genetics of stasis and punctuation. Annu. Rev. Genet. 17, 11–25. ( 10.1146/annurev.ge.17.120183.000303) [DOI] [PubMed] [Google Scholar]
- 4.Gould SJ. 2003. The structure of evolutionary theory. Cambridge, MA: Harvard University Press. [Google Scholar]
- 5.Bateson P. 2010. The evolution of evolutionary theory. Eur. Rev. 18, 287–296. ( 10.1017/S1062798710000049) [DOI] [Google Scholar]
- 6.Eldredge N, Gould SJ. 1972. Punctuated equilibrium: an alternative to phyletic gradualism. In Models in palaeobiology (ed. Schopf TJM.), pp. 82–115. San Francisco, CA: Freeman. [Google Scholar]
- 7.Vrba ES, Eldredge N. 1984. Individuals, hierarchies and processes—towards a more complete evolutionary theory. Paleobiology 10, 146–171. [Google Scholar]
- 8.Simpson GG. 1944. Tempo and mode in evolution: a synthesis of paleontology and genetics. New York, NY: Columbia University Press. [Google Scholar]
- 9.Simpson GG. 1953. The major features of evolution. New York, NY: Columbia University Press. [Google Scholar]
- 10.Goldschmidt R. 1940. Evolution above the species level. New Haven, CT: Yale University Press. [Google Scholar]
- 11.Rensch B. 1947. The material basis of evolution. New York, NY: Columbia University Prress. [Google Scholar]
- 12.De Vries H. 1910. The mutation theory. Chicago, IL: The Open Court Publishing Company. [Google Scholar]
- 13.Kane NC, Barker MS, Zhan SH, Rieseberg LH. 2011. Molecular evolution across the Asteraceae: micro- and macroevolutionary processes. Mol. Biol. Evol. 28, 3225–3235. ( 10.1093/molbev/msr166) [DOI] [PubMed] [Google Scholar]
- 14.Erwin DH. 2000. Macroevolution is more than repeated rounds of microevolution. Evol. Dev. 2, 78–84. ( 10.1046/j.1525-142x.2000.00045.x) [DOI] [PubMed] [Google Scholar]
- 15.Maruvka YE, Shnerb NM, Kessler DA, Ricklefs RE. 2013. Model for macroevolutionary dynamics. Proc. Natl Acad. Sci. USA 110, E2460–E2469. ( 10.1073/pnas.1220014110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IPCC. 2014. Climate Change 2014: synthesis report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, pp. 1–155. Geneva, Switzerland: IPCC.
- 17.Diamond J. 1992. The third chimpanzee. New York, NY: Perrenial. [Google Scholar]
- 18.Grant BR, Grant PR. 1989. Natural selection in a population of Darwin's finches. Am. Nat. 133, 377–393. ( 10.1086/284924) [DOI] [Google Scholar]
- 19.Allen WL, Stevens M, Higham JP. 2014. Character displacement of Cercopithecini primate visual signals. Nat. Commun. 5, 4266 ( 10.1038/ncomms5266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osada N. 2004. Inferring the mode of speciation from genomic data: a study of the great apes. Genetics 169, 259–264. ( 10.1534/genetics.104.029231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faria R, Navarro A. 2010. Chromosomal speciation revisited: rearranging theory with pieces of evidence. Trends Ecol. Evol. 25, 660–669. ( 10.1016/j.tree.2010.07.008) [DOI] [PubMed] [Google Scholar]
- 22.Costello MJ, May RM, Stork NE. 2013. Can we name Earth's species before they go extinct? Science 339, 413–416. ( 10.1126/science.1230318) [DOI] [PubMed] [Google Scholar]
- 23.Ouma C, Roca AL, Were T, Raballah EO. 2010. Genetic structure of hartebeest populations straddling a transition zone between morphotypes. J. Basic Appl. Sci. Res. 1, 131–149. [Google Scholar]
- 24.Xing J, Wang H, Zhang Y, Ray DA, Tosi AJ, Disotell TR, Batzer MA. 2007. A mobile element-based evolutionary history of guenons (tribe Cercopithecini). BMC Biol. 5, 5 ( 10.1186/1741-7007-5-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashley-Ross MA, Hsieh ST, Gibb AC, Blob RW. 2013. Vertebrate land invasions—past, present, and future: an introduction to the symposium. Integr. Comp. Biol. 53, 192–196. ( 10.1093/icb/ict048) [DOI] [PubMed] [Google Scholar]
- 26.Ruben J. 1995. The evolution of endothermy in mammals and birds: from physiology to fossils. Annu. Rev. Physiol. 57, 69–95. ( 10.1146/annurev.ph.57.030195.000441) [DOI] [PubMed] [Google Scholar]
- 27.Smithsoniam Program in Terrestrial Paleoecology. 1992. Evolutionary palaeoecology of terrestrial plants and animals. Chicago, IL: University of Chicago Press. [Google Scholar]
- 28.Conway Morris S. 2003. Life solutions. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 29.Maynard Smith J, Szathmáry E. 1997. Major transitions in evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 30.Szathmáry E. 2015. Toward major evolutionary transitions theory 2.0. Proc. Natl Acad. Sci. USA 112, 10 104–10 111. ( 10.1073/pnas.1421398112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haldane JBS. 1949. Suggestions as to quantitative measurement of rates of evolution. Evolution 3, 51–56. ( 10.2307/2405451) [DOI] [PubMed] [Google Scholar]
- 32.Kurten B. 1960. Chronology and faunal evolution of the earlier European glaciations. Soc. Sci. Fenn. Comment. Biol. 21, 40–62. [Google Scholar]
- 33.Stanley SM. 1979. Macroevolution: pattern and process. San Francisco, CA: Freeman. [Google Scholar]
- 34.Foley RA. 2005. Species diversity in human evolution: challenges and opportunities. Trans. R. Soc. S. Afr. 60, 67–72. ( 10.1080/00359190509520479) [DOI] [Google Scholar]
- 35.Wood B, Lonergan N. 2008. The hominin fossil record: taxa, grades and clades. J. Anat. 212, 354–376. ( 10.1111/j.1469-7580.2008.00871.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinchliff CE, et al. 2015. Synthesis of phylogeny and taxonomy into a comprehensive tree of life. Proc. Natl Acad. Sci. USA 112, 12 764–12 769. ( 10.1073/pnas.1423041112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolpoff MH. 1984. Evolution in Homo erectus: the question of stasis. Palaeobiology 10, 389–406. [Google Scholar]
- 38.Grabowski M, Hatala KG, Jungers WL, Richmond BG. 2015. Body mass estimates of hominin fossils and the evolution of human body size. J. Hum. Evol. 85, 75–93. ( 10.1016/j.jhevol.2015.05.005) [DOI] [PubMed] [Google Scholar]
- 39.Jungers WL, Grabowski M, Hatala KG, Richmond BG. 2016. The evolution of body size and shape in the human career. Phil. Trans. R. Soc. B 371, 20150247 ( 10.1098/rstb.2015.0247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lockwood CA, Kimbel WH, Johanson DC. 2000. Temporal trends and metric variation in the mandibles and dentition of Australopithecus afarensis. J. Hum. Evol. 39, 23–55. ( 10.1006/jhev.2000.0401) [DOI] [PubMed] [Google Scholar]
- 41.Scally A, Durbin R. 2012. Revising the human mutation rate: implications for understanding human evolution. Nat. Rev. Genet. 13, 745–753. ( 10.1038/nrg3295) [DOI] [PubMed] [Google Scholar]
- 42.Hahn MW, Demuth JP, Han SG. 2007. Accelerated rate of gene gain and loss in primates. Genetics 177, 1941–1949. ( 10.1534/genetics.107.080077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scally A, et al. 2012. Insights into hominid evolution from the gorilla genome sequence. Nature 483, 169–175. ( 10.1038/nature10842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raghavan M, et al. 2015. Genomic evidence for the Pleistocene and recent population history of Native Americans. Science 349, paab3884. ( 10.1126/science.aab3884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tattersall I. 2005. Species and paleoanthropology. Theory Biosci. 123, 371–379. ( 10.1016/j.thbio.2004.10.001) [DOI] [PubMed] [Google Scholar]
- 46.Kimbel W. 1991. Species, species concepts and hominid evolution. J. Hum. Evol. 20, 355–371. ( 10.1016/0047-2484(91)90016-O) [DOI] [Google Scholar]
- 47.Simpson GG. 1951. The species concept. Evolution 5, 285–298. ( 10.2307/2405675) [DOI] [Google Scholar]
- 48.Brunet M, et al. 2004. Sahelanthropus tchadensis: the facts. S. Afr. J. Sci. 100, 443–446. [Google Scholar]
- 49.Ward C, Leakey M, Walker A. 1999. The new hominid species Australopithecus anamensis. Evol. Anthropol. Issues News Rev. 7, 197–205. () [DOI] [Google Scholar]
- 50.Villmoare B, Kimbel WH, Seyoum C, Campisano CJ, DiMaggio EN, Rowan J, Braun DR, Arrowsmith JR, Reed KE. 2015. Early Homo at 2.8 Ma from Ledi-Geraru, Afar, Ethiopia. Science 347, 1352–1355. ( 10.1126/science.aaa1343) [DOI] [PubMed] [Google Scholar]
- 51.Walker AC, Leakey RE (eds). 1993. The Nariokotome skeleton. Dortrecht, The Netherlands: Springer. [Google Scholar]
- 52.Mounier A, Marchal F, Condemi S. 2009. Is Homo heidelbergensis a distinct species? New insight on the Mauer mandible. J. Hum. Evol. 56, 219–246. ( 10.1016/j.jhevol.2008.12.006) [DOI] [PubMed] [Google Scholar]
- 53.McDougall I, Brown FH, Fleagle JG. 2005. Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature 433, 733–736. ( 10.1038/nature03258) [DOI] [PubMed] [Google Scholar]
- 54.Maslin MA, Brierley CM, Milner AM, Shultz S, Trauth MH, Wilson KE. 2014. East African climate pulses and early human evolution. Quat. Sci. Rev. 101, 1–17. ( 10.1016/j.quascirev.2014.06.012) [DOI] [Google Scholar]
- 55.Foley RA. 1994. Speciation, extinction and climatic change in hominid evolution. J. Hum. Evol. 26, 275–289. ( 10.1006/jhev.1994.1017) [DOI] [Google Scholar]
- 56.Grove M. 2011. Change and variability in Plio-Pleistocene climates: modelling the hominin response. J. Archaeol. Sci. 38, 3038–3047. ( 10.1016/j.jas.2011.07.002) [DOI] [Google Scholar]
- 57.Potts R. 1998. Variability selection in hominid evolution. Evol. Anthropol. Issues News Rev. 7, 81–96. () [DOI] [Google Scholar]
- 58.Vrba E. 1988. Late Pliocene climatic events and human evolution. In Evolutionary history of the ‘Robust’ Australopithecines (ed. Grine F.), pp. 405–426. New York, NY: Aldine de Gruyter. [Google Scholar]
- 59.Foley RA. 1984. Early man and the Red Queen: tropical African community evolution and ecology. In Hominid evolution and community ecology: prehistoric human adaptation in biological perspective (ed. Foley RA.), pp. 85–110. New York, NY: Academic Press. [Google Scholar]
- 60.Ezard THG, Aze T, Pearson PN, Purvis A. 2011. Interplay between changing climate and species’ ecology drives macroevolutionary dynamics. Science 332, 349–351. ( 10.1126/science.1203060) [DOI] [PubMed] [Google Scholar]
- 61.Gilpin W, Feldman MW, Aoki K. 2016. An ecocultural model predicts Neanderthal extinction through competition with modern humans. Proc. Natl Acad. Sci. USA 113, 2134–2139. ( 10.1073/pnas.1524861113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finlayson C, Fa DA, Finlayson G, Pacheco FG, Vidal JR. 2004. Did the moderns kill off the Neanderthals? A reply to the comments by d'Errico and Sánchez Goni. Quat. Sci. Rev. 23, 1205–1216. ( 10.1016/j.quascirev.2003.12.017) [DOI] [Google Scholar]
- 63.Sanchez-Quinto F, Lalueza-Fox C. 2014. Almost 20 years of Neanderthal palaeogenetics: adaptation, admixture, diversity, demography and extinction. Phil. Trans. R. Soc. B 370, 20130374 ( 10.1098/rstb.2013.0374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sankararaman S, Mallick S, Patterson N, Reich D. 2016. The combined landscape of Denisovan and Neanderthal Ancestry in present-day humans. Curr. Biol. 26, 1241–1247. ( 10.1016/j.cub.2016.03.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foley RA. 1999. The evolutionary geography of Pliocene hominids. In African biogeography, climatic change, and hominid evolution (eds Bromage T, Schrenk F), pp. 328–348. Oxford, UK: Oxford University Press. [Google Scholar]
- 66.Strait DS, Wood BA. 1999. Early hominid biogeography. Proc. Natl Acad. Sci. USA 96, 9196–9200. ( 10.1073/pnas.96.16.9196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grine FE (ed). 1988. The evolutionary history of the ‘robust australopithecines’. Chicago, IL: Aldine. [Google Scholar]
- 68.Krause J, et al. 2007. The derived FOXP2 variant of modern humans was shared with Neandertals. Curr. Biol. 17, 1908–1912. ( 10.1016/j.cub.2007.10.008) [DOI] [PubMed] [Google Scholar]
- 69.Meyer M, et al. 2016. Nuclear DNA sequences from the Middle Pleistocene Sima de los Huesos hominins. Nature 531, 504–507. ( 10.1038/nature17405) [DOI] [PubMed] [Google Scholar]
- 70.Foley RA. 2013. Comparative evolutionary models and the ‘australopithecine radiations’. In Paleobiology of Australopithecus (eds Reed K, Fleagle J, Leakey RE), pp. 163–174. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 71.White TD, Suwa G, Asfaw B. 1994. Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia. Nature 375, 88–92. ( 10.1038/371306a0) [DOI] [PubMed] [Google Scholar]
- 72.Sponheimer M, et al. 2013. Isotopic evidence of early hominin diets. Proc. Natl Acad. Sci. USA 110, 10 513–10 518. ( 10.1073/pnas.1222579110) [DOI] [Google Scholar]
- 73.Harmand S, et al. 2015. 3.3-million-year-old stone tools from Lomekwi 3, West Turkana, Kenya. Nature 521, 310–315. ( 10.1038/nature14464) [DOI] [PubMed] [Google Scholar]
- 74.McPherron SP, Alemseged Z, Marean CW, Wynn JG, Reed D, Geraads D, Bobe R, Béarat HA. 2010. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature 466, 857–860. ( 10.1038/nature09248) [DOI] [PubMed] [Google Scholar]
- 75.Thompson JC, McPherron SP, Bobe R, Reed D, Barr WA, Wynn JG, Marean CW, Geraads D, Alemseged Z. 2015. Taphonomy of fossils from the hominin-bearing deposits at Dikika, Ethiopia. J. Hum. Evol. 86, 112–135. ( 10.1016/j.jhevol.2015.06.013) [DOI] [PubMed] [Google Scholar]
- 76.Leakey MG, Spoor F, Dean MC, Feibel CS, Antón SC, Kiarie C, Leakey LN. 2012. New fossils from Koobi Fora in northern Kenya confirm taxonomic diversity in early Homo. Nature 488, 201–204. ( 10.1038/nature11322) [DOI] [PubMed] [Google Scholar]
- 77.Antón SC. 2003. Natural history of Homo erectus. Yearb. Phys. Anthropol. 46, 126–170. ( 10.1002/ajpa.10399) [DOI] [PubMed] [Google Scholar]
- 78.Toth N, Schick K. 2009. The Oldowan: the tool making of early hominins and chimpanzees compared. Annu. Rev. Anthropol. 38, 289–305. ( 10.1146/annurev-anthro-091908-164521) [DOI] [Google Scholar]
- 79.Domínguez-Rodrigo M, et al. 2014. On meat eating and human evolution: a taphonomic analysis of BK4b (Upper Bed II, Olduvai Gorge, Tanzania), and its bearing on hominin megafaunal consumption. Quat. Int. 322–323, 129–152. ( 10.1016/j.quaint.2013.08.015) [DOI] [Google Scholar]
- 80.Rightmire GP, Lordkipanidze G. 2010. Fossil skulls from Dmanisi: a paleodeme representing earliest Homo in Eurasia. In Out of Africa 1: the first hominin colonisation of Eurasia (eds Fleagle JG, Baden AL, Grine FE, Shea JJ, Leakey RE), pp. 225–244. Berlin, Germany: Springer. [Google Scholar]
- 81.Mirazón Lahr M. 2010. Saharan corridors and their role in the evolutionary geography of ‘Out of Africa I’. In Out of Africa 1: the first hominin colonisation of Eurasia (eds Fleagle JG, Baden AL, Grine FE, Shea JJ, Leakey RE), pp. 27–46. Berlin, Germany: Springer. [Google Scholar]
- 82.Stringer CB, Buck LT. 2014. Diagnosing Homo sapiens in the fossil record. Ann. Hum. Biol. 41, 312–322. ( 10.3109/03014460.2014.922616) [DOI] [PubMed] [Google Scholar]
- 83.Foley RA, Mirazón-Lahr M. 2011. The evolution of the diversity of cultures. Phil. Trans. R. Soc. B 366, 1080–1089. ( 10.1098/rstb.2010.0370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Foley RA, Gamble C. 2009. The ecology of social transitions in human evolution. Phil. Trans. R. Soc. B 364, 3267–3279. ( 10.1098/rstb.2009.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mellars P. 2006. Going east: new genetic and archaeological perspectives on the modern human colonization of Eurasia. Science 313, 796–800. ( 10.1126/science.1128402) [DOI] [PubMed] [Google Scholar]
- 86.Clark JD. 1992. African and Asian perspectives on the origins of modern humans. Phil. Trans. R. Soc. B 337, 148–178. ( 10.1098/rstb.1992.0098) [DOI] [PubMed] [Google Scholar]
- 87.Mellars P, French JC. 2013. Tenfold population increase in Western Europe at the Neandertal–to–modern human transition. Science 333, 623–627. ( 10.1126/science.1206930) [DOI] [PubMed] [Google Scholar]
- 88.Powell A, Shennan S, Thomas MG. 2009. Late Pleistocene demography and the appearance of modern human behavior. Science 324, 1298–1301. ( 10.1126/science.1170165) [DOI] [PubMed] [Google Scholar]
- 89.Henshilwood CS. 2002. Emergence of modern human behavior: Middle Stone Age engravings from South Africa. Science 295, 1278–1280. ( 10.1126/science.1067575) [DOI] [PubMed] [Google Scholar]
- 90.Lyons KS, et al. 2015. Holocene shifts in the assembly of plant and animal communities implicate human impacts. Nature 529, 80–83. ( 10.1038/nature16447) [DOI] [PubMed] [Google Scholar]
- 91.Foley RA. 1995. Causes and consequences in human evolution. J. R. Anthropol. Inst. 1, 67–86. ( 10.2307/3034229) [DOI] [Google Scholar]
- 92.McHenry HM. 1975. Fossils and the mosaic nature of human evolution. Science 190, 425–431. ( 10.1126/science.809842) [DOI] [PubMed] [Google Scholar]
- 93.Hublin JJ. 2009. The origin of Neandertals. Proc. Natl Acad. Sci. USA 106, 16 022–16 027. ( 10.1073/pnas.0904119106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mirazón Lahr M. 2016. The shaping of human diversity: filters, boundaries and transitions. Phil. Trans. R. Soc. B 371, 20150241 ( 10.1098/rstb.2015.0241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bateson PPG. 1988. The active role of behaviour in evolution. In Evolutionary processes and metaphors (eds Ho MW, Fox SW), pp. 191–207. New York, NY: John Wiley. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.