Abstract
Body size is a fundamental biological property of organisms, and documenting body size variation in hominin evolution is an important goal of palaeoanthropology. Estimating body mass appears deceptively simple but is laden with theoretical and pragmatic assumptions about best predictors and the most appropriate reference samples. Modern human training samples with known masses are arguably the ‘best’ for estimating size in early bipedal hominins such as the australopiths and all members of the genus Homo, but it is not clear if they are the most appropriate priors for reconstructing the size of the earliest putative hominins such as Orrorin and Ardipithecus. The trajectory of body size evolution in the early part of the human career is reviewed here and found to be complex and nonlinear. Australopith body size varies enormously across both space and time. The pre-erectus early Homo fossil record from Africa is poor and dominated by relatively small-bodied individuals, implying that the emergence of the genus Homo is probably not linked to an increase in body size or unprecedented increases in size variation. Body size differences alone cannot explain the observed variation in hominin body shape, especially when examined in the context of small fossil hominins and pygmy modern humans.
This article is part of the themed issue ‘Major transitions in human evolution’.
Keywords: body size, body shape, fossil hominins, pygmies
1. Introduction
Dobzhanksy's [1] famous aphorism about the centrality of evolution in biology was recently reworded as ‘nothing in biology makes sense except in the light of [body mass]’ [2, p. 1]. This whimsical paraphrasing underscores the common belief that body size is a fundamental property of an organism that impacts almost all aspects of its biology, including behaviour, life history, ecology and anatomy. Evolutionary morphologists also require reliable measures of body size for standardization before meaningful comparisons of shape and inferences about function are possible [3,4]. Body size is a feature that is frequently estimated by palaeontologists trying to contextualize their fossils and compare them with living species [5,6]. Human palaeontologists are no exception, and estimating the body size of fossils hominins has long been something of a cottage industry in palaeoanthropology (see [7,8] for recent reviews of the literature).
Estimating body size for a fossil seems like a straightforward and tractable enterprise, but is laden, in practice, with theoretical and pragmatic assumptions about best predictors and the most appropriate reference samples [9–11]. It is indeed ‘deceptively simple’ [12, p. 284]. Two influential early attempts to summarize body size evolution in fossil hominins are the landmark papers by McHenry [13] and Ruff et al. [14], although the latter was concerned primarily with changes within the genus Homo. The hominin fossil record has improved dramatically in some ways since these seminal papers were published [15], and two recent, broad-scale analyses have attempted to update and expand upon them using very different methods and databases [7,8]. We focus here primarily on the new data generated in Grabowski et al. [7], consolidating and reorganizing the major results, and we explore their implications for the evolution of body ‘shape’ with special reference to small-bodied fossil hominins and modern human pygmies.
The analysis of Grabowski et al. [7] differs substantially from that of Will & Stock [8] in scope, methods, reference samples (known as ‘training samples’ in regression and calibration) and overall results. For example, using a training sample of individuals of known mass permits one to calculate true prediction intervals on estimates that are arguably more appropriate than confidence intervals from equations based on group means or sex-specific means. Whereas Grabowski et al. [7] used a training sample of modern humans of known body mass, including many small-bodied individuals with skeletons comparable in size to those of the fossil hominin sample, and relied primarily on multivariate estimates explicitly constrained by shared similarities in scaling, Will & Stock [8] relied exclusively on mass estimates from univariate regressions using femoral head diameters (FHDs) as the predictor variable. Many of their FHDs were themselves estimated from other elements. They acknowledge that this indirect procedure introduces another level of cumulative uncertainty. The impact of these different analytical strategies is discussed below.
2. Synopsis of body size evolution in the human career
We are especially interested in examining the body size of early Homo and Homo erectus, because there is a widely held belief that the emergence of the genus Homo and the successful dispersion of H. erectus out of Africa are linked causally to increased body size from some smaller, ancestral australopith [16–24]. Larger body size in Homo is believed to have ‘important energetic, locomotor and survival consequences’ [18, p. S272] and could involve changes in home range, foraging strategies, energy budgets and encephalization [20]. These are plausible links, but it is less clear how a relatively modest increase in overall body size in a striding bipedal hominin favours dispersion and colonization outside of Africa. Regardless, reliable body mass estimates for African early Homo and H. erectus sensu lato are needed in order to validate this evolutionary scenario, and data on australopith antecedents are also very relevant. Body size evolution in later stages of the human career is beyond the scope of our study here, and we refer the reader to other recent sources for body size predictions of Middle Pleistocene hominins [25,26].
Table 1 summarizes new estimates of body size from fossils developed by Grabowski et al. [7], excluding numerous specimens that we judge to be of uncertain taxonomic affinities or subadult status. This trims the sample size of new (mostly multivariate) estimates from 90 specimens to 68, but we have added the body mass estimates for the newly described Homo naledi (n = 8, forensic estimates in table 3 of reference [27]). These results are presented graphically in figure 1. Our results for the two earliest species, Orrorin tugenensis and Ardipithecus ramidus, are smaller than most published estimates at 36.3 and 32.1 kg, respectively (compare with Nakatsukasa et al. [28] and White et al. [29]), because we are modelling them here as bipeds with a modern human training sample as the informative prior. Using a chimpanzee (quadrupedal) reference sample would increase our size estimates considerably, especially for Ar. ramidus [4]. If the human-based, lower estimates are accurate, this suggests that the earliest members of our clade were small-bodied, at the lower end of the range of modern human pygmies from Africa and Asia (see electronic supplementary material, table S1; also see [30–32]). Regardless of whether a modern human or chimpanzee reference sample is more accurate, it seems safe to rule out ‘giants’ among the earliest hominins [33–35]. We believe that Ororrin and Ardipithecus are basal hominins with at least an incipient bipedal adaptation, but because these earliest putative hominins are so ancient, their body mass estimates are less relevant to the central question being addressed in this study, the transition from australopiths to Homo.
Table 1.
Body mass estimates for fossil hominins.
| taxa (composition of sample) | n | mass mean (kg) | mass range (kg) |
|---|---|---|---|
| Orrorin tugenensis (BAR1002′00, BAR1003′00) | 2 | 36.3 | 30–42.5 |
| Ardipithecus ramidus (ARA-VP-6/500) | 1 | 32.1 | |
| Australopithecus anamensis (KNM-KP29285) | 1 | 46.3 | |
| Australopithecus afarensis (A.L.152-2, A.L.211-1, A.L.288-1, A.L.330-6, A.L.333-131, A.L.33-142, A.L.333-95, A.L.333w-40, A.L.333w-43, A.L.333x-26, A.L.827-1, KSD-VP-1/1) | 13 | 41.0 | 24.5–63.6 |
| Australopithecus africanus (MLD17, MLD25, MLD46, Sts14, Stw121, Stw25, Stw300, Stw31, Stw34, Stw361, Stw392, Stw403, Stw431, Stw443, Stw479, Stw501, Stw522, Stw527, Stw 598) | 19 | 30.7 | 22.8–43.3 |
| Australopithecus sediba (MH2, MH4) | 2 | 25.9 | 22.7–29.1 |
| African Homo sp.a (KNM-ER1472, KNM-ER1481, KNM-ER5881) | 3 | 40.6 | 35.5–45.4 |
| Homo habilis (OH62, OH35, KNM-ER3735) | 3 | 33.7 | 27.3–38.4 |
| Homo erectus (Dmanisi) (D4167/3901) | 1 | 40.7 | |
| H. erectus (Africa) (BSN49-P27, KNM-ER1808, KNM-ER3228, OH28 KNM-WT15000) | 5 | 48.9 | 29.4–64.4b |
| H. erectus (Asia) (ZhoukoudianI, ZhoukoudianIV, ZhoukoudianVI, Trinil-I, Trinil-II, Trinil-III, Trinil-IV) | 7 | 51.9 | 49.3–54.8 |
| Homo floresiensis (LB1) | 1 | 27.5 | |
| Homo naledi (U.W.101-002, U.W.101-003, U.W.101-018, U.W.101-226, U.W.101-1136, U.W.101-1391, U.W.101-1475, U.W.101-1482) | 8 | 44.0 | 39.7–54.5c |
| Paranthropus boisei (OH80-12) | 1 | 46.4 | |
| Paranthropus robustus (SK14024, SK3121, SK3155B, SK50, SK82, SK97, SKW19, SWT1/LB2, TM1605) | 9 | 31.7 | 24.0–42.6 |
Table 3.
Humerus length, femur length, humerofemoral index and femoral head diameter (FHD) in human pygmies and selected fossil hominins. All FHDs for the fossils are provided in the supplementary information in Grabowski et al. [7]. Long bone lengths for the fossils are from [42,62–64] or from the authors’ direct measurements (A.L.288-1, LB1).
| group/specimen | humerus length (mm) | femur length (mm) | humerofemoral Index (100 × H/F) | femoral head diameter (mm) |
|---|---|---|---|---|
| African pygmies | ||||
| mean | 276.8 | 376.9 | 73.5 | 37.8 |
| s.d. | 14.9 | 20.4 | 1.8 | 3.1 |
| n | 23 | 23 | 23 | 23 |
| Andaman Islanders | ||||
| mean | 268.7 | 385.7 | 69.7 | 37.8 |
| s.d. | 15.7 | 18.6 | 2.6 | 2.6 |
| n | 32 | 32 | 32 | 32 |
| Filipino negritos | ||||
| mean | 265.2 | 378.4 | 70.1 | 37.1 |
| s.d. | 13.6 | 19.7 | 1.6 | 2.0 |
| n | 14 | 14 | 14 | 14 |
| Khoesan | ||||
| mean | 287.0 | 408.6 | 70.4 | 38.9 |
| s.d. | 22.2 | 27.4 | 2.3 | 2.9 |
| n | 52 | 70 | 52 | 70 |
| ARA-VP-6/500 | ∼278 | ∼312 | ∼89 | 31.7–37.1 |
| A.L.288-1 | 239 | 280 | 85.4 | 28.5 |
| A.L.827-1 | 368 | 38.0 | ||
| D4507/4167 | 295 | 386 | 76.4 | 40.2 |
| KNM-ER1481 | 396 | 43.0 | ||
| KNM-ER1472 | 401 | 40.2 | ||
| LB1 | 243 | 280 | 86.8 | 30.0 |
Figure 1.
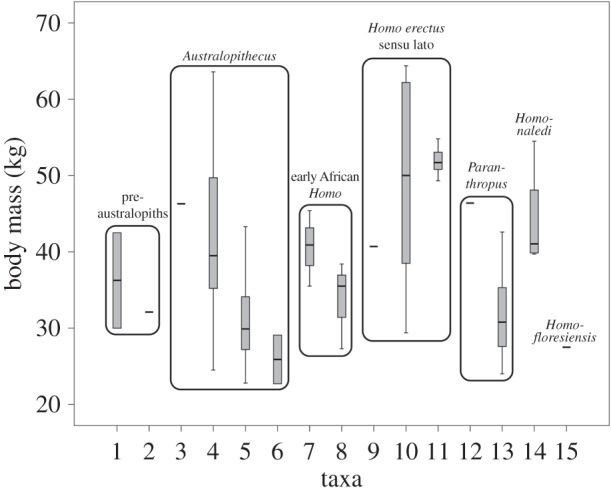
Body mass estimates for fossil hominins presented as boxplots summarizing the data in table 1. The numbers along the horizontal axis refer to taxa: (1) Orrorin tugenensis, (2) Ardipithecus ramidus, (3) Australopithecus anamensis, (4) Au. afarensis, (5), Au. africanus, (6) Au. sediba, (7) early African Homo sp., (8) Homo habilis, (9) Dmanisi H. erectus, (10) African H. erectus, (11) Asian H. erectus, (12) Paranthropus boisei, (13) P. robustus, (14) H. naledi, and (15) H. floresiensis.
The single estimate of body mass in Australopithecus anamensis (46.3 kg) is substantially larger, but still well within the range of small-bodied modern humans. Its descendent Au. afarensis is well represented in the fossil record and in our sample. The average estimated mass of Au. afarensis (41.0 kg) is slightly smaller, but the observed range is very large (24.5–63.6 kg) and includes some of the biggest estimates in the entire fossil sample. Their South African congeners, Au. africanus (mean is 30.7 kg, range 22.8–43.3 kg) and Au. sediba (mean is 25.9 kg, range 22.7–29.1 kg), are very small on average, again at the low end of adult body mass in living human pygmies. Our Au. africanus mean value is similar to the 33 kg estimate offered by McHenry & Berger [36] based on hindlimb dimensions; our Au. sediba estimate of 25.9 kg is substantially smaller than originally proposed [37]. If the East African australopiths are ancestral to the earliest Homo, then body size either remained the same or declined slightly; if Homo is derived instead from South African forms (which we regard as possible but less likely), then body size may have increased slightly with the emergence of our genus (see below). We also note that relative variation as captured by the coefficient of variation (CV) also distinguishes Au. afarensis from Au. africanus; the value of 28.8 in the former is significantly greater that the CV of 16.1 seen in the latter (Fligner–Killeen test, p < 0.03).
We placed three specimens within Homo habilis (OH35 is added here to OH62 and KNM-ER 3735 from Grabowski et al. [7]), and the average is again very small at 33.7 kg (range 27.3–38.4 kg). Eliminating OH35 reduces the average by less than 1 kg. There are other early Homo specimens that might be part of the H. habilis hypodigm (e.g. the femora KMM-ER 1472 and KNM-ER 1481), but they could also pertain to either H. rudolfensis or even H. erectus [16,38]. Indeed, the shapes of the proximal ends of KNM-ER 1472 and KNM-ER 1481 resemble KNM-WT 15000 [39,40], which is securely attributed to H. erectus s.l. We have placed these two femora and KNM-ER 5881 into early African Homo sp., which brings the mean mass of that group to 40.6 kg (range 35.5–45.4), slightly less than that estimated for Au. afarensis. If we combine these three specimens with the three for H. habilis, then the mean estimate of early African Homo drops slightly to 37.2 kg; if we transfer KNM-ER 3228 to this pooled sample from African H. erectus, then the average increases slightly to 38.8 kg. We have no reliable estimates for H. rudolfensis, because there are no definitively associated postcrania for this species, but we doubt that its body size would have been much larger than that of other early Homo based on the size of the craniodental remains attributed to the species. In sum, Australopithecus species and early African Homo are all relatively small-bodied on average, but Au. afarensis does include some large-bodied, presumptive males. Paranthropus robustus is also remarkably small at 31.7 kg (n = 9; range 24.0–42.6 kg), consistent with McHenry's characterization of them as having ‘petite bodies’ [41]. The single specimen that we accept as P. boisei (OH80-12) is larger than its South African congeners and similar to Au. anamensis at 46.4 kg. However, there are eight other ‘possible P. boisei’ specimens considered in Grabowski et al. [8], and their mean estimated mass is only 33 kg. It would therefore be premature to infer that P. boisei was significantly larger in body size than its South African congener.
Pooling the relevant fossils from Dmanisi, Africa and Asia (Indonesia and China) into H. erectus sensu lato, the average of 13 specimens is 49.9 kg (range 29.4–64.4 kg), a value at the high end of body masses for human pygmies (electronic supplementary material, table S1). Although roughly 9 kg higher than the average for Au. afarensis, the difference between these two samples does not quite reach statistical significance (Mann–Whitney U, p = 0.06). With CV at 28.4 for Au. afarensis and 18.7 for H. erectus s.l., variability in the two groups is also not significantly different (Fligner–Killeen test, p > 0.3); if variability is a valid surrogate for adaptability [21], there appears to be no adaptive advantage within the pooled H. erectus group or within African H. erectus. In contrast, the pooled H. erectus sample is significantly larger than the pooled early African Homo (plus H. habilis) sample (p < 0.01). If one breaks down the H. erectus sample geographically, then the picture becomes much more complicated [8], and small sample sizes preclude meaningful statistical tests. The single adult specimen from Dmanisi that we consider here is relatively small at 40.7 kg, and it is one of the earliest securely dated individuals in the pooled H. erectus sample; this value is at the low end of the original estimates of body mass for the Dmanisi specimens [42] and is smaller than our averages for both African and Asian H. erectus. The African H. erectus sample is small and complicated both by the inclusion of KNM-ER 3228 (the earliest specimen in our H. erectus s.l. sample) and by the use of a projected adult body mass for the juvenile KNM-WT 15000. The KNM-ER 3228 pelvis may [43] or may not [16] belong to H. erectus, and the same taxonomic ambiguity is attached to KNM-ER 1481 and KNM-ER 1472 as noted above. We have also included the Gona pelvis in our African H. erectus sample [44,45], but this attribution has also been questioned [46]. Three other possible African H. erectus specimens (KNM-ER 736, KNM-ER 737, KNM-ER 803A) discussed in Grabowski et al. [7] have uncertain species attribution, and these are all relatively large (65.5, 64.1, 54.8 kg, respectively). Accordingly, our African H. erectus sample mean of 48.9 kg based on five individuals may be too conservative, but it is noteworthy that all except KNM-ER 3228 postdate Dmanisi [47]. The seven specimens from Indonesia and China are much less variable and slightly larger (mean of 51.9 kg, range 49.3–54.8) than our limited African H. erectus sample, but they also come from a much later time and do not bear directly on the question of whether or not increased body size (and correlated changes in anatomy) facilitated dispersal out of Africa.
Inspection of figure 1 and table 1 reveals that a surprisingly large percentage of early fossil hominins are small-bodied, and most fit within the known ranges of variation in modern human pygmies from Africa and Asia (table 2 and electronic supplementary material, table S1). Populations with average adult male statures of less than 1.5 m are considered ‘pygmy’; this is not a pejorative term [31]. It is also apparent that body size changes over time have been nonlinear in the human career, and some of the largest body masses appear early in Au. afarensis (and perhaps as early as Ar. ramidus [4,29]). The earliest African Homo sample—no matter how constituted—is small-bodied, thereby implying that the emergence of our genus is not driven by or correlated with larger body size, one of the major take-home messages from [7] that undermines the aforementioned consensus. This conclusion would obtain even if Homo habilis sensu stricto is really an australopith and not ancestral to H. erectus [15,49]. The earliest member of H. erectus outside of Africa (Dmanisi) may have also been small-bodied, and the increase in body mass in African H. erectus probably occurred later in time and is complicated by possible regional differences within the continent [8].
Table 2.
Body mass, stature, body mass index and Ponderal index in human pygmies and selected fossil hominins. BMI = mass/stature2. Ponderal Index = mass1/3/stature.
| group/specimen | mass (kg) | stature (m) | BMI | Ponderal index |
|---|---|---|---|---|
| African and Asian pygmies (sexes pooled, n = 345) | ||||
| mean | 39.6 | 1.43 | 19.24 | 2.37 |
| s.d. | 5.5 | 0.07 | 2.16 | 0.10 |
| LB1 | 27.5 | 1.05 | 24.9 | 2.87 |
| A.L.288-1 | 26.0 | 1.05 | 23.1 | 2.82 |
| A.L.827-1 | 38.2 | 1.38 | 20.2 | 2.44 |
| D4167/3901 | 40.7 | 1.39 | 20.9 | 2.46 |
| KNM-WT15000a | 64.4 | 1.78 | 20.3 | 2.25 |
| KNM-ER1481 | 40.6 | 1.47 | 18.9 | 2.34 |
| KNM-ER1472 | 45.4 | 1.49 | 20.6 | 24.0 |
| Homo naledib | 44.0 | 1.46 | 20.6 | 2.41 |
Although currently undated, the recently diagnosed hominin species from South Africa, H. naledi [27], is also relatively small-bodied with an estimated mean mass of 44 kg (range 39.7–54.5). If this taxon turns out to be a basal member of Homo or another small-bodied member of H. erectus s.l., this would be consistent with our conclusion that earliest Homo was not especially large-bodied. If H. naledi turns out to be Late Pleistocene in age, then it could imply the existence of another late surviving species of our genus not unlike Homo floresiensis in some respects [50,51] from the Late Pleistocene of Indonesia. Our new estimate for the type specimen of H. floresiensis (LB1) is 27.5 kg, a few kg smaller than reported elsewhere [52]. These results indicate that both H. naledi and H. floresiensis were characterized by body masses within the observed range for modern human pygmies.
We noted major differences in procedures used to estimate body mass between Grabowski et al. [7] and Will & Stock [8]. However, several similar conclusions are drawn from both analyses that should be emphasized. Will & Stock [8, p. 1] conclude that their ‘results demonstrate chronological and spatial variation, but no simple temporal or geographical trends for the evolution of body size among early Homo’. They also note that body size increases within African H. erectus occur after hominins were already in place at Dmanisi, ‘suggesting that migrations into Eurasia were not contingent on larger body sizes’ [8, p. 1]. We concur but note that the body mass estimates in the two studies are very different, with ours being much smaller for all specimens that are considered in both. The average difference in 15 specimens analysed in common is +14.6 kg, with a range of differences from +0.6 to +40.5 kg (electronic supplementary material, table S1; KNM-ER 1808 is the extreme outlier). However, if we eliminate the projected adult size values for the juvenile KNM-WT 15000 and the hugely discordant estimates for KNM-ER 1808, and then apply our [7] univariate regression of mass on femoral head size to their FHD database, the differences in estimates among the remaining 13 specimens diminish greatly to an average of +3.3 kg. Choice of training sample obviously has a huge impact on regression formulae and body mass estimates (also see [53,54]).
As we have demonstrated and discussed elsewhere [7], inverse calibration (ordinary least-squares regression) biases estimates towards the mean body mass of a given training sample. The raw skeletal dimensions of many early fossil hominins are small in comparison with most modern humans, and it was for this reason that we assembled a modern human reference sample of known body masses that was intentionally smaller bodied than most that have been employed before. Its mean mass is roughly 10 kg lower that a worldwide population sample from Ruff et al. [14]. Will and Stock's results are almost certainly biased upwardly relative to ours by use of larger bodied training samples and regressions from [46].
3. Size and ‘shape’ in fossil hominins
In view of the surprisingly consistent finding that many fossil hominins are small-bodied and fall within the range of human pygmy body masses, we can compare how much mass they packed onto their skeletal frame to living pygmies if we also have information on their statures. Stature reconstruction is no less complicated that body mass estimation [9,11]. We rely here on stature estimates derived from calibrations using femoral lengths in human pygmies [9,55,56]; this small-bodied reference sample is preferred here for the same reasons discussed with respect to our preferred body mass regressions. We limit our comparisons with eight fossils: A.L.288-1, LB1, A.L.827-1, D4167/3901, KNM-ER 15000, KNM-ER 1481, KNM-ER 1472 and H. naledi (table 2). We apply inverse calibration (ordinary least-squares regression) to estimate stature in A.L.827-1, KNM-ER 1481 and KNM-ER 1472 using the formula:
Because the Dmanisi specimen also preserves an associated tibia, we predict stature from femur + tibia using the formula [56]
Because extreme extrapolation is required to estimate stature in A.L.288-1 and LB1, we use the midpoint of estimates between inverse and classic calibration for these two very short hominins [9,56]. We combine our estimate of mass in a projected adult version of KNM-WT 15000 with the recent ‘adult’ stature estimate provided in [8,48]. We use the approximate midpoint for stature estimates of H. naledi presented elsewhere [27].
A crude measure of body shape can be created by combining stature and body mass into two very different indices [52], the clinically familiar body mass index (BMI, kg m−2) and the ‘dimensionless’ ponderal index (kg1/3 m−1). Figure 2 plots mass versus stature (a) and BMI versus the Ponderal index (b). The large human pygmy database is provided in electronic supplementary, table S1. All but three fossils fit comfortably within the human pygmy scatters: LB1, A.L.288-1 and KNM-WT 15000. The others, a presumed male australopith (A.L.827-1), an early H. erectus (D4167/3901), two early African Homo (KNM-ER 1472 and KNM-ER 1481) and H. naledi would appear to be similar to modern human pygmies in absolute and relative terms. LB1 and A.L.288-1 plot at the lower end of the pygmies in body mass but far below them in stature; this results in unusually high BMIs and out-of-range ponderal indices. These two small hominins, which are separated by 3 million years and a huge distance, are both packing more mass onto their small skeletal frames than would be predicted by stature alone. They are decidedly short and stocky, and share body shapes that depart from small-bodied modern humans. KNM-WT 15000 is an outlier both absolutely and relatively compared with human pygmies and the other fossils. It is possible that the stature estimate is too large [57,58] or that our body mass estimate is too small (or both), but this individual appears rather different from its counterpart at Dmanisi—but it also occurs considerably later in time. As an important footnote, stature scales almost isometrically with mass within our large sample of human pygmies (log stature on log mass has a reduced major axis slope of 0.3), and this indicates that the ponderal index may be the preferred way to express mass per unit stature rather than the BMI in small-bodied hominins and pygmy humans.
Figure 2.
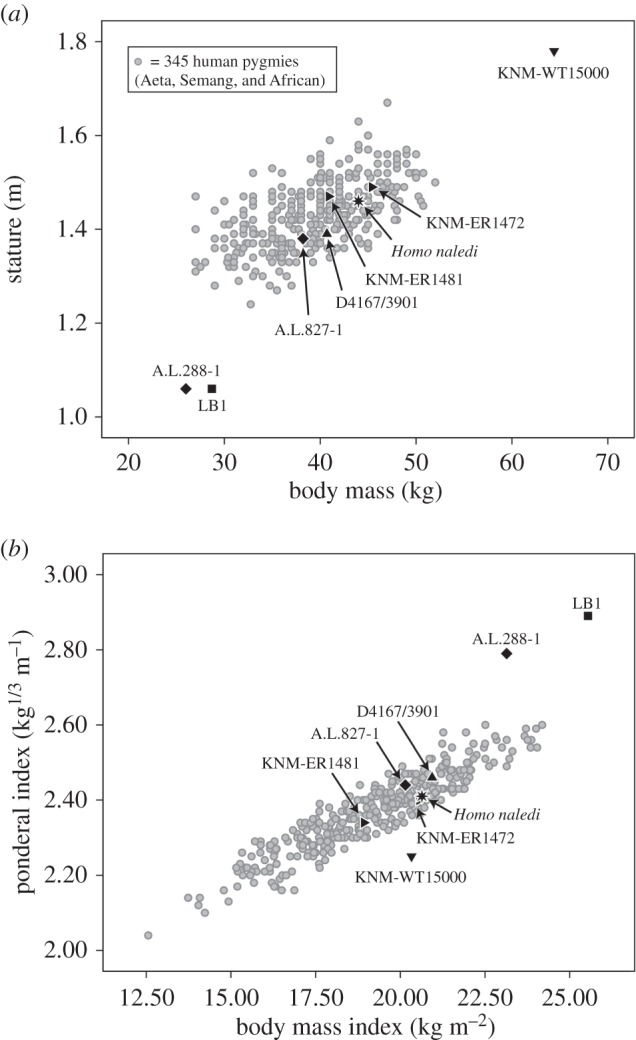
(a) Bivariate scatter of body mass versus stature in a large sample of African and Asian pygmies, with select hominin fossils superimposed. (b) Bivariate scatter of the BMI versus the Ponderal index in human pygmies, with fossil hominins superimposed.
4. Size and limb proportions
Another aspect of body shape that can be evaluated in several ways, including the impact, if any, of body size differences is ‘limb proportions’ [13,20,22,59–61]. Table 3 summarizes our data on interlimb proportions in human pygmies and four small-bodied fossil hominins from Africa (ARA-VP-6/500, A.L.288-1), Dmanisi (D4507/4167) and Indonesia (LB1). Until relatively late in the hominin fossil record, there are remarkably few fossils that preserve both the humerus and femur sufficiently complete, so that lengths can be measured without relying on extreme reconstruction [59,65]. The later fossil record of large-bodied hominins is much more generous in this respect [26,66]. Figure 3a presents boxplots of the humerofemoral index (100× humerus length/femur length) in four groups of modern human pygmies and three of the fossils (A.L.288-1, LB1 and D4509/4167). The adult from Dmanisi exhibits an index of 76.4, at the limit or outside the observed range of the different pygmy groups; its index is unusually high, as is that of the only African H. erectus that preserves both bones (the juvenile KNM-WT 15000 [61]). However, both ‘Lucy’ (A.L.288-1) and LB1 have humerofemoral indices that are so high that they are never observed in modern humans of any body size, including the smallest people on Earth. Based on estimates of humerus length and femur length for Ar. ramidus [62], an approximate estimate of the humerofemoral index in this basal hominin is 89, an outlier even higher than those seen in Au. afarensis and H. floresiensis. Such limb proportions facilitate climbing, even in a bipedal hominin [67]. If we use FHD as a direct size variable rather than to estimate body mass [68], then we can also plot the index directly against ‘size’ (figure 3b). The correlation between the index and FHD is not significant (r = 0.09, p > 0.3); this result would be obtained if we plotted the index versus mass estimated from FHD, because that would simply multiply FHD by a constant. Smaller humans do not have especially high indices; e.g. the larger bodied, cold-adapted Sami (‘Lapps’) have a higher average index than any of the pygmies [61]. The Dmanisi specimen plots close to values seen in pygmies for its FHD, but again lies in the upper part of the distribution. A.L.288-1 and LB1 both plot in the upper left quadrant in an area unoccupied by living humans. In other words, based on what is observed in small-bodied humans, one would not predict the high indices seen in either LB1 or A.L.288-1 based on FHD. The estimates for FHD in ARA-VP-6/500 are broad, ranging from 31.7 to 37.1 mm [7], but at any point in this range, the associated index is far above the human cluster.
Figure 3.
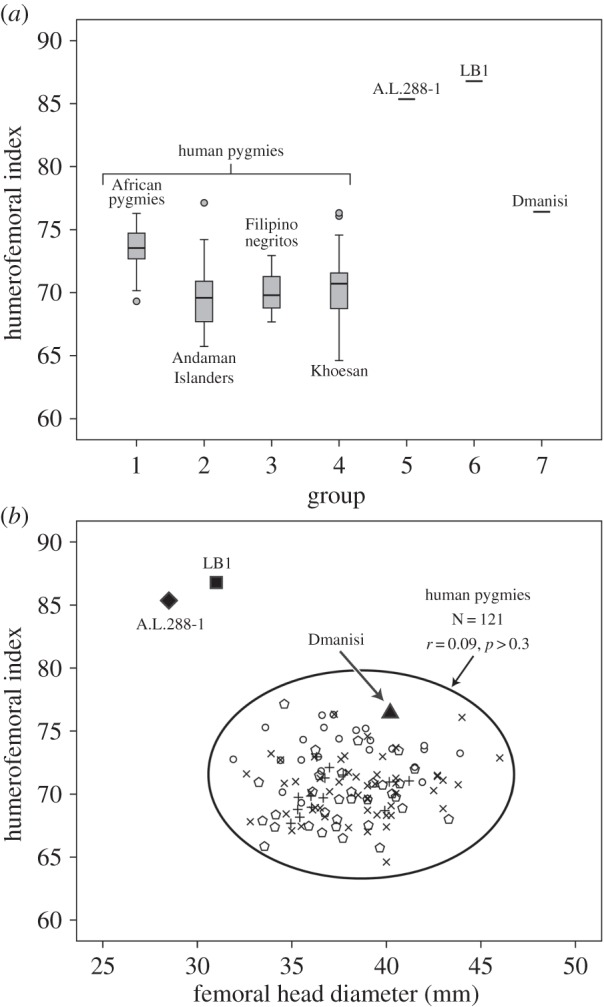
(a) Boxplots of the humerofemoral index in four groups of human pygmies, with three individual fossil hominins indicated to the right. (b) A scatterplot of humerofemoral index versus femoral head diameter in the pooled sample of human pygmies and the three fossil hominins. The circles are African pygmies, the pentagons are Andaman Islanders, the crosses are KhoeSan and the pluses are Flipino negritos.
If we dissect the humerofemoral index via regression analysis, then these inferences are clarified and strengthened (figure 4; data summary in table 3). We can also add femur length to the analysis for three essentially complete adult femora of early hominins. Because we are predicting bone length from FHD and because little to no extrapolation is required, inverse calibration is the preferred method [69]. The regression of humerus length on FHD in our small-bodied human sample predicts well the humerus length in LB1, A.L.288-1 and D4509; all three humeri are close to the regression line and well within the 95% prediction intervals. Owing to uncertainties in the FHD of ARA-VP-5/600, this fossil is not included in the plot, but its humerus would fall either just above or within the upper limits of the prediction intervals along the range of possible FHDs. FHD also predicts femur length reasonably well in D4509, A.L.827-1, KNM-ER 1481 and KNM-ER 1472 in the sense that all four fall within the 95% prediction interval for the human sample; however, all four plot below the regression and are therefore slightly but consistently shorter than predicted. The femur of ARA-VP-5/600 falls below the regression line at all estimates of FHD and below the lower prediction limit for most of the range of its estimated FHD. We suspect that a short estimated femur length is driving the very high index in ARA-VP-5/600, more so than a long estimated humerus length. The femora of LB1 and A.L.288-1 both fall far below and outside the prediction interval for their FHDs; in fact, LB1's femur length is better predicted by the humeral regression than the femoral one. In other words, the femora of these two very small-bodied hominins are especially short, and this accounts for their extremely high humerofemoral indices that approach that of Ardipithecus (contra [70,71]; also see [72]). They are not little humans in their limb proportions. It is also noteworthy that a putative male Au. afarensis femur (A.L.827-1) plots within the modern human scatter, but its conspecific female (A.L.288-1) does not; this could imply an unusual degree of shape dimorphism and possibly reflects sex-related differences in body size, body shape and locomotor function. If Homo floresiensis were a scaled-down H. erectus, which we regard as unlikely [51,73], then LB1's limb lengths and interlimb proportions would imply a homoplastic convergence (i.e. an evolutionary reversal) onto A.L.288-1 that might be driven by the biomechanics of climbing. We also submit that testing predictions about scaling effects in small hominins—which includes the majority of fossils in our study—requires direct comparisons with small-bodied modern humans. LB1 and A.L.288-1 are not only very small; they defy predictions about body shape and limb proportions derived from human pygmies.
Figure 4.
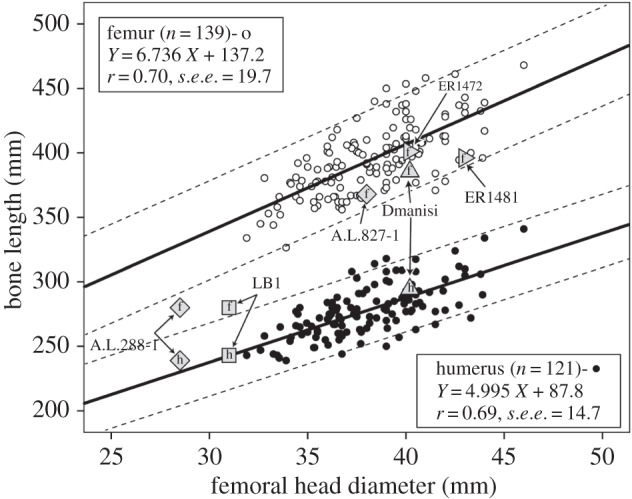
Humerus length and femur length regressed on femoral head diameter in a combined sample of human pygmies. 95% prediction intervals are indicated by dashes lines. Fossil hominins are superimposed, with ‘h’ indicating humerus and ‘f’ indicating femur.
5. Why were so many early hominins small-bodied?
If early hominins were the size of modern human pygmies, as we document here, what does this imply about the origins and biological significance of their small body size? Pfeiffer [74, p. S383] has noted that ‘small body size among fossil forms is difficult to explain because its existence in modern populations is not fully understood’. The competing explanations or adaptive foundations for size reduction (‘dwarfing’) in modern pygmies include thermoregulation, nutrition/diet, locomotion in closed habitats and life-history trade-offs related to early sexual maturation in high mortality environments [31,75]. Each of these explanations has shortcomings or limitations [76], but it seems clear now that the pygmy phenotype has evolved convergently in Africa, Asia and Melanesia [75,77]. Regardless of the adaptive (or not) basis of body size reduction in human pygmies, these dwarfing explanations may not apply to early hominins if small body size is the primitive condition [75]. If there were a step-wise increase in body size from australopiths to early Homo to H. erectus, then the life-history model might imply reduced mortality rates and delayed maturation over time [78]. Unfortunately, our results on the trajectory of body size evolution do not support this scenario, and the pace of development remains quite accelerated in H. erectus [79]. We are currently investigating the evolution of body size in non-human primates and hominins using comparative phylogenetic methods to determine whether or not the earliest hominins retain the body size of the last common ancestor of chimpanzees and humans, and we hope these results will eventually inform us about ‘why’ so many of the early hominins are small-bodied.
6. Conclusion
Because body size influences so many aspects of organismal biology, the quest for plausible body mass estimates in the fossil record of human evolution is an important enterprise—but we note that ‘to influence’ is not the same thing as ‘to determine’. We have summarized results based on a training sample of modern humans of known mass, including small individuals that appear better matched in body size to many fossil hominins. Our methods are primarily multivariate and are constrained by the choice of variables that scale in a manner like the reference sample itself. We submit that this approach has major advantages over other published studies. Like most analyses, we have not only relied upon our ‘best’ estimates, but also acknowledge that all estimates carry with them broad prediction intervals that complicate generalizations. We have found that much of the fossil record of the human career is dominated by relatively small-bodied individuals that fit within observed ranges of the smallest modern humans (characterized as ‘pygmies’ from Africa and Asia). Estimates for the earliest fossils (Orrorin and Ardipithecus) are quite small and perhaps less secure, because we have modelled them as human-like bipeds, an assumption open to debate. Australopiths vary enormously, with East African species much larger than their South African congeners. The Australopithecus afarensis sample is among our largest and reveals tremendous variation, and includes some large-bodied individuals that overlap with later H. erectus. Early African Homo, including H. habilis (no matter how constituted) was roughly the same size or possibly smaller than Au. afarensis. H. erectus sensu lato was also variable in size, but not significantly more so that Au. afarensis. Although the lone estimate from Dmanisi is below the averages for both African and Asian H. erectus, this first cosmopolitan hominin species does appear on average to be appreciably larger than is its early Homo ancestors. Sample composition of African H. erectus is also problematic for reasons discussed above, but it appears that later Asian H. erectus may have been larger on average and much less variable. Regardless, it is clear that the evolutionary changes in body size in hominins was nonlinear, and we see little evidence in our expanded dataset to support the common assumption/inference that the emergence of the genus Homo and/or the out-of-Africa dispersal of H. erectus were necessarily linked to an increase in body size (or to increased variation).
It may also be the case that the increase in size from early Homo to African H. erectus was not due to selection on body size itself. Recent findings suggest that this increase may be linked instead to the substantial increase in brain size. Grabowski [80] found that strong selection to increase brain size probably played a major role in both brain and body size increases throughout human evolution and may have been largely or solely responsible for the major increase in both traits that occurred during the transition to H. erectus. This switch has major implications for adaptive hypotheses on the origins of our genus that assume separate selection pressures led to the substantial increase brain and body size.
Even when contextualized within a large sample of modern human pygmies, two fossils—A.L.288-1 and LB1—stand apart in terms of body shape. Their estimated masses are at the low end of values recorded for human pygmies, but their statures are outside (below) any normal human ever documented. This leads to very high BMIs and especially high ponderal indices; they are packing an unusually large amount of mass onto their small frames. Most other small-bodied hominins examined here fit within the scatter of human pygmies in both absolute and relative terms. The same is true of interlimb proportions. While the humerofemoral index of H. erectus (both the Dmanisi adult and the juvenile from Nariokotome) are very high compared with most modern humans, the indices of both H. floresiensis (LB1) and a female Au. afarensis (A.L.288-1) are simply off the charts, and approach the very high value estimated for the much earlier Ardipithecus (ARA-VP-6/500). These marked departures could be symplesiomorphies that were retained to facilitate climbing, but they cannot be explained away as allometric expectations; the body size of the smallest people on Earth does not predict the body shape of these very short hominins. This should serve as a reminder that although body size probably does influence many important aspects of biology in human evolution, it apparently does not determine everything, including body shape.
Supplementary Material
Supplementary Material
Acknowledgements
We offer our sincere thanks to the organizers of the symposium on ‘Major transitions in human evolution’ for the invitation to participate in this celebration of the career of Richard Leakey. We greatly appreciate the numerous curators in museums who provided access to modern human skeletal material and the hominin fossils in their trust. We are grateful for the thoughtful and constructive comments offered by the reviewers of our contribution.
Data accessibility
Most of the data (specimens, metrics, estimates) can be found in the text and supplementary online information in reference [7]. The remaining raw data are provided here as electronic supplementary material (table S1 and S2).
Authors' contributions
All authors contributed significantly to the conception, design, data collection, analyses, interpretations and drafting of this study.
Competing interests
We have no competing interests.
Funding
Funding for this research was provided by NSF DGE-0801634, NSF BCS-1128170, NSF BCS-1028699, NSF BCS-1515054, NSF SMA-1409612, NSF BCS-1316947, The George Washington University's Selective Excellence Programme and the Fulbright US Scholar Programme.
References
- 1.Dobzhansky T. 1973. Nothing in biology makes sense except in the light of evolution. Am. Biol. Teach. 35, 125–129. ( 10.2307/4444260) [DOI] [Google Scholar]
- 2.Brassey CA, Gardiner JD. 2015. An advanced shape-fitting algorithm applied to quadrupedal mammals: improving volumetric mass estimates. R. Soc. open sci. 2, 150302 ( 10.1098/rsos.150302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel S. 1988. Life's devices: the physical world of animals and plants. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Almécija S, Smaers JB, Jungers WL. 2015. The evolution of human and ape hand proportions. Nat. Commun. 6, 7717 ( 10.1038/ncomms8717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damuth J, MacFadden B (eds). 1990. Body size in mammalian paleobiology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.O'Gorman EJ, Hone DWE. 2012. Body size distribution of the dinosaurs. PLoS ONE 7, e51925 ( 10.1371/journal.pone.0051925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabowski M, Hatala KG, Jungers WL, Richmond BG. 2015. Body mass estimates of hominin fossils and the evolution of human body size. J. Hum. Evol. 85, 75–93. ( 10.1016/j.jhevol.2015.05.005) [DOI] [PubMed] [Google Scholar]
- 8.Will M, Stock JT. 2015. Spatial and temporal variation of body size among early Homo. J. Hum. Evol. 82, 15–33. ( 10.1016/j.jhevol.2015.02.009) [DOI] [PubMed] [Google Scholar]
- 9.Konigsberg LW, Hens SM, Jantz LM, Jungers WL. 1998. Stature estimation and calibration: Bayesian and maximum likelihood perspectives in physical anthropology. Am. J. Phys. Anthropol. Suppl. 27, 65–92. () [DOI] [PubMed] [Google Scholar]
- 10.Auerbach BM, Ruff CB. 2004. Human body mass estimation: a comparison of ‘morphometric’ and ‘mechanical’ methods. Am. J. Phys. Anthropol. 125, 331–342. ( 10.1002/ajpa.20032) [DOI] [PubMed] [Google Scholar]
- 11.Uhl NM, Rainwater CW, Konigsberg LW. 2013. Testing for size and allometric differences in fossil hominin bodymass estimation. Am. J. Phys. Anthropol. 151, 215–229. ( 10.1002/ajpa.22269) [DOI] [PubMed] [Google Scholar]
- 12.Smith RJ. 2002. Lead review: estimation of body mass in paleontology. J. Hum. Evol. 43, 271–287. ( 10.1006/jhev.2002.0573) [DOI] [Google Scholar]
- 13.McHenry HM. 1992. Body size and proportions in early hominids. Am. J. Phys. Anthrop. 87, 407–431. ( 10.1002/ajpa.1330870404) [DOI] [PubMed] [Google Scholar]
- 14.Ruff CB, Trinkaus E, Holliday TW. 1997. Body mass and encephalization in Pleistocene Homo. Nature 387, 173–176. ( 10.1038/387173a0) [DOI] [PubMed] [Google Scholar]
- 15.Wood B, Baker J. 2011. Evolution in the genus Homo. Annu. Rev. Ecol. Evol. 42, 47–69. ( 10.1146/annurev-ecolsys-102209-144653) [DOI] [Google Scholar]
- 16.McHenry HM, Coffing K. 2000. Australopithecus to Homo: transformations in body and mind. Annu. Rev. Anthropol. 29, 125–146. ( 10.1146/annurev.anthro.29.1.125) [DOI] [Google Scholar]
- 17.Dunsworth HM. 2010. Origin of the genus Homo. Evol. Educ. Outreach 3, 353–366. ( 10.1007/s12052-010-0247-8) [DOI] [Google Scholar]
- 18.Aiello LC, Antón SC. 2012. Human biology and the origins of Homo. Curr. Anthropol. 53(Suppl 6), S269–S277. ( 10.1086/667693) [DOI] [Google Scholar]
- 19.Antón SC. 2012. Early Homo. Who, when, and where. Curr. Anthropol. 53(Suppl 6), S278–S298. ( 10.1086/667695) [DOI] [Google Scholar]
- 20.Pontzer H. 2012. Ecological energetics in early Homo. Curr. Anthropol. 53(Suppl 6), S346–S358. ( 10.1086/667402) [DOI] [Google Scholar]
- 21.Antón SC, Potts R, Aiello LC. 2014. Evolution of early Homo an integrated biological perspective. Science 345, 1 236 828-1–1 236 828-13. ( 10.1126/science.1236828) [DOI] [PubMed] [Google Scholar]
- 22.Holliday TW. 2012. Body size, body shape, and the circumscription of the genus Homo. Curr. Anthropol. 53, S330–S345. ( 10.1086/667360) [DOI] [Google Scholar]
- 23.Holliday TW. 2015. The significance of changes in body mass in human evolution. BMSAP 23, 101–109. ( 10.1007/s13219-015-0133-6) [DOI] [Google Scholar]
- 24.Maslin MA, Shultz S, Trauth MH. 2015. A synthesis of the theories and concepts of early human evolution. Phil. Trans. R. Soc. B 370, 20140064 ( 10.1098/rstb.2014.0064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trinkaus E, Ruff CB. 2012. Femoral and tibial diaphyseal cross-sectional geometry in Pleistocene Homo. Paleoanthropology 2012, 13–62. [Google Scholar]
- 26.Arsuaga JL, et al. 2015. Postranial morphology of the middle Pleistocene humans from Sima de los Huesos, Spain. Proc. Natl Acad. Sci. USA 112, 11 524–11 529. ( 10.1073/pnas.1514828112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger L, et al. 2015. Homo naledi, a new species of the genus Homo from the Dinaledi Chamber, South Africa. eLife 4, e09560 ( 10.7554/eLife.09560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakatsukasa M, Pickford M, Egi N, Senut B. 2007. Femur length, body mass, and stature estimates of Orrorin tugenensis, a 6 Ma hominid from Kenya. Primates 48, 171–178. ( 10.1007/s10329-007-0040-7) [DOI] [PubMed] [Google Scholar]
- 29.White TD, Asfaw B, Beyene Y, Haile-Selassie Y, Lovejoy CO, Suwa G, WoldeGabriel G. 2009. Ardipithecus ramidus and the paleobiology of early hominids. Science 326, 75–86. [PubMed] [Google Scholar]
- 30.Cavalli-Sforza L (ed.). 1986. African pygmies. Orlando, FL: Academic Press. [Google Scholar]
- 31.Migliano AB, Vinicius L, Mirazon Lahr M. 2007. Life history trade-offs explain the evolution of human pygmies. Proc. Natl Acad. Sci. USA 104, 20 216–20 219. ( 10.1073/pnas.0708024105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry GH, Dominy NJ. 2009. Evolution of the human pygmy phenotype. Trends Ecol. Evol. 24, 218–225. ( 10.1016/j.tree.2008.11.008) [DOI] [PubMed] [Google Scholar]
- 33.Weidenreich F. 1946. Apes, giants and man. Chicago, IL: University of Chicago Press. [Google Scholar]
- 34.Eckhardt RB. 1973. Gigantopithecus as a hominid ancestor. Anthropol. Anz. 34, 1–8. [Google Scholar]
- 35.Frayer DW. 1973. Gigantopithecus and its relationship to Australopithecus. Am. J. Phys. Anthropol. 39, 413–426. ( 10.1002/ajpa.1330390310) [DOI] [PubMed] [Google Scholar]
- 36.McHenry HM, Berger LR. 1998. Body proportions in Australopithecis afarensis and A. africanus and the origin of the genus Homo. J. Hum. Evol. 35, 1–22. ( 10.1006/jhev.1997.0197) [DOI] [PubMed] [Google Scholar]
- 37.Berger LR, de Ruiter DJ, Churchill SE, Schmid P, Carlson KJ, Dirks PHGM, Kibii JM. 2010. Australopithecus sediba: a new species of Homo-like australopith from South Africa. Science 328, 195–204. ( 10.1126/science.1184944) [DOI] [PubMed] [Google Scholar]
- 38.Wood B, Leakey M. 2011. The Omo–Turkana basin fossil hominins and their contribution to our understanding of human evolution. Evol. Anthropol. 20, 264–292. ( 10.1002/evan.20335) [DOI] [PubMed] [Google Scholar]
- 39.Richmond BG, Jungers WL. 2008. Orrorin tugenensis femoral morphology and the evolution of hominin bipedalism. Science 319, 1162–1165. ( 10.1126/science.1154197) [DOI] [PubMed] [Google Scholar]
- 40.Almecija SM, Tallman DM, Alba M, Pina S, Moya-Sola M, Jungers WL. 2013. The femur of Orrorin tugenensis exhibits morphometric affinities with both Miocene apes and later hominins. Nat. Commun. 4, 2888 ( 10.1038/ncomms3888) [DOI] [PubMed] [Google Scholar]
- 41.McHenry HM. 1991. Petite bodies of the ‘robust’ australopithecines. Am. J. Phys. Anthropol 86, 445–454. ( 10.1002/ajpa.1330860402) [DOI] [Google Scholar]
- 42.Lordkipanidze D, et al. 2007. Postcranial evidence from early Homo from Dmanisi, Georgia. Nature 449, 305–310. ( 10.1038/nature06134) [DOI] [PubMed] [Google Scholar]
- 43.Rose MD. 1984. A hominine hip bone, KNM-ER 3228, from East Lake Turkana, Kenya. Am. J. Phys. Anthropol. 63, 371–378. ( 10.1002/ajpa.1330630404) [DOI] [PubMed] [Google Scholar]
- 44.Simpson SW, Quade J, Levin NE, Butler R, Dupont-Nivet G, Everett M, Semaw S. 2008. A female Homo erectus pelvis from Gona, Ethiopia. Science 322, 1089–1092. ( 10.1126/science.1163592) [DOI] [PubMed] [Google Scholar]
- 45.Simpson SW, Quade J, Levin NE, Semaw S. 2014. The female Homo pelvis from Gona: response to Ruff (2010). J. Hum. Evol. 68, 32–35. ( 10.1016/j.jhevol.2013.12.004) [DOI] [PubMed] [Google Scholar]
- 46.Ruff CB. 2010. Body size and body shape in early hominins: implications of the Gona pelvis. J. Hum. Evol 58, 166–178. ( 10.1016/j.jhevol.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 47.Lepre CJ, Kent DV. 2015. Chronostratigraphy of KNM-ER 3733 and other Area 104 hominins from Koobi Fora. J. Hum. Evol. 86, 99–111. ( 10.1016/j.jhevol.2015.06.010) [DOI] [PubMed] [Google Scholar]
- 48.Ruff CB, Burgess ML. 2015. How much more would KNM-WT 15000 have grown? J. Hum. Evol. 80, 74–82. ( 10.1016/j.jhevol.2014.09.005) [DOI] [PubMed] [Google Scholar]
- 49.Wood B, Collard M. 1999. The genus Homo. Science 284, 65–71. ( 10.1126/science.284.5411.65) [DOI] [PubMed] [Google Scholar]
- 50.Brown P, Sutikna T, Morwood MJ, Soejono RP, Jatmiko, Saptomo EW, Due RA. 2004. A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature 431, 1055–1061. ( 10.1038/nature02999) [DOI] [PubMed] [Google Scholar]
- 51.Morwood MJ, et al. 2005. Further evidence for small-bodied hominins from the Late Pleistocene of Flores, Indonesia. Nature 437, 1012–1017. ( 10.1038/nature04022) [DOI] [PubMed] [Google Scholar]
- 52.Jungers WL, Baab K. 2009. The geometry of hobbits: Homo floresiensis and human evolution. Significance 6, 159–164. ( 10.1111/j.1740-9713.2009.00389.x) [DOI] [Google Scholar]
- 53.Lorkiwicz-Muszynska D, Przystanska A, Kociemba W, Sroka A, Rewekant A, Zaba C, Paprzycki W. 2013. Body mass estimation in modern population using anthropometric measurements from computed tomography. Forens. Sci. Int. 231, 405.e1–405.e6. [DOI] [PubMed] [Google Scholar]
- 54.Elliott M, Kurki H, Weston PA, Collard M. 2015. Estimating body mass from postcranial variables: an evaluation of current equations using a large known-mass sample of modern humans. Arch. Anthropol. Sci. 1–6. ( 10.1007/s12520-015-0251-6) [DOI] [Google Scholar]
- 55.Jungers WL. 1988. Lucy's length: stature reconstruction in Australopithecus afarensis (A.L.288-1) with implications for other small-bodied hominids. Am. J. Phys. Anthropol. 76, 227–231. ( 10.1002/ajpa.1330760211) [DOI] [PubMed] [Google Scholar]
- 56.Baab K, Brown P, Falk D, Richtsmeier JT, Hildebolt CF, Smith K, Jungers W. In press A critical evaluation of the Down syndrome diagnosis for LB1, type specimen of Homo floresiensis. PLoS ONE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohman J, Wood C, Wood B, Crompton RH, Günther MM, Yu L, Savage R, Wang W. 2002. Stature-at-death of KNM-ER 15 000. Hum. Evol. 17, 129–142. ( 10.1007/BF02436366) [DOI] [Google Scholar]
- 58.Graves RR, Lupo AC, McCarthy RC, Wescott DJ, Cunningham DL. 2010. Just how strapping was KNM-WT 15 000? J. Hum. Evol. 59, 542–554. ( 10.1016/j.jhevol.2010.06.007) [DOI] [PubMed] [Google Scholar]
- 59.Richmond BG, Aiello LC, Wood BA. 2002. Early hominin limb proportions. J. Hum. Evol. 43, 529–548. ( 10.1016/S0047-2484(02)90594-4) [DOI] [PubMed] [Google Scholar]
- 60.Green DJ, Gordon AD, Richmond BG. 2007. Limb-size proportions in Australopithecus afarensis and Australopithecus africanus. J. Hum. Evol. 52, 187–200. ( 10.1016/j.jhevol.2006.09.001) [DOI] [PubMed] [Google Scholar]
- 61.Jungers WL. 2009. Interlimb proportions in humans and fossil hominins: variability and scaling. In The first humans: origin of the genus Homo (eds Grine FE, Leakey RE, Fleagle JG), pp. 93–98. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 62.Lovejoy CO, Suwa G, Simpson SW, Matternes JH, White TD. 2009. The great divides: Ardipithecus ramidus reveals the postcrania of our last common ancestors with African apes. Science 326, 100–1006. [PubMed] [Google Scholar]
- 63.Ward CV, Kimbel WH, Harmon EH, Johanson DC. 2012. New postcranial fossils of Australopithecus afarensis from Hadar, Ethiopia (1990–2007). J. Hum. Evol. 63, 1–51. ( 10.1016/j.jhevol.2011.11.012) [DOI] [PubMed] [Google Scholar]
- 64.McHenry HM. 1991. Femoral lengths and stature in Plio-Pleistocene hominids. Am. J. Phys. Anthropol 85, 149–158. ( 10.1002/ajpa.1330850204) [DOI] [PubMed] [Google Scholar]
- 65.Korey KA. 1990. Deconstructing reconstruction: the OH 62 humerofemoral index. Am. J. Phys Anthropol 83, 25–33. ( 10.1002/ajpa.1330830104) [DOI] [PubMed] [Google Scholar]
- 66.Holliday TW. 1997. Body proportions in Late Pleistocene Europe and modern human origins. J. Hum. Evol. 32, 423–447. ( 10.1006/jhev.1996.0111) [DOI] [PubMed] [Google Scholar]
- 67.Jungers WL. 1985. Body size and scaling of limb proportions in primate. In Size and scaling in primate biology (ed. Jungers WL.), pp. 345–381. New York, NY: Plenum Publishing Co. [Google Scholar]
- 68.Smith RJ, et al. 1996. Biology and body size in human evolution: statistical inference misapplied [and comments and reply]. Curr. Anthropol. 37, 309–349. ( 10.1086/204505) [DOI] [Google Scholar]
- 69.Smith RJ. 2009. Use and misuse of the reduced major axis for line-fitting. Am. J. Phys. Anthropol. 140, 476–486. ( 10.1002/ajpa.21090) [DOI] [PubMed] [Google Scholar]
- 70.Franciscus RG, Holliday TW. 1992. Hindlimb skeletal allometry in Plio-Pleistocene hominids with special reference to A.288-1 (‘Lucy’). Bull. Mem. Soc. Anthropol. Paris 4, 5–20. ( 10.3406/bmsap.1992.2300) [DOI] [Google Scholar]
- 71.Holliday TW, Franciscus RG. 2009. Body size and its consequences: allometry and the lower limb length of Liang Bua 1 (Homo floresiensis). J. Hum. Evol. 57, 223–228. ( 10.1016/j.jhevol.2009.04.007) [DOI] [PubMed] [Google Scholar]
- 72.Holliday TW, Franciscus RG. 2012. Humeral length allometry in frican hominids (sensu lato) with special reference to AL 288-1 and Liang Bua 1. Paleoanthropology 2012, 1–12. [Google Scholar]
- 73.Jungers WL. 2013. Homo floresiensis. In Companion to paleoanthropology (ed. Begun DR.), pp. 582–598. Malden, MA: Wiley-Blackwell. [Google Scholar]
- 74.Pfeiffer S. 2012. Conditions for evolution of small adult body size in Southern Africa. Curr. Anthropol 53, S383–S394. ( 10.1086/667521) [DOI] [Google Scholar]
- 75.Migliano AB, et al. 2013. Evolution of the pygmy phenotype: evidence of positive selection from genome-wide scans in African, Asian, and Melanesian pygmies. Hum. Biol. 85, 12. ( 10.3378/027.085.0313) [DOI] [PubMed] [Google Scholar]
- 76.Meazza C, Pagani S, Bozzola M. 2011. The pygmy short stature enigma. Ped. Endocrin. Rev. 8, 394–399. [PubMed] [Google Scholar]
- 77.Ramirez Rozzi FV, Kondou Y, Froment A, LeBouc Y, Botton J. 2015. Growth pattern from birth to adulthood in African pygmies of known age. Nat. Commun. 6, 7672 ( 10.1038/ncomms8672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Migliano AB, Bamberg A, Guillon M. 2012. The effects of mortality, subsistence, and ecology on human adult height and implications for Homo evolution. Curr. Anthropol. 53, S359–S368. ( 10.1086/667694) [DOI] [Google Scholar]
- 79.Dean C, Leakey MG, Reid D, Schrenk F, Schwartz GT, Stringer C, Walker A. 2001. Growth processes in teeth distinguish modern humans from Homo erectus and early hominids. Nature 414, 628–631. ( 10.1038/414628a) [DOI] [PubMed] [Google Scholar]
- 80.Grabowski M. 2016. Bigger brains led to bigger bodies? The correlated evolution of human brain and body size. Curr. Anthropol. 57, 174–196. ( 10.1086/685655) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Most of the data (specimens, metrics, estimates) can be found in the text and supplementary online information in reference [7]. The remaining raw data are provided here as electronic supplementary material (table S1 and S2).


