Abstract
Although the transition from Australopithecus to Homo is usually thought of as a momentous transformation, the fossil record bearing on the origin and earliest evolution of Homo is virtually undocumented. As a result, the poles of the transition are frequently attached to taxa (e.g. A. afarensis, at ca 3.0 Ma versus H. habilis or H. erectus, at ca 2.0–1.7 Ma) in which substantial adaptive differences have accumulated over significant spans of independent evolution. Such comparisons, in which temporally remote and adaptively divergent species are used to identify a ‘transition’, lend credence to the idea that genera should be conceived at once as monophyletic clades and adaptively unified grades. However, when the problem is recast in terms of lineages, rather than taxa per se, the adaptive criterion becomes a problem of subjectively privileging ‘key’ characteristics from what is typically a stepwise pattern of acquisition of novel characters beginning in the basal representatives of a clade. This is the pattern inferred for species usually included in early Homo, including H. erectus, which has often been cast in the role as earliest humanlike hominin. A fresh look at brain size, hand morphology and earliest technology suggests that a number of key Homo attributes may already be present in generalized species of Australopithecus, and that adaptive distinctions in Homo are simply amplifications or extensions of ancient hominin trends.
This article is part of the themed issue ‘Major transitions in human evolution’.
Keywords: early Homo, Australopithecus, transition
Whether primeval man, when he possessed very few arts of the rudest kind, and when his power of language was extremely imperfect, would have deserved to be called man, must depend on the definition which we employ. In a series of forms graduating insensibly from some ape-like creature to man as he now exists it would be impossible to fix on any definite point when the term ‘man’ ought to be used.
—Charles Darwin [2, p. 235]
The descent of man, and selection in relation to sex.
It seems to me more likely that H[omo] habilis and H. erectus, as well as some of the australopithecines, were all evolving along their own distinct lines by Lower Pleistocene times. This would mean that their shared common ancestor must be sought in the more remote past and that when such examples of the parent stock are found they will not much resemble any one of the three subsequent branches.
—Louis Leakey [3, p. 1280]
‘Homo habilis, Homo erectus and the australopithecines’.
1. Introduction
The origin and earliest evolution of the genus Homo perennially fascinate and frustrate in equal measure. Our fascination stems from the near-mythic qualities of uniqueness with which we tend to imbue the evolution of our lineage [4], whereas the frustration stems from critical gaps in the fossil record that actually bears on the first appearance of these features. By almost all accounts, the earliest populations of the Homo lineage emerged from a still unknown ancestral species in Africa at some point between approximately 3 and approximately 2 million years ago (Ma; [5–7], but see [8]). This temporal interval reaches forward in time from the latest known occurrences of ‘generalized’ Australopithecus species (A. afarensis in eastern Africa, A. africanus in southern Africa) to the earliest known records of two, perhaps three, species commonly attributed to the genus Homo (H. habilis, H. rudolfensis and H. erectus). Between them lies a million years of rare, isolated or fragmentary fossils that constitute the hard evidence for the origin of Homo. That this time period also contains some of the earliest undisputed evidence for stone-tool manufacture is of no small consequence for scenarios about the origin of characteristics thought to be critical human adaptations, including proclivities for meat consumption, hunting, mobility, cooking, prosocial behaviour, etc., usually in novel ‘open’ habitats of the Early Pleistocene [9–15]. It also witnessed the rise and diversification of the craniodentally specialized ‘robust’ australopiths, which means that isolated traces of behaviour and taxonomically uninformative pieces of skeletal anatomy often reside in a phylogenetic limbo between these lineages.
As a result of this ambiguity, hypotheses about the origin of Homo commonly transport adaptive suites observed in later populations backwards into the sparsely populated zones on the time chart, where they are tautologically deployed to explain the emergence of the lineage. Large brains, stone-tool technology, derived life-history traits and complex social behaviours have at one time or another all been seen as ‘defining’ of the genus Homo. It is not uncommon to see the transition to the genus Homo imaginatively reconstructed from a comparison of the relatively derived morphology and behaviour of Pleistocene H. erectus to the much more generalized morphology and behaviour of early Australopithecus—even though no such radical transition ever actually occurred. We have previously pointed out [5] the similarity of this situation to the conundrum nineteenth-century evolutionists faced, when, in the absence of an early fossil record, they drew conclusions about foundational events in human origins from differences between extant great apes and humans, the only sources of insight available to them. Absent the chronicle of events provided by the fossil record, they assumed (as we tend to do today) that constellations of characters constitute adaptively linked complexes whose origins in deep time are equivalent to the sum of the differences accumulated in sister taxa since their last common ancestor (this is equivalent to the ‘referential model’ of Tooby & DeVore [16]).
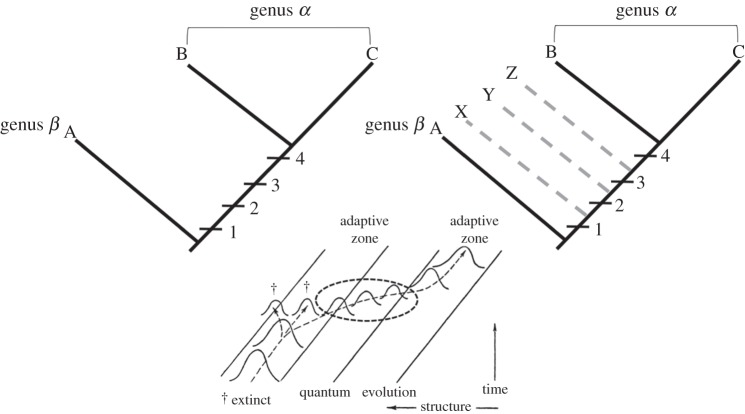
This essay is predicated on two arguments. First, the idea that a ‘major transition’ from Australopithecus to Homo is moot, pending significant new additions to the record. Second, the concept of a major transition as it applies to the difference between two closely related genera (e.g. sister taxa) is itself open to question. While the genus is a nomenclatural necessity in the Linnaean system of species-naming, ‘the genus’, unlike ‘the species’, plays no role in evolutionary theory. It is simply the least inclusive taxonomic category in the Linnaean hierarchy meant to hold collections of species. Biologists nearly universally agree that monophyly is the one essential criterion for grouping species in a genus [17,18].1 Because of the atheoretical nature of the genus, however, there are no other principles governing how genera ought to be delineated, only popular rules of thumb. There is a general sense that genera should be separated from one another by a ‘decided gap’ in morphology or behaviour [19], which some systematists have refined to mean that genera, in addition to being monophyletic, should contain species occupying a distinctive adaptive zone or plateau (‘a more fundamental difference in ecology than that between ecological niches of species’, in the words of Mayr [20, p. 110]). According to this construal, a genus is both a clade and an adaptive grade [8]. However, a significant complication arises when the concept of lineage is brought into the discussion. In many examples of evolutionary change within a lineage, transitional fossils narrow gaps between terminal taxa by bridging the character complexes that distinguish them [21,22]. In the common case of a stepwise or piecemeal acquisition of novel characters, the problem becomes where to draw a line between adaptive grades without leaving a rump of relatively generalized (potentially ancestral) species grouped into paraphyletic taxa (or set off as monotypic genera; figure 1). Whereas evidence of monophyly objectively specifies clades, grades can only be defined arbitrarily whenever intermediates appear in the fossil record (see [23] for additional discussion).
Figure 1.
The effect of transitional fossils on distinct adaptive suites inferred from the comparison of terminal taxa. Top left: genus α is distinguished from genus β by a suite of characters [1–4] thought to establish distinct adaptive grades. (In practice, characters 1–4 might be treated as a single functionally integrated character depending on the nature of the inferred adaptation.) Top right: fossil taxa (X–Z) bearing transitional forms show that, in reality, the pattern of acquisition of the characters was stepwise, defying clear delineation of adaptive grades along the lineage. Bottom: fig. 31 from Simpson [21] shows transitional populations (added dotted oval) linking species that occupy different ‘adaptive zones’. Although Simpson depicted this transition as an example of phyletic evolution (as opposed to speciation), the pattern of character acquisition is neutral with respect to evolutionary mode.
The case of early Homo illustrates this issue well. In 1999, Wood & Collard [24] concluded that H. erectus was sufficiently similar to H. sapiens in the adaptive zone it occupied that it could reasonably be included in our genus. In the intervening years, however, data on dental growth and development (and, by inference, life-history profile; [25]), sexual dimorphism ([26], but see [27]), the shoulder and pelvis [28–30], variation in endocranial volume and encephalization quotient [31,32], and technological complexity [33] leave little doubt that H. erectus (especially its early populations) occupied a rather distinctive adaptive zone compared with H. sapiens—certainly one more fundamental than a mere difference in ecological niche, to borrow from Mayr's [20] formulation. Today, it is pretty clear, on the ‘clade + grade’ definition of the genus category, that a case can be made for resuscitating the genus Pithecanthropus for fossils assigned to H. erectus, at least as judged by the long retrospective view from the adaptive peak occupied by modern H. sapiens.2 Wood & Baker [34] have contrasted this ‘top-down’ view, which searches backwards in time for traces in the fossil (and, perhaps, archaeological) record of key adaptations that mirror those of specialized extant Homo sapiens, with a ‘bottom-up’ perspective, the view from the base of the lineage, which seeks evidence of a monophyletic relationship and adaptive unity with modern humans in an initial climb through temporally remote and relatively generalized precursors. From the latter view, following time's vector, the adaptive profile of H. erectus in some ways now appears more similar to that of phylogenetically more basal species of the Homo clade, including H. habilis, which Wood & Collard [8,24] have argued are sufficiently dissimilar in adaptive profile to Homo to warrant their removal from the genus. This change in perspective is because the pattern of acquisition of the suite of ‘human-like’ adaptive features in Early Pleistocene Homo appears to have been much more piecemeal (not to say ‘gradual’) than previously appreciated, underscoring the essentially arbitrary nature of adaptive grades once their mode of construction in phylogeny is taken into account.
These issues have been raised afresh in Schwartz & Tattersall's [35] call for a new ‘definition’ of the genus Homo, which, they argue (echoing reference [8]), has been expanded beyond acceptable morphological limits by the inclusion of relatively primitive-looking fossils suggested to sample early time periods in the divergence of the group. In seeking a fixed morphological definition of our genus, however, Schwartz and Tattersall invert the meaning of the genus category. Because a genus ‘exists’ only in virtue of the monophyletic relationship among its constituent species, genera do not have a ‘definition’ other than the set of synapomorphies (of minimum number one) that unites these species with their most recent common ancestor. Thus, the ‘definition of the genus’ should be the outcome of a phylogenetic exercise, not a phenetic one in which researchers must agree a priori on some ‘satisfactory’ concept of its morphological limits. (What these limits should be Schwartz and Tattersall decline to say, but we are reminded of a quote from G. G. Simpson [36, p. 16]: ‘The question, ‘Precisely how large is the scope of a genus, a family, or an order?’ is not much more determinate than the question, ‘Precisely how far is up?’.’) Palaeoanthropologists have a set of tools that enable them to identify objectively monophyletic groups; nowhere in this Hennigean toolbox can be found a definition of the genus or of its limits (or that of any other supraspecific taxonomic category, for that matter).
Schwartz & Tattersall [35, p. 932] seem to be concerned that including early species of the Homo lineage within the genus Homo inaccurately represents past taxonomic diversity: ‘In contrast to … austere linearity, we may find that human evolution rivaled that of other mammals in its evolutionary experimentation and luxuriant diversity’. As we review below, however, a linear hominin phylogenetic tree was vigorously rejected more than three decades ago, as the number of fossil hominin species identified, each with a unique morphological and adaptive profile, has grown significantly as the result of new discoveries coupled with conceptual and methodological advances in systematics. In any event, a more inclusive genus Homo neither increases nor decreases diversity at the species level, the level at which Schwartz and Tattersall seem to aim their critique. Our taxonomy first and foremost needs to reflect the underlying branching pattern, and the utility of a more, rather than less, inclusive, genus Homo to accommodate 2 Myr old and older fossils representing populations uniquely related to living humans still stands as an important marker for the starting point of our own unique evolutionary trajectory.
2. The changing idea of early Homo
The current consensus on the early evolution of Homo is the outgrowth of an approximately 30-year-old movement away from the concept of a single, gradually evolving lineage leading inexorably from some Pliocene australopith to modern humans. During the 1950s–early 1960s, it was possible to see Australopithecus africanus of southern Africa, inferred to be a generalized tool-using (if not tool-making) bipedal omnivore, as a link to ‘man the hunter’, whose earliest manifestation was Homo erectus, a cosmopolitan, relatively encephalized, technologically sophisticated creature [37–40]. The recognition, in 1964, of the species Homo habilis by Leakey et al. [41] for non-robust hominin material from Bed I and lower Bed II (1.85–1.65 Ma) in Olduvai Gorge, Tanzania, inserted a new stage in the single-file march toward modernity. Leakey et al. argued that the co-occurrence at Olduvai of brain sizes greater than 600 cc, lightly built skulls housing small, narrow cheek teeth, a dexterous hand capable of both power- and precision-grips, implied occupation of an adaptive peak fundamentally closer to that of later species in the genus Homo, including modern humans, than to that of Australopithecus.3 Collard & Wood [8] have suggested that this attribution of a new species to the genus Homo was one in a series of cases stretching back in history to the recognition of H. neanderthalensis (by William King in 1864) in which the morphological limits of our genus (particularly in brain size and masticatory form) were unduly stretched by the inclusion of progressively older fossils based on the ad hoc use of ‘key’ characteristics thought to foreshadow modern humans. In the case of H. habilis, humanlike manual dexterity (as gleaned from the hand bones of type specimen OH 7) and fully upright bipedal locomotion were clearly paramount considerations: these were the first two characteristics Leakey et al. [41, p. 9] listed in their revised diagnosis of the genus Homo, which, notably, dropped Arthur Keith's ‘Cerebral Rubicon’ of 750 to 600 cc to accommodate the new Olduvai material.
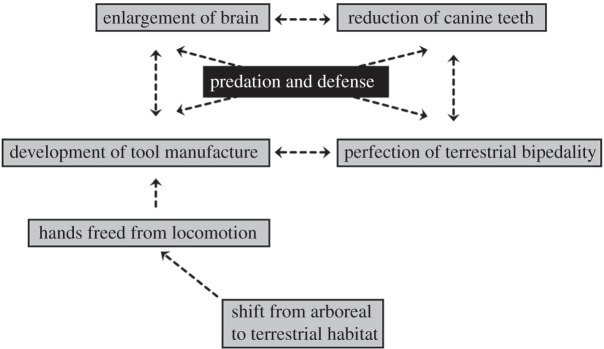
Leakey, typical of most palaeoanthropologists of his era, was under the powerful influence of the Charles Darwin's model of human origins. Darwin [2] had outlined a theory causally linking freedom of the hands from locomotion, granted by terrestrial bipedality to manual dexterity, tool manufacture, reduced canine teeth and an armed, predatory lifestyle (figure 2). So dominant was this scenario that, in the centennial year of the Origin of species, archaeologist Kenneth Oakley [43, p. 5] was able to write, ‘If man is defined as a tool-making animal, then the problem of the antiquity of man resolves itself into the question of the geological age of the earliest known artifacts…’ At the end of their paper, Leakey et al. [41, p. 9] linked Homo habilis to the crudely chipped (Oldowan) stone tools that had been found in abundance in Bed I sediments and previously assumed by Leakey to have been left by the robust australopith species Zinjanthropus boisei (i.e. Australopithecus boisei, found in Bed I in 1959; [44]). ‘While it is possible that Zinjanthropus and Homo habilis both made stone tools’, Leakey et al. wrote, ‘it is probable that the latter was the more advanced tool maker and that the Zinjanthropus skull represents an intruder (or a victim) on the Homo habilis living site’. Leakey and co-workers here committed to the prevailing Darwinian paradigm while erasing the lower limit on brain size thought to be consistent with it.4
Figure 2.
In The Descent of Man, Darwin [2] presented a model, sketched here, to account for the changes that needed to be invoked to account for human divergence from great apes. Darwin postulated environmental change as the spark and natural selection for attributes enhancing skill in defence and hunting as the engine of change, and he envisioned positive feedback linking adaptive changes in technology, intelligence and anatomical structure as the key to the human species' success. He proposed this, of course, in the absence of a fossil record that could have provided a chronology of events for these changes.
The gulf between ape-man and true-man was at the same time dramatically being narrowed on another front, which we suggest may have been influential in Leakey and co-workers’ thinking about the classification of the new Olduvai fossils. In 1960, with Leakey's encouragement and support, Jane Goodall started the first long-term study of chimpanzees in the wild, at Gombe in Tanzania. Almost immediately, she observed termite-fishing by chimps using twigs deliberately modified to function as tools [46]. In reply to an excited letter from Goodall, Leakey famously telegrammed: ‘Now we must redefine tool, redefine Man, or accept chimpanzees as humans!’ [47]. From this perspective, it is easy to see how the new Olduvai material could be enfolded within Homo under a theoretically motivated expansion (as opposed to ad hoc: pace 8) of the human genus. If tool-making no longer separated Pan from Homo, then certainly an extinct human ancestor with twice the brain of a chimp and the cognitive ability and manual dexterity to make flaked stone tools was ‘man enough’ to be included in our genus.
Mayr's [20] invocation of a universal hominin ‘despecialized’ ecological niche in a theoretical argument (from competitive exclusion) against true speciation in the hominin lineage led to a trimming of the hominin taxonomic inventory even among the majority of palaeoanthropologists who did not embrace the ‘single species hypothesis’. While most students of the hominin record [37–40,48] upheld a distinction between Australopithecus and Homo (and in some cases Paranthropus), the prevailing depiction of evolution in Homo was unilinear, with one species grading into its temporal successor in a progression towards modern humans. (Tobias's [40] phylogenetic tree is particularly evocative of this idea.) Into this chain was inserted H. habilis, ‘the last remaining major gap in the Pleistocene story of human evolution’ [40, p. 33].
The discovery, in 1972, of the large-brained KNM-ER 1470 cranium in sediments of the upper Burgi Member of the Koobi Fora Formation (approx. 2.0 Ma) revived the importance of large brain size in the identification of earliest Homo and, simultaneously, opened a now 40-year-old debate regarding species-level diversity in non-robust African hominins. This edentulous cranium possessed an unusually massive, deep face, an anteriorly flattened maxilla and inferred large tooth size—all distinctly ‘non-human’—but the exceptionally large endocranial volume—about 50% larger than that of Australopithecus—stood out [49]. Walker & Leakey [50] thought that the Homo species represented by KNM-ER 1470 was ancestral to H. erectus, whereas smaller-brained non-robust crania, such as KNM-ER 1813 (approx. 510 cc), represented a contemporary lineage of Australopithecus or Homo.5 Meanwhile, stone artefacts and broken bones of large mammals had been found together on sedimentary surfaces and in situ in the upper Burgi Member, where they were presumed to be functionally related to sites of hominin occupation [51]. The spatio-temporal juxtaposition of what was thought to be among the earliest sources of evidence for tool manufacture and large-brained Homo spawned new ideas about the emergence of uniquely human forms of resource utilization, tool use, complex cognition, and social organization: ‘Occupation sites containing stone artifacts and introduced, broken-up bones probably imply a significant evolutionary intensification of certain behavioral characteristics, which may occur sporadically in nonhuman primates but which became especially prominent characteristics of Pleistocene Hominidae. These are manufacture of tools, hunting or scavenging (or both), food sharing, and the organization of movements around an archeologically recognizable home base’ [51, p. 133]. Here, the Darwinian edifice was refashioned into an explanation for the transformation of a bipedal australopith ancestor with a great ape's limited cultural capacity into a direct modern human ancestor endowed with nascent linguistic and altruistic tendencies built around food sharing [52,53] (figure 3). Underpinning this scenario was a strongly progressivist stance on the emergence of unique human qualities that sees modern human cognitive capabilities and social behaviour writ small in the earliest stone-tool makers [54].
Figure 3.
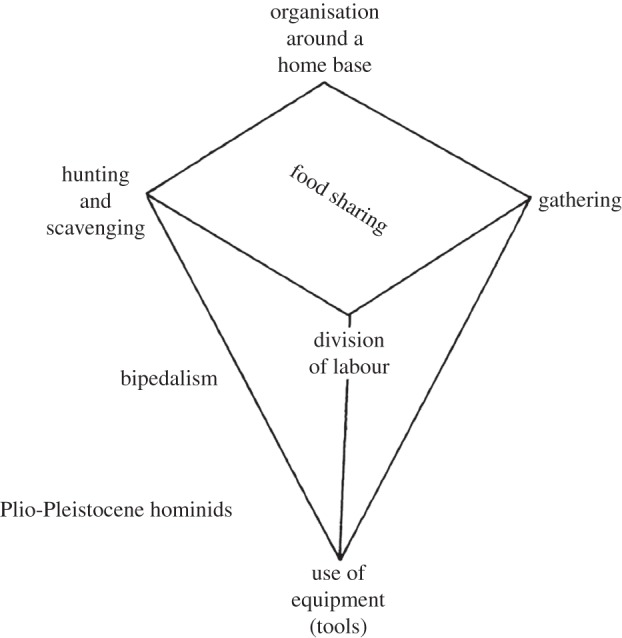
Isaac's [52] food-sharing model of ‘proto-human’ behavioural evolution in the Plio-Pleistocene.
In the 1970s, the introduction of cladistic methods of phylogenetic inference in palaeoanthropology led to greater concern with detailed character study, quantitative comparison of inter- versus intraspecific variation, and a reassessment of fossil hominin morphology under explicit hypotheses of common ancestry. One can see in these early exercises the roots of discontent with a unilinear evolution of the genus Homo (for example in the perception of the ill fit of derived H. erectus cranial morphology into a Middle-to-Late Pleistocene sequence of emerging modern human features), but the main message was familiar: the Homo lineage emerged out of a generalized ancestor like A. africanus to embark on an evolutionary path marked by key long-term trends, including perfection of bipedality, decrease in tooth size, increase in brain size, advances in lithic technology and the transition to a hunting lifestyle [55,56].
The current consensus on lineage diversity in Homo around 2.0–1.7 Ma is less a product of new discoveries than an outgrowth of these conceptual and methodological shifts in the field. After all, the major pieces of fossil evidence have been with us for at least four decades. Although the contrast between KNM-ER 1470 and the smaller-brained KNM-ER 1813 (discovered in 1972 and 1973, respectively) has played an outsized role in how diversity in early Homo is perceived, the fact that significant discontinuities in time or geography could be discounted as explanations for the differences between these Koobi Fora crania meant that hypotheses invoking sexual dimorphism or other sources of intraspecific variation could be tested explicitly by reference to variation in modern hominoid species [57,58]. The domed frontal devoid of a supraorbital torus and supratoral sulcus, the vertically deep face with broad, forwardly sloping zygomatics, and the flat, vertical subnasal plate observed in KNM-ER 1470 were deemed unlikely to represent male morphology in a sexually dimorphic species—in which KNM-ER 1813 exhibited more generalized female morphology—based on extant primate models. Wood [57] reintroduced the species name H. rudolfensis—coined by Alexeev [59] for KNM-ER 1470—and grouped KNM-ER 1813 in the hypodigm of H. habilis with all of the Olduvai fossils originally linked to holotype specimen OH 7 by Leakey et al. [41].6
Cranium KNM-ER 3733 (discovered in 1975), from the lower part of the KBS Member of the Koobi Fora Formation at East Turkana, has exemplified the best and oldest evidence for a third cranial morph—attributed to early Homo erectus (or H. ergaster by some)—in eastern Africa [50,60,61]. Recent refinement in the age framework for the KBS Member has pushed up the age estimate for this specimen from as old as 1.78 to 1.7–1.6 Ma [62,63], which makes it approximately contemporary with Homo habilis in upper Bed I and lower Bed II at Olduvai Gorge. KNM-ER 2598, an occipital bone unmistakably H. erectus-like in its greatly thickened, strongly flexed squama surmounted by a prominent occipital torus ([61] and figure 4), now stands as potentially the earliest evidence for H. erectus in Africa at approximately 1.9 Ma, approximately contemporary with KNM-ER 1470 and KNM-ER 1813.7
Figure 4.
The partial occipital bone KNM-ER 2598 from the upper Burgi Member of the Koobi Fora Formation (a, posterior view; b, left lateral view). Thick bone (seen in cross section), a well-developed occipital torus (especially centrally), and the low position of the internal occipital protuberance relative to inion affiliate this specimen with the Homo erectus morphological pattern [61]. The widely divergent limbs of the lambdodial suture are also characteristic of many African and Asian H. erectus crania. Photos courtesy of Fred Spoor (scale bar, 1 cm). (Online version in colour.)
Association of postcranial fossils with early Homo skull morphs is an urgent but risky venture, because so many of the potential associations are based on incomplete and extremely fragmentary remains (e.g. OH 62, KNM-ER 3735, both attributed to H. habilis). Many of the better preserved postcranial fossils from eastern Africa between approximately 2.0 and approximately 1.5 Ma are isolated, completely unmoored to taxonomic evidence from the skull. Yet morphological and perhaps functional differentiation does appear to characterize at least the hip and thigh of early Homo. Ward et al.'s [69] revealing analysis of the fragmentary KNM-ER 5881 femur and pelvis from Koobi Fora (upper Burgi Member, approx. 1.9 Ma) found a derived, distinctly Homo-like proximal femur (large head compared with neck; rounded neck cross section) and ilium (laterally flexed anterior border; distinct, craniocaudally directed iliac pillar), but a femoral shaft that lacks the derived mediolateral expansion characteristic of all early and modern Homo femora except that of OH 62 and two femora in the Dmanisi sample. The KNM-ER 5881 pelvis is much smaller than the similar-age KNM-ER 3228, which is usually attributed to Homo; an argument that they represent the same species would imply a gorilla-like level of sexual size-dimorphism in the early Homo pelvis [69]. Inferred pelvic inlet shape is distinct from that of the Gona H. erectus pelvis [30], which itself is more humanlike (circular) than fossil pelves typically assigned to early Homo (i.e. KNM-ER 3228, OH 28). This mixed pattern of affinities in the hip and thigh of early Homo mirrors the morphological differentiation within a derived Homo-like phylogenetic context conveyed by the skulls of this group. It may be difficult to read adaptive differentiation from these fragmentary remains, but they nonetheless underscore the likelihood that a single major adaptive transformation in body form did not accompany a ‘transition’ from Australopithecus to Homo.
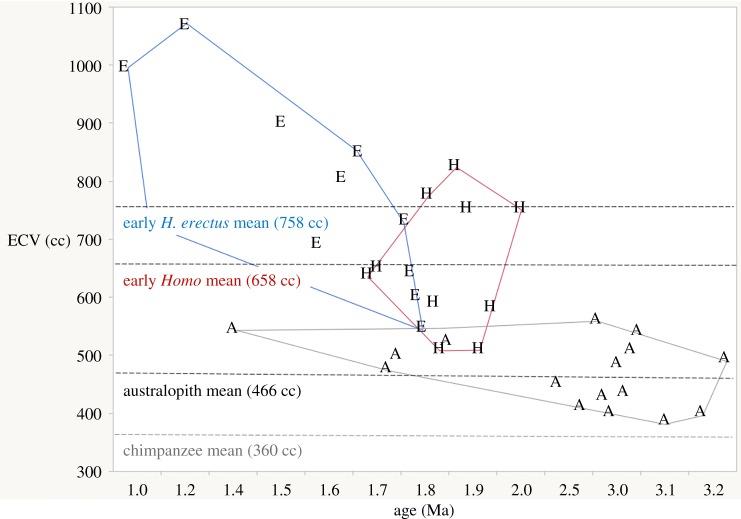
Although Spoor et al.'s [6] new range of estimates for the endocranial volume of OH 7 (729–824 ml) suggests that, in absolute terms, H. habilis (sensu stricto) brain size overlapped that of H. rudolfensis and early H. erectus, there is great uncertainty regarding the range of body size and body-size dimorphism for any of these species. The view is further clouded by the recent recovery of extremely large postcranial elements in association with diagnostic dental remains of A. boisei at 1.34 Ma [70], which implies that large body size may not be a distinguishing feature of Early Pleistocene Homo relative to contemporary australopiths, as is often suggested ([15,71,72], but see [27]). While the relationship between body mass and brain size is critical in ecology and life history, the relevance of relative brain size to cognitive capacity within the primates may be overstated [73,74]. The documented increase in absolute brain size in early Homo appears to be a continuation of an enlargement that is already apparent in Australopithecus. Brain size in Australopithecus (sensu lato) averages approximately 470 cc (based on data in [75]), which is about one-third larger than the average chimpanzee brain (363 cc; data from [76] Pan troglodytes troglodytes individuals in [77]). Recent discoveries confirm what has long been hinted by the estimated endocranial volume (ECV) of 727 cc for H. erectus cranium OH 12 (Olduvai Gorge, Bed IV, approx. 1.0 Ma), which is that brain size in early Homo is highly variable—even within fairly narrow time bands—with some early H. erectus crania (e.g. D4500) falling into the Australopithecus range [6,32,66]. Thus, while the early Homo mean ECV excluding H. erectus is just shy of 40% larger than that of Australopithecus, the mean for crania usually attributed to early African/Eurasian H. erectus represents only a further 14% increase. The gap in ECV data for Homo earlier than about 2.0 Ma means that the pattern of brain size increase (if any) with the origin and earliest evolution of the Homo clade is completely undocumented. But based on evidence presently available, we argue that the adaptive shift represented by the ECV of Australopithecus is at least as significant as the one represented by the ECV of early Homo, and that a major ‘grade-level’ leap in brain size with the advent of H. erectus is probably illusory (figure 5).
Figure 5.
Early hominin endocranial volumes (ECVs) over time. The average ECV for chimpanzees is shown for comparison (based on data in [77]). A, Australopithecus (sensu lato); H, H. habilis and H. rudolfensis; E, H. erectus. Data for fossil hominins from references [6,31,32,75]. (Online version in colour.)
3. Homo before 2.0 Ma
The occurrence of multiple craniofacial, and perhaps postcranial, morphs attributable to the Homo clade at 2.0–1.8 Ma implies a common ancestral form older than 2.0 Ma. Collard & Wood [8] have recently updated their review of evidence for monophyly of the genus Homo that includes the three fossil species discussed above. They note the common finding of parsimony analysis that H. habilis and H. rudolfensis are part of a monophyletic Homo clade, but emphasize the relatively weak statistical support for this result, which they interpret as evidence against the hypothesis of monophyly. Weak support, however, is support nonetheless. An alternative interpretation acknowledges a high degree of homoplasy at the species level within hominins, and argues that the basal species of a clade are inherently unlikely to possess many of the synapomorphies present in its subsequent constituents. These factors may be expected to result in the unstable rooting of basal taxa of a clade in species-level phylogenetic analyses (see also [78]).
In its craniofacial configuration, Homo habilis is usually found to be the most symplesiomorphic of these early Homo species [6,76,79,80]. Yet, among the oldest specimens clearly diagnosable as Homo, the A.L. 666-1 maxilla (Hadar, 2.35 Ma) already shows derived nasal cavity and subnasal morphology seen in SK 847 (Swartkrans; 2.0–1.5 Ma) and D2282 and D4500 (Dmanisi; 1.8 Ma) crania [32,76], which are usually affiliated with H. erectus. The Dmanisi hominin assemblage, which, in our judgement, probably represents a population early in the emergence of H. erectus, is approximately contemporaneous with H. habilis and slightly younger than the earliest candidate H. erectus from Koobi Fora (KNM-ER 2598; see above and footnote 7). A small, mesiodistally elongated and steep-walled M1 crown from 2.34 Myr-old sediments at Lokalalei, West Turkana, compares closely with H. habilis homologues in Beds I and II, Olduvai Gorge [81]. Mandible UR 501 from the Chiwondo Beds near Lake Malawi is thought to be as old as 2.4 Ma (but could be as young as 1.9 Ma) and is attributed to H. rudolfensis based on derived mandibular corpus morphology shared with many later Homo jaws, and its large, but buccolingually narrow, M1 crown and broad-crowned, multirooted lower premolars [82]. A clear implication of these observations is that diversity in Homo may be expected to extend back into the Late Pliocene.
Very limited fossil evidence substantiates Homo older than about 2.4 Ma. If we set aside the candidacy of A. garhi (2.5 Ma; [83]), whose type (and only craniofacial) specimen shares virtually no derived features exclusively with Homo, then we have only a few jaws and isolated teeth from members C–E of the Shungura Formation of the Omo River basin and a half-mandible with teeth from the Ledi-Geraru site (both in Ethiopia) that arguably document early Homo between 2.8 and 2.4 Ma [7,84,85].8 These remains show departures in jaw and dental structure from generalized Australopithecus conditions. For example, the P3 crown has a more symmetrical occlusal profile, a mesially directed anterior fovea sealed by a stronger, continuous mesial marginal ridge, and macrowear that is more evenly distributed across the occlusal surface (perhaps implicating derived upper canine morphology and function) compared with A. afarensis. The lower molars, though equivalent in overall crown size to those of A. afarensis, lack the bulging buccal sides common in this and other Australopithecus species. The mandible's filled-out lateral corpus under the premolars, subequal anterior and posterior corpus depths, posteriorly directed mental foramen, and posteriorly positioned anterior margin of the ascending ramus all signal a departure from the most common Australopithecus morphology.
That many of these derived features occur regularly in early Homo jaws and dentitions between 2.3 and 1.9 Ma implies a phylogenetic connection, and based on considerations of monophyly, these early fossils can reasonably be placed in the genus Homo alongside them [7]. Can a corresponding adaptive shift be discerned in these features, one that might satisfy advocates of a ‘clade + grade’ criterion for generic unity? These early fossils hint at some kind of functional shift from generalized australopith conditions in the dentognathic system, perhaps involving the canine/premolar complex at least, but the evidence is still far too sketchy to move beyond such speculation.
As noted above, A. africanus of southern Africa has long figured in scenarios regarding the origin of Homo, and, indeed, relative to the more generalized A. anamensis–A. afarensis lineage, this species shares some derived dentognathic morphology with early Homo. In fact, a few specimens included in the A. africanus hypodigm resemble those of later species of Homo so closely that claims of taxonomic heterogeneity have occasionally been aired though not widely accepted (reviewed in [89]). Comprehensive phylogenetic analyses typically position A. africanus basal to a clade that unites Homo and robust australopiths (Paranthropus; reviewed in [78]). South African Australopithecus sediba (approx. 2.0 Ma) has also been claimed to have been a direct ancestor to Homo, possibly even to H. erectus [90], but is more plausibly considered a close relative of A. africanus [91–93].
4. Conclusion
The fossil record bearing on the ancestry of Pleistocene Homo is poor. However, the more we learn about early Homo, the less compelling is the case that an adaptive shift can be read from currently documented skull and skeletal anatomy as a ‘major transition’ from generalized Australopithecus precursors. Early, phylogenetically basal species of the Homo clade resemble generalized australopiths more than they do later species of the clade—as expected from a Darwinian pattern of descent with modification. These and subsequent ‘transitional’ species of the Homo lineage (e.g. H. erectus) erode the impression of distinct adaptive suites created by comparisons between terminal taxa or those separated by large temporal gaps. The epigraph heading this paper indicates that Darwin [2] himself recognized this as a probable outcome of descent with modification, given a grown fossil record. (The argument has recently been updated by Cartmill [94].) This recognition is not, however, a paean to wholesale phyletic or ‘gradual’ evolution in Homo, as was commonly asserted half a century ago. The African fossil record of Homo demonstrates diversity quite clearly between 2.0 and 1.7 Ma, and there are hints of it as far back as 2.4 Ma. It is, rather, an argument against ‘adaptive unity’ as a biologically necessary adjunct to monophyly in the definition of the genus category. Whether or not we choose to adopt the larger number of supraspecific taxonomic ranks (and their associated taxon names) dictated by a bushy hominin clade is not a pressing scientific matter in the quest to understand human origins. Of greater importance can be counted recent arguments for the use or manufacture of stone tools in time periods predating by some half a million years the earliest Homo fossils known so far [95,96], and the potential they have to shrink the adaptive space between Homo and Australopithecus still further. Indeed, the expanded brain size, human-like wrist and hand anatomy [97,98], dietary eclecticism [99] and potential tool-making capabilities of ‘generalized’ australopiths root the Homo lineage in ancient hominin adaptive trends, suggesting that the ‘transition’ from Australopithecus to Homo may not have been that much of a transition at all.9
Acknowledgements
Thanks to Rob Foley, Marta Lahr, Chris Stringer and Lawrence Martin for the invitation (to W.H.K.) to participate in the Royal Society Discussion Meeting in honour of Richard Leakey, and for their forbearance during the preparation of the manuscript. Thanks are due Carol Ward, Fred Spoor, Bernard Wood, Gen Suwa, Luke Delezene and Dave Strait for discussion about the issues at stake in thinking about early Homo. The manuscript also benefitted from the constructive comments of two anonymous referees.
Endnotes
We interpret monophyly in its inclusive sense of incorporating an ancestral species and all the descendants of that species (=holophyly).
The monophyly of the genus Pithecanthropus would be suspect if it were to be determined that African populations (sometimes attributed to H. ergaster) yielded descendants whereas Asian ones did not. This problem is avoided by maintaining these groups within Homo.
Here we are deliberately glossing over the debate of the mid-1960s to early 1970s regarding the taxonomic distinctiveness of H. habilis relative to A. africanus (see [42]).
Montagu [45] had already urged dropping Keith's Rubicon to 600 cc on the stratigraphic association of stone tools with the Zinjanthropus cranium in Bed I.
At this time, the age but not the stratigraphic provenience of KNM-ER 1470 was debated, whereas the stratigraphic placement of the sediments that yielded KNM-ER 1813 was unresolved (see below.) In any case, it is the large-brained 1470 cranium that was usually invoked in scenarios of brain size and the origin of stone-tool making.
Included in the H. rudolfensis hypodigm were KNM-ER 1590 (parietal bones and a mixed deciduous and permanent dentition), KNM-ER 1802 (mandible with teeth) and KNM-ER 3732 (partial cranium), among other less complete fossils. In addition to the originally attributed (OH 4, OH 6, OH 7, OH 8, OH 13) or referred (OH 14, OH 16) specimens in the Olduvai sample, the deformed cranium OH 24, and the badly fragmented partial skeleton of OH 62 were included in the hypodigm of H. habilis, along with some fragmentary mandibles from Koobi Fora.
These specimens all come from surface sediments below the KBS Tuff at Koobi Fora. The best estimate of the age of KNM-ER 1813 is 1.86 ± 0.08 Ma [64], and for KNM-ER 1470 it is 2.058 ± 0.034 Ma [65]. The KNM-ER 62000 maxilla, which reiterates ER 1470s distinctive facial morphology [66], is constrained in age between 1.95 and 1.98 Ma [65]. The find spot of KNM-ER 2598 occurs 4 m below the KBS Tuff [67]; its age is estimated to be 1.9 Ma [68]. As seen in figure 4, the latter specimen is heavily weathered, raising the possibility that it originally derived from younger sediments than those on which it was found (i.e. from the overlying KBS Member). However, KBS Member exposures are very limited in the vicinity the hominin locality [68], and contamination by younger sediments is considered unlikely (C. Feibel, personal communication; M. Leakey, personal communication).
A 2.4 Myr-old temporal bone (KNM-BC 1) from the Chemeron Formation in the Baringo basin, Kenya, has been said to represent Homo [86,87], possibly H. rudolfensis. In contrast, Lockwood et al. [88] found KNM-BC 1 to have a mixed pattern of morphometric affinities but no derived features that uniquely link it to undoubted fossils of the Homo clade.
While this paper was in proof, Grabowski et al. [100] published results of a new scaling analysis of brain and body size in primates that support our contention that early australopiths were significantly more encephalized than extant great apes, and that no significant advance in encephalization occurred with the initial appearance of H. erectus.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.McBrearty S, Brooks AS. 2000. The revolution that wasn't: a new interpretation of the origin of modern human behavior. J. Hum. Evol. 39, 453–563. ( 10.1006/jhev.2000.0435) [DOI] [PubMed] [Google Scholar]
- 2.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 3.Leakey LSB. 1966. Homo habilis, Homo erectus, and the australopithecines. Nature 209, 1279–1281. ( 10.1038/2091279a0) [DOI] [PubMed] [Google Scholar]
- 4.Cartmill M, Pilbeam DR, Isaac GL. 1986. One hundred years of paleoanthropology. Am. Sci. 74, 410–420. [Google Scholar]
- 5.Kimbel WH. 2009. The origin of Homo. In The first humans—origin and early evolution of the genus Homo, pp. 31–37. Dordretch, The Netherlands: Springer. [Google Scholar]
- 6.Spoor F, Gunz P, Neubauer S, Stelzer S, Scott N, Kwekason A, Dean MC. 2015. Reconstructed Homo habilis type OH 7 suggests deep-rooted species diversity in early Homo. Nature 519, 83–86. ( 10.1038/nature14224) [DOI] [PubMed] [Google Scholar]
- 7.Villmoare B, Kimbel WH, Seyoum C, Campisano CJ, DiMaggio EN, Rowan J, Braun DR, Arrowsmith JR, Reed KE. 2015. Early Homo at 2.8 Ma from Ledi-Geraru, Afar, Ethiopia. Science 347, 1352–1355. ( 10.1126/science.aaa1343) [DOI] [PubMed] [Google Scholar]
- 8.Collard M, Wood BA. 2015. Defining the genus Homo. In Handbook of Paleoanthropology, 2nd edn (eds Henke W, Tattersall I), pp. 2108–2144. Berlin, Germany: Springer. [Google Scholar]
- 9.Brain CK. 1981. The evolution of man in Africa: Was it the result of Cainozoic cooling? Trans. Geol. Soc. S. Afr. Annexure 84, 1–21. [Google Scholar]
- 10.Stanley SM. 1992. An ecological theory for the origin of Homo. Paleobiology 18, 237–257. [Google Scholar]
- 11.Vrba E. 1994. An hypothesis of heterochrony in response to climatic cooling and its relevance to early hominid evolution. In Integrative paths to the past-paleoanthropological advances in Honor of F. Clark Howell (eds Corruccini RS, Ciochon RL), pp. 345–376. Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- 12.Aiello LC, Wells JCK. 2002. Energetics and the evolution of the genus Homo. Annu. Rev. Anthropol. 31, 323–338. ( 10.1146/annurev.anthro.31.040402.085403) [DOI] [Google Scholar]
- 13.Dunsworth HM. 2010. Origin of the genus Homo. Evol. Edu. Outreach 3, 353–366. ( 10.1007/s12052-010-0247-8) [DOI] [Google Scholar]
- 14.deMenocal PB. 2011. Climate and human evolution. Science 331, 540–541. ( 10.1126/science.1190683) [DOI] [PubMed] [Google Scholar]
- 15.Antón SC, Josh Snodgrass J. 2012. Origins and evolution of genus Homo: new perspectives. Curr. Anthropol. 53, S496–S479. ( 10.1086/667692) [DOI] [Google Scholar]
- 16.Tooby J, DeVore I. 1987. The reconstruction of hominid behavioral evolution through strategic modeling. In The evolution of human behavior: primate models (ed. Kinzey W.), pp. 183–237. Albany, NY: SUNY Press. [Google Scholar]
- 17.Simpson GG. 1961. The principles of animal taxonomy. New York, NY: Columbia University Press. [DOI] [PubMed] [Google Scholar]
- 18.Wiley EO. 1981. Phylogenetics: the theory and practice of phylogenetic systematics. New York, NY: Wiley Interscience. [Google Scholar]
- 19.Mayr E, Linsley EG, Usinger RL. 1953. Methods and principles of systematic zoology. New York, NY: McGraw Hill. [Google Scholar]
- 20.Mayr E. 1951. Taxonomic categories in fossil hominids. Cold Spring Harbor Symp. Quant. Biol. 15, 109–118. ( 10.1101/SQB.1950.015.01.013) [DOI] [PubMed] [Google Scholar]
- 21.Simpson GG. 1944. Tempo and mode in evolution. New York, NY: Columbia University Press. [Google Scholar]
- 22.Simpson GG. 1963. The meaning of taxonomic statements. In Classification and human evolution (ed. Washburn SL.), pp. 1–31. Chicago, IL: Aldine. [Google Scholar]
- 23.de Queiroz K, Gauthier J. 1992. Phylogenetic taxonomy. Annu. Rev. Ecol. Syst. 23, 449–480. ( 10.1146/annurev.es.23.110192.002313) [DOI] [Google Scholar]
- 24.Wood BA, Collard M. 1999. The human genus. Science 284, 65–71. ( 10.1126/science.284.5411.65) [DOI] [PubMed] [Google Scholar]
- 25.Dean MC, Smith BH. 2009. Growth and development of the Nariokotome Youth, KNM-WT 15000. In The first humans–origins and early evolution of the genus Homo (eds Grine FE, Fleagle JG, Leakey RE), pp. 101–120. Berlin, Germany: Springer. [Google Scholar]
- 26.Rightmire GP, Van Arsdale AP, Lordkipanidze D. 2008. Variation in the mandibles from Dmanisi, Georgia. J. Hum. Evol. 54, 904–908. ( 10.1016/j.jhevol.2008.02.003) [DOI] [PubMed] [Google Scholar]
- 27.Plavcan JM. 2012. Body size, size variation, and sexual size dimorphism in early Homo. Curr. Anthropol. 53, S409–S423. ( 10.1086/667605) [DOI] [Google Scholar]
- 28.Larson SG, Jungers WL, Morwood MJ, Sutikna T, Jatmiko T, Saptomo EW, Due RA, Djubiantono T. 2007. Homo floresiensis and the evolution of the human shoulder. J. Hum. Evol. 53, 718–731. ( 10.1016/j.jhevol.2007.06.003) [DOI] [PubMed] [Google Scholar]
- 29.Lordkipanidze D, et al. 2007. Postcranial evidence from early Homo from Dmanisi, Georgia. Nature 449, 305–310. ( 10.1038/nature06134) [DOI] [PubMed] [Google Scholar]
- 30.Simpson SW, Quade J, Levin NE, Butler R, Dupont-Nivet G, Everett M, Semaw S. 2008. A female Homo erectus pelvis from Gona, Ethiopia. Science 322, 1089–1092. ( 10.1126/science.1163592) [DOI] [PubMed] [Google Scholar]
- 31.Spoor F, Leakey MG, Gathogo PN, Brown FH, Antón SC, McDougall I, Kiarie C, Manthi FK, Leakey LN. 2007. Implications of new early Homo fossils from Ileret, east of Lake Turkana, Kenya. Nature 448, 688–691. ( 10.1038/nature05986) [DOI] [PubMed] [Google Scholar]
- 32.Lordkipanidze D, Ponce de León MS, Margvelashvili A, Rak Y, Rightmire GP, Vekua A, Zollikofer CPE. 2013. A complete skull from Dmanisi, Georgia, and the evolutionary biology of early Homo. Nature 342, 326–331. ( 10.1126/science.1238484) [DOI] [PubMed] [Google Scholar]
- 33.Mgeladze A, Lordkipanidze D, Moncel M-H, Despriee J, Chagelishvili R, Nioradze M, Nioradze G. 2011. Hominin occupations at the Dmanisi site, Georgia, southern Caucasus: raw materials and technical behaviours of Europe's first hominins. J. Hum. Evol. 60, 571–596. ( 10.1016/j.jhevol.2010.10.008) [DOI] [PubMed] [Google Scholar]
- 34.Wood B, Baker J. 2011. Evolution in the genus Homo. Annu. Rev. Ecol. Evol. Syst. 42, 47–69. ( 10.1146/annurev-ecolsys-102209-144653) [DOI] [Google Scholar]
- 35.Schwartz JH, Tattersall I. 2015. Defining the genus Homo. Science 349, 931–932. ( 10.1126/science.aac6182) [DOI] [PubMed] [Google Scholar]
- 36.Simpson GG. 1945. The principles of classification and a classification of the mammals. Bull. Am. Mus Nat. Hist. 85, 1–350. [Google Scholar]
- 37.Le Gros Clark WE. 1955. The fossil evidence for human evolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 38.Robinson JT. 1961. The australopithecines and their bearing on the origin of man and of stone tool-making. S. Afr. J. Sci. 57, 3–13. [Google Scholar]
- 39.Pilbeam DR, Simons EL. 1965. Some problems of hominid classification. Am. Sci. 53, 237–259. [PubMed] [Google Scholar]
- 40.Tobias PV. 1965. Early man in East Africa. Science 149, 22–33. ( 10.1126/science.149.3679.22) [DOI] [PubMed] [Google Scholar]
- 41.Leakey LSB, Tobias PV, Napier JR. 1964. A new species of the genus Homo from Olduvai Gorge. Nature 202, 7–9. ( 10.1038/202007a0) [DOI] [PubMed] [Google Scholar]
- 42.Tobias PV. 1991. Olduvai Gorge, volume 4: The skulls, endocasts and teeth of Homo habilis. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 43.Oakley KP. 1959. Man the tool-maker. Chicago, IL: University of Chicago Press. [Google Scholar]
- 44.Leakey LSB. 1959. A new fossil skull from Olduvai. Nature 184, 491–493. ( 10.1038/184491a0)13850382 [DOI] [Google Scholar]
- 45.Montagu MFA. 1961. The ‘cerebral rubicon’: brain size and the achievement of hominid status. Am. Anthropol. 63, 377–378. ( 10.1525/aa.1961.63.2.02a00100) [DOI] [Google Scholar]
- 46.Goodall J. 1964. Tool-using and aimed throwing in a community of free-living chimpanzees. Nature 201, 1264–1266. ( 10.1038/2011264a0) [DOI] [PubMed] [Google Scholar]
- 47.Goodall J. 1998. Learning from the chimpanzees: a message humans can understand. Science 282, 2185 ( 10.1126/science.282.5397.2184) [DOI] [Google Scholar]
- 48.Washburn SL, Patterson B. 1951. Evolutionary importance of the South African ‘man-apes’. Nature 167, 650–651. ( 10.1038/167650a0) [DOI] [PubMed] [Google Scholar]
- 49.Leakey REF. 1973. Evidence for an advanced Plio-Pleistocene hominid from East Rudolf, Kenya. Nature 242, 447–450. ( 10.1038/242447a0) [DOI] [PubMed] [Google Scholar]
- 50.Walker AC, Leakey REF. 1978. The hominids of East Turkana. Sci. Am. 239, 54–66. ( 10.1038/scientificamerican0878-54) [DOI] [PubMed] [Google Scholar]
- 51.Isaac GL, Leakey REF, Behrensmeyer AK. 1971. Archeological traces of early hominid activities, east of Lake Rudolf, Kenya. Science 173, 1129–1134. ( 10.1126/science.173.4002.1129) [DOI] [PubMed] [Google Scholar]
- 52.Isaac GL. 1978. Food sharing and human evolution: archaeological evidence from the Plio-Pleistocene of East Africa. J. Anthropol. Res. 34, 311–325. [Google Scholar]
- 53.Leakey REF, Lewin R. 1981. The making of mankind. New York, NY: Penguin. [Google Scholar]
- 54.Binford LR. 1985. Human ancestors: changing views of their behavior. J. Anthropol. Arch. 4, 292–327. ( 10.1016/0278-4165(85)90009-1) [DOI] [Google Scholar]
- 55.Eldredge N, Tattersall I. 1975. Evolutionary models, phylogenetic reconstruction, and another look at hominid phylogeny. In approaches to primate paleobiology (ed. Szalay FS.), pp. 218–242. Basel, Switzerland: Karger. [PubMed] [Google Scholar]
- 56.Tattersall I, Eldredge N. 1977. Fact, theory, and fantasy in human paleontology. Am. Sci. 65, 204–211. [PubMed] [Google Scholar]
- 57.Wood BA. 1992. Origin and evolution of the genus Homo. Nature 355, 783–790. ( 10.1038/355783a0) [DOI] [PubMed] [Google Scholar]
- 58.Wood BA, Li Y, Willoughby C. 1991. Intraspecific variation and sexual dimorphism in cranial and dental variables among higher primates and their bearing on the hominid fossil record. J. Anat. 174, 185–205. [PMC free article] [PubMed] [Google Scholar]
- 59.Alexeev VP. 1986. The origin of the human race. USSR: Progress Publishers. [Google Scholar]
- 60.Rightmire GP. 1990. The evolution of Homo erectus. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 61.Wood BA. 1991. Koobi fora research project, volume 4: hominid cranial remains. Oxford, UK: Oxford University Press. [Google Scholar]
- 62.Lepre CJ, Kent DV. 2010. New magnetostratigraphy for the Olduvai Subchron in the Koobi Fora formation, northwest Kenya, with implications for early Homo. Earth Planet. Sci. Lett. 290, 362–374. ( 10.1016/j.epsl.2009.12.032) [DOI] [Google Scholar]
- 63.McDougall I, Brown FH, Vasconselos PM, Cohen BE, Thiede DS, Buchanan MJ. 2012. New single crystal 40Ar/39Ar ages improve time scale for the deposition of the Omo Group, Omo-Turkana Basin, East Africa. J. Geol. Soc. Lond. 169, 213–226. ( 10.1144/0016-76492010-188) [DOI] [Google Scholar]
- 64.Feibel CS, Lepre CJ, Quinn RL. 2009. Stratigraphy, correlation and age estimates for fossils from Area 123, Koobi Fora. J. Hum. Evol. 57, 112–122. ( 10.1016/j.jhevol.2009.05.007) [DOI] [PubMed] [Google Scholar]
- 65.Joordens JCA, et al. 2013. Improved age control on early Homo fossils from the upper Burgi Member at Koobi Fora, Kenya. J. Hum. Evol. 65, 731–745. ( 10.1016/j.jhevol.2013.09.002) [DOI] [PubMed] [Google Scholar]
- 66.Leakey MG, Spoor F, Dean MC, Feibel CS, Antón SC, Kiarie C, Leakey LN. 2012. New fossils from Koobi Fora in northwestern Kenya confirm taxonomic diversity in early Homo. Nature 488, 201–204. ( 10.1038/nature11322) [DOI] [PubMed] [Google Scholar]
- 67.Feibel CS, Brown FH, McDougall I. 1989. Stratigraphic context of fossil hominids from the Omo group deposits: northern Turkana Basin, Kenya and Ethiopia. Am. J. Phys. Anthropol. 78, 595–622. ( 10.1002/ajpa.1330780412) [DOI] [PubMed] [Google Scholar]
- 68.Gathogo PN, Brown FH. 2006. Revised stratigraphy of Area 123, Koobi Fora, Kenya, and new age estimates of its fossil mammals, including hominins. J. Hum. Evol. 51, 471–479. ( 10.1016/j.jhevol.2006.05.005) [DOI] [PubMed] [Google Scholar]
- 69.Ward CV, Feibel CS, Hammond AS, Leakey LN, Moffett EA, Plavcan JM, Skinner MM, Spoor F, Leakey MG. 2015. Associated ilium and femur from Koobi Fora, Kenya, and postcranial diversity in early Homo. J. Hum. Evol. 81, 48–67. ( 10.1016/j.jhevol.2015.01.005) [DOI] [PubMed] [Google Scholar]
- 70.Domínguez-Rodrigo M, et al. 2013. First partial skeleton of a 1.34-million-year-old Paranthropus boisei from Bed II, Olduvai Gorge, Tanzania. PLOS ONE 8, e80347 ( 10.1371/journal.pone.0080347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holliday TW. 2012. Body size, body shape and the circumscription of the genus Homo. Curr. Anthropol. 53, S330–S345. ( 10.1086/667360) [DOI] [Google Scholar]
- 72.Pontzer H. 2012. Ecological energetics in early Homo. Curr. Anthropol. 53, S346–S358. ( 10.1086/667402) [DOI] [Google Scholar]
- 73.Deaner RO, Isler K, Burkart J, van Schaik C. 2007. Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav. Evol. 70, 115–124. ( 10.1159/000102973) [DOI] [PubMed] [Google Scholar]
- 74.Herculano-Houzel S. 2009. The human brain in numbers: a linearly scaled-up primate brain. Front. Neurosci. 3, 1–11. ( 10.3389/neuro.09.031.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holloway RL, Broadfield DC, Yuan MS. 2004. The human fossil record, volume 3: brain endocasts–the paleoneurological evidence. Hoboken, NJ: Wiley-Liss. [Google Scholar]
- 76.Kimbel WH, Johanson DC, Rak Y. 1997. Systematic assessment of a maxilla of Homo from Hadar, Ethiopia. Am. J. Phys. Anthropol. 103, 235–262. ( 10.1002/(SICI)1096-8644(199706)103:2%3C235::AID-AJPA8%3E3.0.CO;2-S) [DOI] [PubMed] [Google Scholar]
- 77.Isler K, Christopher Kirk E, Miller JMA, Albrecht GA, Gelvin BR, Martin RD. 2008. Endocranial volumes of primate species: scaling analyses using a comprehensive and reliable data set. J. Hum. Evol. 55, 967–978. ( 10.1016/j.jhevol.2008.08.004) [DOI] [PubMed] [Google Scholar]
- 78.Strait D, Grine FE, Fleagle JG. 2015. Analyzing hominin phylogeny: cladistic approach. In Handbook of paleoanthropology, 2nd edn (eds Henke W, Tattersall I), pp. 1990–2014. Berlin, Germany: Springer. [Google Scholar]
- 79.Strait DS, Grine FE, Moniz MA. 1997. A reappraisal of early hominid phylogeny. J. Hum. Evol. 32, 17–82. ( 10.1006/jhev.1996.0097) [DOI] [PubMed] [Google Scholar]
- 80.Strait DS, Grine FE. 2004. Inferring hominoid and early hominin phylogeny using craniodental characters: the role of fossil taxa. J. Hum. Evol. 47, 399–452. ( 10.1016/j.jhevol.2004.08.008) [DOI] [PubMed] [Google Scholar]
- 81.Prat S, et al. 2005. First occurrence of early Homo in the Nachukui formation (West Turkana, Kenya) at 2.3–2.4 myr. J. Hum. Evol. 49, 230–240. ( 10.1016/j.jhevol.2005.03.009) [DOI] [PubMed] [Google Scholar]
- 82.Bromage TG, Schrenk F, Zonneveld FW. 1995. Paleoanthropology of the Malawi Rift: an early hominid mandible from the Chiwondo Beds, northern Malawi. J. Hum. Evol. 28, 71–108. ( 10.1006/jhev.1995.1007) [DOI] [Google Scholar]
- 83.Asfaw B, White TD, Lovejoy CO, Latimer BM, Simpson S, Suwa G. 1999. Australopithecus garhi: a new species of early hominid from Ethiopia. Science 284, 629–635. ( 10.1126/science.284.5414.629) [DOI] [PubMed] [Google Scholar]
- 84.Suwa G. 1990. A comparative analysis of hominid dental remains from the Shungura and Usno Formations, Omo Valley, Ethiopia. Dissertation, University of California, Berkeley.
- 85.Suwa G, White TD, Howell FC. 1996. Mandibular postcanine dentition from the Shungura Formation, Ethiopia: crown morphology, taxonomic allocations, and Plio-Pleistocene hominid evolution. Am. J. Phys. Anthropol. 101, 247–282. ( 10.1002/(SICI)1096-8644(199610)101:2%3C247::AID-AJPA9%3E3.0.CO;2-Z) [DOI] [PubMed] [Google Scholar]
- 86.Hill A, Ward S, Deino A, Curtis G, Drake R. 1992. Earliest Homo. Nature 355, 719–722. ( 10.1038/355719a0) [DOI] [PubMed] [Google Scholar]
- 87.Sherwood RJ, Ward SC, Hill A. 2002. The taxonomic status of the Chemeron temporal (KNM-BC 1). J. Hum. Evol. 42, 153–184. ( 10.1006/jhev.2000.0409) [DOI] [PubMed] [Google Scholar]
- 88.Lockwood CA, Kimbel WH, Lynch JM. 2005. Variation in early hominin temporal bone morphology and its implications for species diversity. Trans Roy. Soc. S. Afr. 60, 73–77. ( 10.1080/00359190509520480) [DOI] [Google Scholar]
- 89.Grine FE. 2013. The alpha taxonomy of Australopithecus africanus. In The paleobiology of Australopithecus (eds Reed KE, Fleagle JG, Leakey RE), pp. 73–104. Dordrecht, The Neherlands: Springer. [Google Scholar]
- 90.Berger L, de Ruiter DJ, Churchill SE, Schmid P, Carlson KJ, Dirks PHGM, Kibii JM. 2010. Australopithecus sediba: a new species of Homo-like austrlopith from South Africa. Science 328, 195–204. ( 10.1126/science.1184944) [DOI] [PubMed] [Google Scholar]
- 91.Irish JD, Guatelli-Steinberg D, Legge SS, de Ruiter DJ, Berger LR. 2013. Dental morphology and the phylogenetic ‘place’ of Australopithecus sediba. Science 340, 1233062-1-4. ( 10.1126/science.1233062) [DOI] [PubMed] [Google Scholar]
- 92.Kimbel WH. 2015. The species and diversity of australopiths. In Handbook of paleoanthropology, 2nd edn (eds Henke W, Tattersall I), pp. 2072–2104. Berlin, Germany: Springer. [Google Scholar]
- 93.Prang TC. 2016. The subtalar joint complex in Australopithecus sediba. J. Hum. Evol. 90, 105–119. ( 10.1016/j.jhevol.2015.10.009) [DOI] [PubMed] [Google Scholar]
- 94.Cartmill M. 2012. Primate origins, human origins, and the end of higher taxa. Evol. Anthropol. 21, 208–220. ( 10.1002/evan.21324) [DOI] [PubMed] [Google Scholar]
- 95.McPherron SP, Alemseged Z, Marean CW, Wynn JG, Reed D, Geraads D, Bobe R, Béarat HA. 2010. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature 466, 857–860. ( 10.1038/nature09248) [DOI] [PubMed] [Google Scholar]
- 96.Harmand S, et al. 2015. 3.3-million-year-old stone tools from Lomekwi 3, West Turkana, Kenya. Nature 521, 310–316. ( 10.1038/nature14464) [DOI] [PubMed] [Google Scholar]
- 97.Alba DM, Moyà-Solà S, Köhler M. 2003. Morphological affinities of the Australopithecus afarensis hand on the basis of manual proportions and relative thumb length. J. Hum. Evol. 44, 225–254. ( 10.1016/S0047-2484(02)00207-5) [DOI] [PubMed] [Google Scholar]
- 98.Tocheri MW, Orr CM, Jacofsky M, Marzke MW. 2008. The evolutionary history of the hominin hand since the last common ancestor of Pan and Homo. J. Anat. 212, 544–562. ( 10.1111/j.1469-7580.2008.00865.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sponheimer M, et al. 2013. Isotopic evidence of early hominin diets. Proc. Natl Acad. Sci. USA 110, 10 513–10 518. ( 10.1073/pnas.1222579110) [DOI] [Google Scholar]
- 100.Grabowski M, Kjetil LV, Hansen TF. 2016. Evolutionary modeling and correcting for observation error support a 3/5 brain-body allometry for primates. J. Hum. Evol. 94, 106–116. ( 10.1016/j.jhevol.2016.03.001) [DOI] [PubMed] [Google Scholar]