Abstract
The effects from multigenerational exposures to engineered nanoparticles (ENPs) in their pristine and transformed states are currently unknown despite such exposures being an increasingly common scenario in natural environments. Here, we examine how exposure over 10 generations affects the sensitivity of the nematode Caenorhabditis elegans to pristine and sulfidized Ag ENPs and AgNO3. We also include populations that were initially exposed over six generations but kept unexposed for subsequent four generations to allow recovery from exposure. Toxicity of the different silver forms decreased in the order AgNO3, Ag ENPs and Ag2S ENPs. Continuous exposure to Ag ENPs and AgNO3 caused pronounced sensitization (approx. 10-fold) in the F2 generation, which was sustained until F10. This sensitization was less pronounced for Ag2S ENP exposures, indicating different toxicity mechanisms. Subtle changes in size and lifespan were also measured. In the recovery populations, the sensitivity to Ag ENPs and AgNO3 resulting from the initial multigenerational exposure persisted. Their response sensitivity for all endpoints was most closely related to the last ancestral exposed generation (F5), rather than unexposed controls. The mechanisms of transgenerational transfer of sensitivity are probably organized through the epigenome, and we encourage others to investigate such effects as a priority for mechanistic toxicology.
Keywords: multigenerational exposure, silver, silver sulfide, nanotoxicology, transgenerational effects, epigenetics
1. Introduction
Assessments of the environmental impacts of engineered nanoparticles (ENPs) generally rely on the use of short-term laboratory tests to provide information on the toxicity of the ‘as-produced’ (pristine) materials. This focus lies counter to what is currently known about the likely nature of environmental ENP exposures, as these will often be to ENPs that have undergone transformation processes and for extended time (e.g. over multiple generations).
Common environmental transformations of metal ENPs include oxidation reactions (e.g. from Ag0 to Ag+, and thereafter to complexed Ag(I) species [1,2]). As a class B (soft) metal cation, Ag is particularly susceptible to sulfidation [3–5]. For example, Ag ENPs are completely transformed to Ag2S during wastewater treatment [5–7]. These processes can alter not only the particle surface, but also speciation of the particle core, with effects on environmental behaviour and altered toxicity compared with pristine materials [2,8]. For instance, dissolution can increase toxic potential through the release of toxic ions [9], while chemical speciation changes, such as sulfidation, have been shown to reduce toxicity possibly by suppressing ion release and reducing uptake of intact particles [10–12]. What is currently not known is how these changes in exposure form relate to long-term environmental effects of ENPs (e.g. over multiple generations).
To date, only a limited number of multigenerational exposure studies for ENPs are available, and so far these have considered only pristine materials. In one such study, Völker et al. [13] found a complex pattern of sensitivity in three Daphnia species exposed to Ag ENPs over five generations. Some evidence of greater tolerance in the later generations was found at lower test concentrations; however, this effect was inconsistent and there was some evidence of increased sensitivity at the higher long-term exposure concentrations. For C. elegans exposed to CdSe and CdSe/ZnS quantum dots and CdSO4 over four exposed generations, Contreras et al. [14] found a consistent sensitivity of individual life-cycle traits, fitness and locomotion across all tested generations. Sixteen-generation studies on the effect of uranium to C. elegans found adaptation to exposure conditions for both exposed and control populations. Observed effects on fecundity were consistent between population treatments, whereas in one of the studies effects on body length showed differential evolution over the exposure duration once maternal effects diminished [15,16].
In some ecosystems (e.g. lotic freshwaters) and when pollution is spatially heterogeneous (e.g. soil), species may experience the potential to recover from exposures. The extent of such recovery may affect the way that species respond to future challenges. In C. elegans, adaptation was found to be dependent on the type of pollutant and the persistence of its exposure [17]. Even when exposure is removed for more than a generation, maternal contaminant transfer or potentially epigenetic changes (transgenerational inheritance) could influence the responses of unexposed offspring. Tests of generational recovery from ENP exposure have been conducted. For example, Daphnia magna regained full reproductive output and lifespan in the first unexposed generation for a majority of tested carbon nanomaterials [18]. In C. elegans, exposure to Au ENPs in parents led to increased reproductive tract malformations and egg production failure in unexposed F2 offspring, but not the previous or subsequent generations [19]. These studies highlight the possibility for the generational transfer of effects through unknown mechanisms.
To provide a comprehensive analysis of multigenerational exposure effects, including recovery, we here conduct a continuous 10-generation exposure of the nematode C. elegans to both pristine and sulfidized (‘aged’) Ag ENPs, as well as to ionic Ag as a positive control mimicking full dissolution. Recovery is assessed by transferring nematodes exposed for six generations to clean media for a further four generations before re-exposure. Our aim was to assess how sensitivity was affected by multigenerational exposure and to confirm that any such changes in sensitivity were lost when the continuous exposure was removed.
2. Material and methods
(a). Particle characterization
The polyvinylpyrrolidone (PVP)-coated Ag ENPs (Ag-PVP) and sulfidized Ag2S ENPs were synthesized and supplied as described by Starnes et al. [11] (for synthesis details, see the electronic supplementary material). Transmission electron microscopy (TEM) primary particle sizes were reported to be 58.3 ± 12.9 nm for Ag-PVP and 64.5 ± 19.4 nm for Ag2S [11]. Energy-dispersive X-ray spectroscopy of Ag to S ratios (10 : 1) in the Ag2S ENPs indicated incomplete sulfidation. Ionic Ag as AgNO3 was purchased from Sigma Aldrich Chemicals (Poole, UK).
To determine the ENP stability in the simulated soil pore water (SSPW) exposure medium [20], particle hydrodynamic diameter was characterized over 96 h at 24 h intervals for 10 mg Ag l−1 dispersions in triplicate using Nano Tracking Analysis (NanoSight NS500, Malvern Instruments, Malvern, UK). Data were analysed using NTA v. 2.3. Electrophoretic mobility was determined by phase analysis light scattering using the Zetasizer NanoZS. Zeta potential was estimated from electrophoretic mobility using the Smolokowski model. The 96 h period was chosen as it was the maximum duration of exposure before media renewal, while 10 mg Ag l−1 lay within the tested concentration range for both particle types and above detection limit over the test duration for both techniques. Measurements were conducted in the test media without the bacterial food source Escherichia coli strain OP50 to avoid scattering interferences. Samples of both the pristine and Ag2S ENPs were prepared for TEM analysis to establish Ag : S ratios and primary particle diameter in samples by drying one drop of dispersion solution on a TEM grid for 1 h followed by examination on a JEOL 2010 analytical TEM equipped with an Oxford Instruments LZ5 windowless energy-dispersive X-ray spectrometer.
Actual exposure concentrations were validated in 96 randomly chosen samples (10% of the total number). Particle dissolution after 96 h exposure at concentrations of Ag-PVP = 1.5 mg Ag l−1 and Ag2S = 15 mg Ag l−1 was determined in triplicate by ultrafiltration with 3 kDa ultrafiltration devices (Amicon, Millipore) after pre-conditioning of the membranes with 0.1 M Cu(SO4)2 · 5H2O according to Diez-Ortiz et al. [21]. Additionally, the recovery of AgNO3 = 0.10 mg Ag l−1 after ultrafiltration was measured. Ag concentration of both sample sets was determined by atomic absorbance spectroscopy (Perkin Elmer 1100B) after acidification with aqua regia.
(b). Nematode exposures
Caenorhabditis elegans (N2 Bristol strain) obtained from the C. elegans Genetics Center (University of Minnesota, USA) were initially maintained at 20°C in the dark on nematode growth medium agar plates and fed a uracil-deficient E. coli strain OP50 [22]. To start the multigenerational exposures, initial populations were established on SSPW agar plates, with 17 g bacteriological agar and 2.5 g bacteriological peptone per litre of SSPW, with 1 ml cholesterol (5 mg ml−1 EtOH) added after autoclaving. Large numbers of eggs were obtained from these populations through NaClO egg preparation [23] and immediately exposed to single concentrations of AgNO3, Ag-PVP or Ag2S ENPs (AgNO3 = 0.1 mg Ag l−1, Ag-PVP = 1.5 mg Ag l−1 and Ag2S = 15 mg Ag l−1). Selected concentrations corresponded to the EC30 values for reproduction for each Ag form [11,20]; the actual effect level was assessed in a reproductive toxicity test with the parent generation (see below). Continuous exposures of these concentrations were conducted in 9 cm Petri plates containing 4 ml SSPW exposure medium with OP50 at O.D. 0.35 on 10 ml SSPW agar.
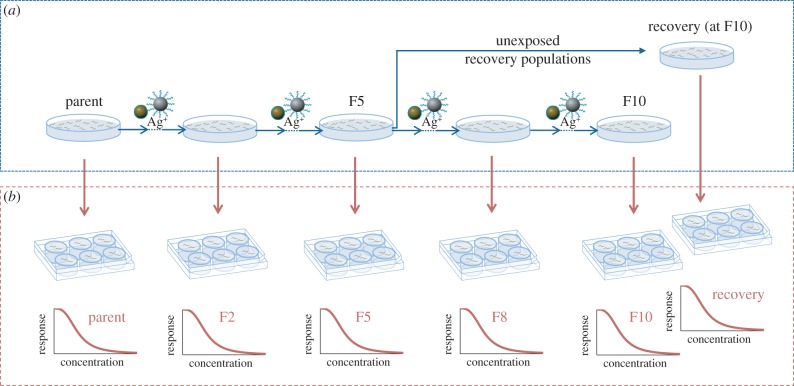
After 96 h, the next generation of eggs was isolated through NaClO bleaching egg preparation and transferred to respective freshly prepared exposure media. This procedure was repeated for each generation. After the F5 generation, each population was split and half of the remaining individuals were further exposed until the F10 generation was reached. The other half of the individuals was maintained in clean medium for four further generations to assess the potential for recovery after cessation of exposure and subsequent re-exposure in offspring generation 10 (figure 1). Throughout the entire test, unexposed control populations were reared through generations as for the exposed lines. Life-cycle traits of individuals in these reference control populations were assessed at the same intervals as the treated populations. Throughout the study, two types of control were used and different abbreviations have been assigned to identify these in the analysis: (i) continuously unexposed reference populations to account for culturing effects, referred to as unexposed reference populations ‘UnExp-Reference’; and (ii) individuals taken from continuously exposed ancestral generations that are transferred into clean medium and served as control within the toxicity test conducted for each phenotyped generation, referred to as multigenerational exposed control population ‘MGExp-Control’.
Figure 1.
Experimental design for multigenerational study. (a) Continuous exposures were carried out in clean medium (UnExp-Reference) and at AgNO3 = 0.10 mg Ag l−1, Ag-PVP = 1.5 mg Ag l−1 and Ag2S = 15 mg Ag l−1. Recovery populations were transferred to clean medium after exposure until generation F5. (b) Effect of exposure to different concentrations of the respective Ag treatment and in clean medium (MGExp-control) on reproduction, lifespan and size was tested at parent (P), F2, F5, F8, F10 offspring generation and for populations unexposed after F5 until F10 (recovery generation). (Online version in colour.)
(i). Toxicity test to measure life-cycle traits
Toxicity bioassays were conducted for the parental (P) and offspring generations F2, F5, F8 and F10, and the recovery populations at offspring generation 10 (R). For this test, a subset of eggs was exposed in six-well plates (1 ml SSPW on 2 ml SSPW agar, E. coli strain OP50 O.D. 0.35, 20°C in constant dark) to concentration ranges of 0.05–1.52 mg Ag l−1 AgNO3, 0.75–24 mg Ag l−1 Ag-PVP and 7.5–240 mg Ag l−1 Ag2S. The concentration ranges were adjusted in the course of the assay to account for increases in sensitivity at later generations (AgNO3, Ag-PVP for F10 and Ag2S for F5–F10, R) by dropping the highest concentrations and adding another twofold dilution of the lowest concentration.
Initial exposures were conducted for a cohort of L1 juveniles for each biological replicate population in bulk for 24 h. This initial bulk exposure limited loss of replicates due to mechanical injury of the fragile eggs during the distribution and allowed for transfer of only viable juveniles for brood size assessment. After 24 h exposure, two individuals per replicate population were randomly selected and transferred to the corresponding Ag treatment concentrations in one well of a six-well plate (five replicates per test condition). Thereafter at 48 h intervals, adults were transferred into fresh medium to ensure constant exposure conditions. After adults were removed, eggs and hatched juveniles were counted. Reproductive toxicity was measured as decrease in the total number of offspring produced per nematode compared with the respective control (MGExp-control), as average between the two individuals in each well. Lifespan of the 10 individuals per treatment was assessed by recording the mortality for each individual daily.
To determine the effect of the six tested Ag concentrations per material on growth, 10–20 individuals were taken from each of the five replicate bulk exposures per concentration 48 h after egg preparation. These nematodes were killed/preserved in 5 µl 10% (w/v) sodium azide and photographed using an EVOS core XL photo microscope. The area and length of five individuals were measured per replicate (i.e. a total of 25 individuals per concentration) with Image-Pro Express v. 4.5 (Media cybernetics, Rockville, MD, USA) and their volumetric length (cubic root of body volume) was calculated.
(c). Statistical analysis
As each Ag form was shown to have greatly differing toxicity, analysis was carried out separately for each material. Results of the reproductive toxicity tests were analysed for concentration–response relationships in Sigmaplot v. 12.0 (Systat Software, San Jose, CA) and fitted a nonlinear three parameter logistic regression estimating upper asymptote, EC50 and slope parameters for each of the generations and Ag treatments. Responses were compared across generations using the F-test to define F- and p-values for the difference between concentration–response curves [24]. No regression curves could be fitted to lifespan and body size data. Hence, analysis was conducted by two-way analysis of variance (ANOVA) using general linear models (GLM) in Minitab v. 16, with ‘exposure generation’ and ‘tested Ag concentration’ as fixed factors and ‘generation × tested Ag concentration’ as the interaction term. Where significant treatment differences were found, Tukey's pairwise comparisons were used to identify significant differences between generations and conditions. Assessment using Kolmogorov–Smirnov and Leven tests showed some statistically significant deviations from assumptions of normality and homoscedasticity. As these had the potential to affect the validity of the GLM results, we further conducted non-parametric tests to validate findings. In all cases, observation of significance was in full agreement. Thus for simplicity we refer to GLM results. Results of all statistical tests are reported in the electronic supplementary material.
3. Results
(a). Exposure validation and characterization
Ag concentrations in the measured 10% of all test solutions showed close agreement to stated nominal concentrations for both AgNO3: 93.1 ± 2.5% and Ag2S: 90.8 ± 3.4%. Given this agreement, all treatments and calculated values are for simplicity hereafter given with reference to their stated nominal concentrations. Ag concentrations for Ag-PVP exposures were only 60.2 ± 4.2% of nominals across all measured samples; they were therefore recalculated using the average recovered percentage and these values were used for subsequent analyses.
NTA analysis of Ag-PVP showed the number averaged hydrodynamic diameter (79 nm) of the dispersed ENPs immediately after addition to the SSPW not to be significantly different to that of the primary ENPs (60 nm). The Ag2S ENPs immediately aggregated (hereafter referred to as clustered) from 85 nm in the primary particle suspension to 243 nm after addition to the medium. Over the 96 h exposure, a slight increase in the cluster size of the dispersed ENPs was observed and only minor change to the zeta potential (electronic supplementary material, table S1). NTA showed sizes for Ag-PVP ranging from 79 nm at the start of the exposure to a maximum hydrodynamic diameter of 105 nm after 72 h. Analysis of the Ag2S ENPs showed a temporal increase in mean hydrodynamic diameter from 243 nm at the start of the exposure to 298 nm at 48 h followed by a decrease to 213 nm at 96 h. Zeta potential of both ENPs in the test media shifted from −2 to −5 mV over the test duration indicating an unstable suspension compared with the stock zeta potential (Ag-PVP −11.6 mV, Ag2S −19.2 mV).
Measurements of dissolution rate over 96 h showed 1.5 ± 0.1% dissolution of Ag-PVP and 0.023 ± 0.002% of Ag2S. A separate analysis of the level of AgNO3 from test solutions indicated a recovery of only 72.9 ± 0.4% after ultrafiltration. This suggests some loss of dissolved Ag species to the filter membrane potentially due to incomplete pre-conditioning of the membrane with Cu [25] or at other points in the sample preparation.
(b). Nematode life-cycle trait response to exposure
(i). Reproductive toxicity: parental generation
Reproductive output of the parental (P) generation was decreasing in a concentration dependent manner with increasing concentration. Ag exposure significantly (p < 0.05) reduced reproduction compared with unexposed controls for AgNO3 concentrations above 0.30 mg Ag l−1, Ag-PVP concentrations above 9.6 mg Ag l−1 and Ag2S concentrations above 15 mg Ag l−1 (electronic supplementary material, figure S1). Reproduction EC50 values (±s.e.) were ordered ionic Ag > pristine Ag-PVP > sulfidized Ag2S, being 0.23 ± 0.07 mg Ag l−1 for AgNO3, 4.22 ± 1.43 mg Ag l−1 for Ag-PVP and 12.02 ± 3.05 mg Ag l−1 for Ag2S, respectively (p < 0.05). The concentrations used for continuous exposures corresponded to an EC35 value for AgNO3, an EC25 for Ag-PVP and an EC56 for Ag2S in the parent generation instead of the anticipated EC30 (which were 0.07 mg Ag l−1, 1.32 mg Ag l−1 and 6.0 mg Ag l−1, respectively). Hence, there were slight differences in the initial toxic pressure among the Ag forms.
(ii). Multigenerational exposure
Continuous multigenerational exposure to Ag (ionic and particulate) gradually increased time to first egg laying. By generation F9, the egg-laying period before age synchronization had to be extended from 96 to 120 h in the silver-exposed populations to produce sufficient offspring for toxicity testing in F10. This delay was not seen in the reference population (UnExp-Reference) nor in any of the recovery populations, and gave an important indication of the impact of multigenerational exposure on a life-cycle trait (time to reproduction) not measured in the short-term toxicity bioassay. Further, one of the five Ag2S-exposed populations stopped reproducing entirely at F9 and therefore could not be included in further testing, reducing replicate in the Ag2S F10 test to four biological replicates.
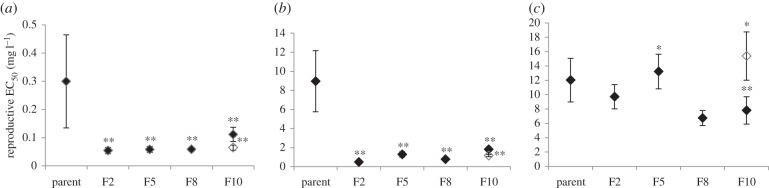
No difference in number of offspring was found between UnExp-reference and the nematodes from MGExp-control in the short-term bioassays for each of the F2, F5, F8 and F10 generations (GLM: treatment F3,94 = 0.97 p = 0.412, generation F5,94 = 4.36 p = 0.001, interaction treatment × generation F15,94 = 1.01 p = 0.370; electronic supplementary material, figure S3). There was a slight initial increase from the parent to the offspring generations that was similar for UnExp-reference and MGExp-control (no significant difference for ‘treatment’ or the ‘interaction element’), potentially caused by adaptation to the experimental conditions, yet as it was stable thereafter it was deemed biologically insignificant. In all generations exposed to all Ag forms, a significant concentration-dependent decrease for reproduction was found. Comparison of reproductive toxicity concentration–response relationships between generations (F- and p-values in electronic supplementary material, table S2) revealed a significant increase of sensitivity compared with P generation in the F2, and that remained stable over all subsequently tested offspring generations for both AgNO3 (figure 2a; electronic supplementary material, figure S3a) and Ag-PVP exposure (figure 2b; electronic supplementary material, figure S3b). EC50 values were up to 7.3-fold lower for AgNO3 and up to 18.6-fold lower for Ag-PVP compared with P population values. In both the AgNO3 and Ag-PVP treatments, a small reduction in sensitivity was indicated for F10s. Changes in sensitivity in multigenerational exposed populations changed the expected effect of the continuous exposure concentration from an EC35 to EC63–EC66 for AgNO3 and an EC25 to an EC55–EC73 for Ag-PVP-exposed F2–F8 generations (electronic supplementary material, table S3). A slight reduction in F10 sensitivity reduced this effect severity for the multigenerational exposure to an EC19 and EC13 for AgNO3 and Ag-PVP, respectively. This resulted from changes in the shape of the response curves with EC50 levels remaining lower than the P generation.
Figure 2.
(a) AgNO3, (b) Ag-PVP, (c) Ag2S reproductive EC50 average ± s.e.; asterisks indicate significant differences of dose–response regression curves compared with parent generation (F-ratio test p = 0.05*, p = 0.01**), Filled diamond, continuous exposure; unfilled diamond, recovery generation.
Ag2S exposure resulted in a concentration-dependent decrease in reproductive output in each generation (F- and p-values in electronic supplementary material, table S2). However, comparison of generational responses showed a significant change in the concentration–response for Ag2S only in the F5, F10 and recovery (R) generations (figure 2; electronic supplementary material, figure S3c). This change was associated with differences in the slope parameter, not the EC50 values. EC50 values for reproduction were lower than P generation only in the F8 and F10 generation (approximately twofold). While suggesting a possible increase in sensitivity, this observation alone cannot unequivocally support a multigenerational increase in sensitivity without extension of the exposure and testing for additional generations. The effect of the continuous exposure concentration varied over the course of the experiment, being greater than EC50 in all tested generations, however, with no clear pattern (electronic supplementary material, table S3).
Toxicity testing for AgNO3 or Ag-PVP in the R generation nematodes (previously placed in a recovery environment) did not show a recovery of sensitivity compared to the P populations (F- and p-values in electronic supplementary material, table S2). Sensitivity was even increased compared with the simultaneously maintained continuously exposed F10 populations (F = 16.202, p < 0.001 and F = 18.609, p < 0.001, respectively). Thus, while the EC50 of the F10 populations increased, those of the R populations were similar to those of the F5 (i.e. their last exposed ancestral generation). This suggests a transfer of sensitivity across the multiple unexposed generations. Ag2S again induced a different response pattern compared with AgNO3 and Ag-PVP in the R populations. A significantly different concentration–response was observed in the R nematodes compared with the P generation (F = 3.677, p = 0.02). However, this difference was associated with an increase in the slope rather than a change in the median effect concentrations as observed for AgNO3 and Ag-PVP. Indeed, there was no significant difference in sensitivity expressed by EC50 in the R generation compared with the continuously exposed F10 and last exposed ancestral F5 generation.
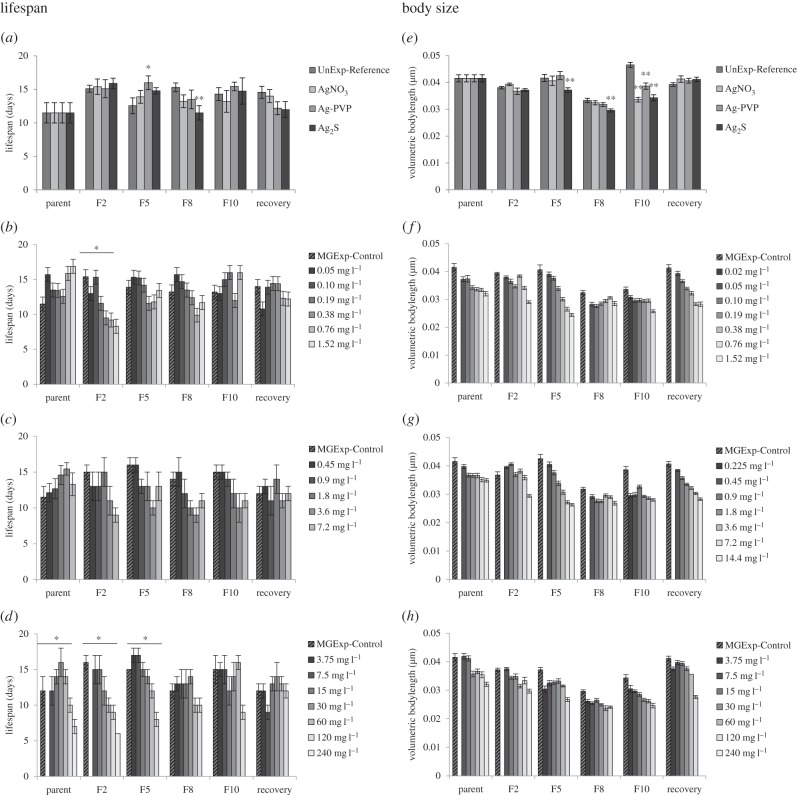
(iii). Lifespan
Multigenerational exposure to different Ag forms did not significantly alter the lifespan of nematodes in MGExp-Control for the silver-exposed populations compared with the UnExp-Reference (figure 3a; GLM: treatment: F3,205 = 0.31 p = 0.818, generation F5,205 = 5.29 p < 0.001, interaction treatment × generation F15,205 = 0.97 p = 0.489). In only two cases, there was a change in the average MGExp-Control nematode lifespan (increase in F5 for Ag-PVP, decrease in F8 for Ag2S); however, with no clear underlying pattern.
Figure 3.
(a) Lifespan (days) and (e) volumetric body lengths of controls for each generation after different generations of continuous exposure, average ± s.e. Asterisks indicate significant differences of MGExp-control compared with UnExp-Reference, *p = 0.05, **p = 0.01. (b–d) Lifespan (days) of nematodes exposed to (b) AgNO3, (c) Ag-PVP and (d) Ag2S exposure after different generations of continuous exposure, average ± s.e. (f–h) Volumetric body length of nematodes exposed to (f) AgNO3, (g) Ag-PVP and (h) Ag2S at 48 h after age synchronization after different generations of continuous exposure, average ± s.e. Asterisks indicate generation with a significant (p = 0.05) concentration-dependent effect on lifespan within a generation.
Multigenerational exposure of nematodes to each Ag form caused a concentration-dependent reduction in lifespan in several generations (figure 3b–d; for model fits, d.f., p- and F-test values see electronic supplementary material, table S4). Overall, these effects were strongest in early generations and lost in later generations (i.e. after F8 for AgNO3, F10 for Ag2S and in all R generations). This may have been the result of various mechanisms such as microevolution based on genetic variation resulting from mutation occurring in the test system.
(iv). Body size
Comparison of the MGExp-Control nematodes across generations found that sustained exposure significantly reduced the size of offspring for AgNO3 and Ag-PVP after 10 generations, while Ag2S induced such effects from F5 onwards (figure 3e; GLM: treatment: F3,585 = 11.54 p < 0.001, generation F5,585 = 37.22 p < 0.001, interaction treatment × generation F15,585 = 6.30 p < 0.001). Exposure to AgNO3 and both Ag ENP forms resulted in reductions in the size (measured as volumetric body length) of exposed nematodes at 48 h after age synchronization of eggs in each tested generation. The multigenerational exposure had a highly significant impact on the nature of these concentration–response relationships (GLM: interaction treatment × generations AgNO3: F20,702 = 9.39 p < 0.001, Ag-PVP: F20,747 = 12.0 p < 0.001, Ag2S: F20,667 = 3.70 p < 0.001). In the AgNO3- and Ag-PVP-exposed nematodes, concentration-dependent decreases in size were found for P, F2 and F5 generations (figure 3f,g). At F8 and F10, this response was altered to a threshold concentration–response pattern, such that there was very little difference in the severity of response between silver concentrations (figure 3f,g). This change in response pattern was not seen as clearly for Ag2S (figure 3h). The R populations revealed a strong concentration-dependent decrease in size for both AgNO3 and Ag-PVP with a response pattern similar to the F5 nematodes (i.e. their last exposed ancestral generation) in both cases. The R generation from the previously Ag2S-exposed nematodes showed a similar concentration-dependent decrease in size to the parent generation, suggesting recovery after the series of unexposed generations.
4. Discussion
The persistence of ENPs in natural environments, in different physically and/or chemically modified forms (e.g. sulfidation in the case of Ag ENPs), means that multigenerational exposure of organisms is a highly relevant exposure scenario. Understanding such effects, including responses following the removal of the exposure over generations as a study of the ‘memory’ effect of past exposure on traits, should therefore be a key area of research for environmentally relevant ENP effect assessment. Here, in such a study, nematode exposure of parental and subsequent multigenerational exposed cohorts of nematodes to all forms of Ag showed a strong concentration-dependent effect on brood size and final body size, but not consistently on lifespan. While these general patterns of effects were similar, the manner in which different traits respond to the multigenerational exposure differed between Ag forms.
For Ag-PVP- and AgNO3-exposed worms, patterns of response to continuous population exposure were broadly similar for all assessed endpoints. Continued exposure clearly changed population sensitivity. The apparent ‘sensitization’ was not recovered (i.e. did not return to that of previous unexposed worms) by further extension of the exposure except in the F10 generation, where slightly reduced reproductive sensitivity was observed. This slight recovery in the F10 population is unlikely to result from the development of tolerance given the need for such development to occur through similar functional mutations occurring in replicate populations, leaving the cause of the change at present uncertain and emphasizing the need for further research in this area. Increases in time to egg laying in later generations required a slight change to the test protocol (extension of exposure per generation from 96 to 120 h) prior to egg isolation. Currently, we cannot exclude the possibility that this subtle change may have affected offspring in an as-yet uncharacterized way with an effect on tolerance.
The sulfidized Ag2S ENPs produced a different multigenerational effect pattern from the greatly reduced reproductive sensitivity over generations observed for AgNO3 and Ag-PVP. Similarly, while there were subtle changes in concentration-related effects on body length, the overall pattern of the concentration-related response remained consistent across generations. Studies in plants and invertebrates have indicated differences in the mechanisms of action of pristine Ag and transformed Ag2S ENPs [11]. The parallels in the multigenerational response to the Ag-PVP and AgNO3 point to an effect driven by Ag ions, which are recognized as the cause of ENP toxicity following release by dissolution [26–28], while the absence of such a parallel for Ag2S points to a different mechanism, perhaps a particle-specific effect. This difference in mode of action was previously indicated in C. elegans by Starnes et al. [11], who found that the toxicity pathway for Ag2S differed dramatically from AgNO3 and Ag ENP, and did not involve Ag uptake for Ag2S, but rather probable cuticle damage. Further, the slight differences in the initial toxicity level in the multigenerational exposure (parental reproductive EC55 for Ag2S, approximate EC30 for AgNO3 and Ag-PVP) may also contribute to the difference in multigenerational sensitization. At these different effect levels, different biological pathways may be disrupted, especially given the possible differences in mode of action between the silver forms.
Ecotoxicological risk assessment of chemicals has traditionally relied on the use of short-term experimental toxicity data, which are subsequently extrapolated to derive predicted no-effect concentrations aimed to protect against the long-term ecological effects of pollution on populations in the field. To make this extrapolation, under some jurisdictions various assessment factors may be applied to the determined laboratory-derived effect concentrations (although this is not always the case). Such assessment factors can range from the division of toxicity test statistics (e.g. ECx (concentration needed for x% effect), no observed effect concentration) by a factor of 1000 down to division by a factor of 3 [29,30]. The observed greater-than-10-fold increase in sensitivity from P generation nematodes to the multigenerationally Ag-PVP- and AgNO3-exposed cohorts challenges this assessment factor-based approach. The effects of multigenerational exposure alone account for one order of magnitude difference between short- and long-term exposure effects (i.e. the environmental risk may in fact be much greater than estimated from short-term testing). Further, in C. elegans, mitigation of maternal effects was only found after at least four generations of exposure to uranium [16,17]. The result may be a failure of environmental protection by environmental quality standards derived from single generation toxicity tests in cases (such as for Ag ENPs and Ag ions) where multigenerational exposure is relevant, especially in those cases where standards are derived without use of assessment factors (e.g. US EPA aquatic life ambient water quality criteria). Because an increase in sensitivity was observed within the first-tested offspring generation, already an extension of short-term tests to include the second offspring generation could prove a valuable tool for assessing long-term exposure where longer tests are not possible.
Investigating the potential for recovery from multigenerational exposure remarkably showed a high similarity in the concentration–response pattern of the recovery populations to that of their last exposed F5 ancestral generation independent of the nature of the Ag exposure form. This suggests a transfer of the underlying sensitivity through the unexposed generations rather than any recovery. Most studies examining the chronic effect of exposure test transgenerational effect by studying the response of endpoints over several unexposed generations after only a single exposed generation. These studies have tended to indicate a persistence of the effects to unexposed generations such as for gold nanoparticles [19] and metals in C. elegans [31], and to some extent for carbon nanotubes in D. magna [31]. In C. elegans, even increased negative effects on individuals after cessation of γ-irradiation compared with continuously exposed ones were observed [32]. To our knowledge, none have so far looked at recovery from multigenerational exposure. Hence, we believe this observation of the transfer of sensitivity across so many unexposed generations to be a novel finding of fundamental interest for researchers interested in understanding both mechanisms of toxicity and also their implications for continuously and periodically exposed populations.
There are various underlying mechanisms that may cause the observed changes in sensitivity following the multigenerational exposure of C. elegans to AgNO3 and Ag ENPs. The possibility that continued culturing alone or an artificial selection towards more sensitive individuals caused a shift in sensitivity can be excluded based on the unchanged reproductive output and largely unaffected growth of unexposed continuously cultured cohorts and absence of multigenerational sensitization for the Ag2S-exposed populations. The relatively rapid change in sensitivity seen between the ancestral and F2 populations does not point to a role of mutation in observed sensitization, especially as these effects would need to arise independently in multiple populations. This requirement for similar changes to arise also probably precludes a role for mutation in the slight increases in EC50 values observed in later continuously exposed generations. Maternal transfer of Ag from one generation to the next is another possible mechanism of increased sensitization. It has previously been shown in C. elegans that maternal transfer of Ag ENPs is possible [33]. However, if this were the case then sensitization should decrease fairly dramatically with each generation in the recovery populations after the source of exposure is removed because the quantity of Ag transferred to offspring is only a fraction of the maternal body burden.
Given that the changes in sensitivity were retained over multiple unexposed generations, with toxicity levels matching those of the last exposed ancestral generation, a likely mechanism is through the epigenome. Epigenetic mechanisms such as DNA methylation, histone tail modification (e.g. acetylation, methylation) and microRNA expression can alter genome function in response to external stressors [34,35]. All of these processes have been found to be affected by ENP exposure, although to date most studies have been carried out in cell lines and have yet to be confirmed in vivo [34]. While epigenetic effects of ENP exposure have not yet been studied in C. elegans, microRNA has been found to be involved in the transgenerational effects of starvation on C. elegans for at least three generations and histone modification in the transfer of longevity and germline mortality [36–38]. If, as is possible, the effects of AgNO3 and Ag-PVP on life-cycle traits are mediated through effects on metabolism and resource acquisition, then our results of the transfer of sensitization may in part parallel these transgenerational effects observed for starvation.
As well as microRNA, other epigenetic mechanisms may also play a role in inherited sensitivity. DNA methylation at the fifth position in cytosine (5mc) as well as homologues of cytosine methyltransferase have not been identified in C. elegans, and it was generally accepted that DNA methylation does not occur in this species [39]. However, a recently published study [40] confirmed DNA methylation on N-6 Adenine (6ma) and it raised a possibility for these changes to be associated with epigenetic inheritance. The second epigenetic mechanism, histone methylation, has been shown in C. elegans and the role of histone H3K36 methylation has been suggested in the epigenetic memory [41]. Also interesting is the finding of the crosstalk between 6ma and histone methylation, which enhances the possibility of both mechanisms in transferring epigenetic information [40]. A further mechanism also found in C. elegans that may be involved in epigenetic modulation is through the polycomb group protein complex (PcG), associated with maintaining the so-called ‘developmental memory’, which is a memory of transcriptional states for important developmental genes [39]. With a range of mechanisms potentially contributing to the retention of sensitivity, further work is clearly warranted to investigate the range of mechanisms possibly involved. Such studies may include sequencing and analysing microRNA expression levels or comparison of the chromatin state and possible methylation structure across different generations in each of the continuous exposure and the recovery populations as contributors to retained epigenetic toxicity in C. elegans, or indeed any other in vivo system. Given the novelty of our findings, we would hope that our work will encourage others to both validate our observations and also extend such work to further chemicals and nanomaterials with a focus also on understanding mechanisms of this striking effect.
Supplementary Material
Acknowledgements
We thank Rudo Verweij at Vrije Universiteit, Amsterdam for conducting the AAS measurement, and Kerstin Jurkschat at the University of Oxford for the TEM analysis. Any opinions, findings, conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF or the EPA. This work has not been subjected to EPA review and no official endorsement should be inferred.
Data accessibility
Data supporting this article have been uploaded to Dryad and can be found at: http://dx.doi.org/10.5061/dryad.cv2d5.
Authors' contributions
C.L.S. carried out the laboratory work, analysed the data, designed the study and drafted the manuscript. A.W. and O.V.T. carried out laboratory work and designed the experimental set-up. J.M.U. synthesized the nanoparticles. A.C. provided characterization advice. C.S. and D.J.S. designed and coordinated the study and drafted the manuscript. All authors commented on the manuscript.
Competing interests
The authors confirm that they have no competing financial interests.
Funding
C.L.S. was supported by the EU 7th framework programme, Marie Curie Actions, ITN NanoTOES (PITN-GA-2010-264506). Remaining contributors were supported by the joint NERC/US-EPA, the Transatlantic Initiative for Nanotechnology and the Environment (TINE) grant no. (Reference NE/H013679/1, RD83457401). D.J.S. and C.S. receive support from National Capability funding through the UK NERC Centre for Ecology and Hydrology Pollution and Environmental Risk Theme and the EU FP7 project GUIDEnano (CP-FP7 604387). J.M.U., O.V.T. and A.W. were supported by the National Science Foundation (NSF) and the Environmental Protection Agency (EPA) under NSF Cooperative Agreement EF-0830093 and DBI-1266252, Center for the Environmental Implications of NanoTechnology (CEINT).
References
- 1.Lowry GV, Gregory KB, Apte SC, Lead JR. 2012. Transformations of nanomaterials in the environment. Environ. Sci. Technol. 46, 6893–6899. ( 10.1021/es300839e) [DOI] [PubMed] [Google Scholar]
- 2.Levard C, Hotze EM, Lowry GV, Brown GE Jr. 2012. Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ. Sci. Technol. 46, 6900–6914. ( 10.1021/es2037405) [DOI] [PubMed] [Google Scholar]
- 3.Levard C, Reinsch BC, Michel FM, Oumahi C, Lowry GV, Brown GE. 2011. Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: impact on dissolution rate. Environ. Sci. Technol. 45, 5260–5266. ( 10.1021/Es2007758) [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Pennell KG, Hurt RH. 2011. Kinetics and mechanisms of nanosilver oxysulfidation. Environ. Sci. Technol. 45, 7345–7353. ( 10.1021/es201539s) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaegi R, Voegelin A, Sinnet B, Zuleeg S, Hagendorfer H, Burkhardt M, Siegrist H. 2011. Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ. Sci. Technol. 45, 3902–3908. ( 10.1021/es1041892) [DOI] [PubMed] [Google Scholar]
- 6.Ma R, Levard C, Judy JD, Unrine JM, Durenkamp M, Martin B, Jefferson B, Lowry GV. 2013. Fate of zinc oxide and silver nanoparticles in a pilot wastewater treatment plant and in processed biosolids. Environ. Sci. Technol. 48, 104–112. ( 10.1021/es403646x) [DOI] [PubMed] [Google Scholar]
- 7.Lombi E, et al. 2013. Transformation of four silver/silver chloride nanoparticles during anaerobic treatment of wastewater and post-processing of sewage sludge. Environ. Pollut. 176, 193–197. ( 10.1016/j.envpol.2013.01.029) [DOI] [PubMed] [Google Scholar]
- 8.Kent RD, Oser JG, Vikesland PJ. 2014. Controlled evaluation of silver nanoparticle sulfidation in a full-scale wastewater treatment plant. Environ. Sci. Technol. 48, 8564–8572. ( 10.1021/es404989t) [DOI] [PubMed] [Google Scholar]
- 9.Mahendra S, Zhu H, Colvin VL, Alvarez PJ. 2008. Quantum dot weathering results in microbial toxicity. Environ. Sci. Technol. 42, 9424–9430. ( 10.1021/es8023385) [DOI] [PubMed] [Google Scholar]
- 10.Levard C, et al. 2013. Sulfidation of silver nanoparticles: natural antidote to their toxicity. Environ. Sci. Technol. 47, 13 440–13 448. ( 10.1021/es403527n) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starnes DL, Unrine JM, Starnes CP, Collin BE, Oostveen EK, Ma R, Lowry GV, Bertsch PM, Tsyusko OV. 2015. Impact of sulfidation on the bioavailability and toxicity of silver nanoparticles to Caenorhabditis elegans. Environ. Pollut. 196, 239–246. ( 10.1016/j.envpol.2014.10.009) [DOI] [PubMed] [Google Scholar]
- 12.Devi GP, Ahmed KBA, Varsha MKNS, Shrijha BS, Lal KKS, Anbazhagan V, Thiagarajan R. 2015. Sulfidation of silver nanoparticle reduces its toxicity in zebrafish. Aquat. Toxicol. 158, 149–156. ( 10.1016/j.aquatox.2014.11.007) [DOI] [PubMed] [Google Scholar]
- 13.Völker C, Boedicker C, Daubenthaler J, Oetken M, Oehlmann J. 2013. Comparative toxicity assessment of nanosilver on three Daphnia species in acute, chronic and multi-generation experiments. PLoS ONE 8, e75026 ( 10.1371/journal.pone.0075026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contreras EQ, Cho M, Zhu H, Puppala HL, Escalera G, Zhong W, Colvin VL. 2013. Toxicity of quantum dots and cadmium salt to Caenorhabditis elegans after multigenerational exposure. Environ. Sci. Technol. 47, 1148–1154. ( 10.1021/es3036785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goussen B, Parisot F, Beaudouin R, Dutilleul M, Buisset-Goussen A, Péry AR, Bonzom J-M. 2013. Consequences of a multi-generation exposure to uranium on Caenorhabditis elegans life parameters and sensitivity. Ecotoxicology 22, 869–878. ( 10.1007/s10646-013-1078-5) [DOI] [PubMed] [Google Scholar]
- 16.Goussen B, Péry ARR, Bonzom J-M, Beaudouin R. 2015. Transgenerational adaptation to pollution changes energy allocation in populations of nematodes. Environ. Sci. Technol. 49, 12 500–12 508. ( 10.1021/acs.est.5b03405) [DOI] [PubMed] [Google Scholar]
- 17.Dutilleul M, Bonzom J-M, Lecomte C, Goussen B, Daian F, Galas S, Réale D. 2014. Rapid evolutionary responses of life history traits to different experimentally-induced pollutions in Caenorhabditis elegans. BMC Evol. Biol. 14, 1–14. ( 10.1186/s12862-014-0252-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arndt DA, Chen J, Moua M, Klaper RD. 2014. Multigeneration impacts on Daphnia magna of carbon nanomaterials with differing core structures and functionalizations. Environ. Toxicol. Chem. 33, 541–547. ( 10.1002/etc.2439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SW, Kwak JI, An Y-J. 2013. Multigenerational study of gold nanoparticles in Caenorhabditis elegans: transgenerational effect of maternal exposure. Environ. Sci. Technol. 47, 5393–5399. ( 10.1021/es304511z) [DOI] [PubMed] [Google Scholar]
- 20.Tyne W, Lofts S, Spurgeon DJ, Jurkschat K, Svendsen C. 2013. A new medium for Caenorhabditis elegans toxicology and nanotoxicology studies designed to better reflect natural soil solution conditions. Environ. Toxicol. Chem. 32, 1711–1717. ( 10.1002/etc.2247) [DOI] [PubMed] [Google Scholar]
- 21.Diez-Ortiz M, Lahive E, George S, Ter Schure A, Van Gestel CAM, Jurkschat K, Svendsen C, Spurgeon DJ. 2015. Short-term soil bioassays may not reveal the full toxicity potential for nanomaterials; bioavailability and toxicity of silver ions (AgNO3) and silver nanoparticles to earthworm Eisenia fetida in long-term aged soils. Environ. Pollut. 203, 191–198. ( 10.1016/j.envpol.2015.03.033) [DOI] [PubMed] [Google Scholar]
- 22.Brenner S. 1974. Genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiernagel T. 1999. Maintenance of C. elegans. In C. elegans: a practical approach (ed. Hope I.), pp. 60–63. Oxford, UK: Oxford University Press. [Google Scholar]
- 24.Motulsky H, Christopoulos A. 2004. Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. Oxford, UK: Oxford University Press. [Google Scholar]
- 25.Whitley AR, Levard C, Oostveen E, Bertsch PM, Matocha CJ, Kammer FVD, Unrine JM. 2013. Behavior of Ag nanoparticles in soil: effects of particle surface coating, aging and sewage sludge amendment. Environ. Pollut. 182, 141–149. ( 10.1016/j.envpol.2013.06.027) [DOI] [PubMed] [Google Scholar]
- 26.Li L, Wu H, Ji C, van Gestel CAM, Allen HE, Peijnenburg WJGM. 2015. A metabolomic study on the responses of Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Ecotoxicol Environ. Saf. 119, 66–73. ( 10.1016/j.ecoenv.2015.05.005) [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Gondikas AP, Marinakos SM, Auffan M, Liu J, Hsu-Kim H, Meyer JN. 2012. Mechanism of silver nanoparticle toxicity is dependent on dissolved silver and surface coating in Caenorhabditis elegans. Environ. Sci. Technol. 46, 1119–1127. ( 10.1021/es202417t) [DOI] [PubMed] [Google Scholar]
- 28.Gomes SIL, Hansen D, Scott-Fordsmand JJ, Amorim MJB. 2015. Effects of silver nanoparticles to soil invertebrates: oxidative stress biomarkers in Eisenia fetida. Environ. Pollut. 199, 49–55. ( 10.1016/j.envpol.2015.01.012) [DOI] [PubMed] [Google Scholar]
- 29.Bodar CM, Pronk MEJ, Sijm DTHM. 2005. The European Union risk assessment on zinc and zinc compounds: the process and the facts. Integr. Environ. Assess. Manag. 1, 301–319. ( 10.1002/ieam.5630010401) [DOI] [PubMed] [Google Scholar]
- 30.EC. 2003. Technical guidance document in support of commission directive 93/67/EEC on risk assessment for new notified substances and commission regulation (EC) No 1488/94 on risk assessment for existing substances. Brussels, Belgium: European Commission.
- 31.Yu Z, Chen X, Zhang J, Wang R, Yin D. 2013. Transgenerational effects of heavy metals on L3 larva of Caenorhabditis elegans with greater behavior and growth inhibitions in the progeny. Ecotoxicol Environ. Saf. 88, 178–184. ( 10.1016/j.ecoenv.2012.11.012) [DOI] [PubMed] [Google Scholar]
- 32.Buisset-Goussen A, Goussen B, Della-Vedova C, Galas S, Adam-Guillermin C, Lecomte-Pradines C. 2014. Effects of chronic gamma irradiation: a multigenerational study using Caenorhabditis elegans. J. Environ. Radioact. 137, 190–197. ( 10.1016/j.jenvrad.2014.07.014) [DOI] [PubMed] [Google Scholar]
- 33.Meyer JN, Lord CA, Yang XY, Turner EA, Badireddy AR, Marinakos SM, Chilkoti A, Wiesner MR, Auffan M. 2010. Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans. Aquat. Toxicol. 100, 140–150. ( 10.1016/j.aquatox.2010.07.016) [DOI] [PubMed] [Google Scholar]
- 34.Stoccoro A, Karlsson HL, Coppedè F, Migliore L. 2013. Epigenetic effects of nano-sized materials. Toxicology 313, 3–14. ( 10.1016/j.tox.2012.12.002) [DOI] [PubMed] [Google Scholar]
- 35.Vandegehuchte MB, Janssen CR. 2011. Epigenetics and its implications for ecotoxicology. Ecotoxicology 20, 607–624. ( 10.1007/s10646-011-0634-0) [DOI] [PubMed] [Google Scholar]
- 36.Greer EL, Maures TJ, Ucar D, Hauswirth AG, Mancini E, Lim JP, Benayoun BA, Shi Y, Brunet A. 2011. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature 479, 365–371. ( 10.1038/nature10572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katz DJ, Edwards TM, Reinke V, Kelly WG. 2009. A C. elegans LSD1 demethylase contributes to germline immortality by reprogramming epigenetic memory. Cell 137, 308–320. ( 10.1016/j.cell.2009.02.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rechavi O, Houri-Ze'evi L, Anava S, Goh WS, Kerk SY, Hannon GJ, Hobert O. 2014. Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell 158, 277–287. ( 10.1016/j.cell.2014.06.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wenzel D, Palladino F, Jedrusik-Bode M. 2011. Epigenetics in C. elegans: facts and challenges. Genesis 49, 647–661. ( 10.1002/dvg.20762) [DOI] [PubMed] [Google Scholar]
- 40.Greer EL, et al. 2015. DNA methylation on N6-adenine in C. elegans. Cell 161, 868–878. ( 10.1016/j.cell.2015.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersen EC, Horvitz HR. 2007. Two C. elegans histone methyltransferases repress lin-3 EGF transcription to inhibit vulval development. Development 134, 2991–2999. ( 10.1242/dev.009373) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this article have been uploaded to Dryad and can be found at: http://dx.doi.org/10.5061/dryad.cv2d5.