Abstract
Termite colonies are typically founded by a pair of sexually reproducing dispersers, which can sometimes be replaced by some of their offspring. Some Reticulitermes and Embiratermes species routinely practice asexual queen succession (AQS): the queen is replaced by neotenic daughters produced by parthenogenesis, which mate with the primary king. Here, to cast light on the evolution of AQS, we investigated another candidate species, Cavitermes tuberosus (Termitinae). Of 95 nests, 39 contained a primary queen and 28 contained neotenic females (2–667 individuals), usually with the primary king. Microsatellite analyses confirmed that colonies were initiated by single pairs after large dispersal flights. More than 80% of the neotenic females were of exclusively maternal origin and completely homozygous, suggesting automictic parthenogenesis with gamete duplication. Conversely, workers, soldiers, and most alates and primary reproductives were produced sexually. AQS often occurs late, after colonies have reached maturity, whereas early AQS in other species may boost the young colony's growth rate. We suggest additional benefits of AQS in C. tuberosus, related with a smaller size, lesser stability and higher mobility of colonies. Our data add to the phylogenetical dispersion and diversity of modalities of AQS in termites, supporting a multiple evolutionary origin of this process.
Keywords: thelytokous parthenogenesis, gamete duplication, reproductive strategies, population structure, Isoptera, Termitidae
1. Introduction
Sexual reproduction is the rule in animals and plants. Fully parthenogenetic lineages are known in a variety of species, but remain marginal with respect to the omnipresence of sexual reproduction [1]. Other taxa, such as aphids or cladocerans, display cyclical parthenogenesis: asexual reproduction under favourable conditions, allowing rapid multiplication, alternates with sexual reproduction, which provides genetic variation better suited to produce survivors under harsh or unpredictable conditions [2,3]. Among social insects, a few ant lineages are also fully parthenogenetic, but others have evolved reproductive strategies to combine sexual and asexual reproduction, using either process whenever it is the more advantageous: new queens, well preserved from environmental hazards in the nest, are produced asexually and disperse through colony fission, whereas workers, many of which are constantly exposed to the outside world, are produced sexually (reviewed in [4–6]).
In termites, a typical colony is headed by a pair of dealated imagos (the primary king and queen), which have founded it after the dispersal flight and reproduce sexually. In many species [7], if the primary king and queen die, they can be replaced by some of their sons and daughters, which also inbreed to reproduce sexually. These secondary reproductives are often neotenics, which retain nymphal features as they reach sexual maturity without going through the imago stage. Facultative parthenogenesis (i.e. the ability of termite queens to lay unfertilized parthenogenetic eggs) has been reported in some termite species [8,9], but was long considered anecdotal and restricted to situations when the founding queen fails to mate. Only recently, systematic alternance of sexual and asexual reproduction was documented in three Reticulitermes species (R. speratus [10]; R. virginicus [11]; R. lucifugus [12]). Through this process, called ‘asexual queen succession’ (AQS), the primary queen is systematically replaced, early in the colony's life, by asexually produced neotenic daughters, which mate with the primary king. These neotenics result from automictic parthenogenesis with terminal fusion and are therefore almost fully homozygous for maternal alleles, whereas other castes are produced by conventional sexual reproduction. For these species, AQS is an integral part of their natural life cycle and determines the caste fate of individuals.
AQS presents several advantages over classical queen replacement by sexually produced daughters [10,11]: (i) because the neotenic daughters carry exclusively the primary queen's genes, AQS is genetically equivalent to expanding the primary queen's reproductive capacity and lifespan; (ii) AQS prevents inbreeding and consequently avoids homozygosity in the alate, worker or soldier offspring; (iii) deleterious recessive alleles could be exposed to selection in homozygous neotenics and eliminated at a minor cost; and (iv) AQS might allow the colony to boost its growth rate and size (e.g. in large Reticulitermes colonies, where primary queen fecundity appears to be limiting) [13,14].
In view of the potential benefits of AQS, we hypothesized that this strategy or similar ones might be present in other termite lineages, especially in the Termitidae. This family is the most species-rich and the most ecologically diversified, and multiple replacement reproductives are known from many species (reviewed in [7]). Recent observations [15] showed that AQS occurs in the soil-feeding termite Embiratermes neotenicus (Termitidae, Syntermitinae), a species long notorious for the frequent presence of physogastric neotenic females accompanied by a primary king [16]; in contrast with Reticulitermes species, the thelytokous eggs appear to be produced via automixis with central fusion in E. neotenicus. Parallel sampling of other species in French Guiana revealed the frequent occurrence of numerous neotenic females accompanied by a single primary king in the soil-feeding higher termite Cavitermes tuberosus (Emerson, 1925) (Termitidae, Termitinae), making it a worthy candidate for AQS testing. This species is phylogenetically remote and ecologically very different from Reticulitermes (Rhinotermitidae, wood feeders). It is ecologically close to E. neotenicus, but belongs to a different branch of the Termitidae [17]. Our aim was to elucidate the mechanism and dynamics of neotenic production in C. tuberosus, to cast further light on the evolution of queen replacement strategies in termites. We investigated the genetic architecture and breeding systems of C. tuberosus nests, and studied their genetic structuration at micro- and macro-geographic scales.
2. Material and methods
(a) Sample collection
Cavitermes tuberosus lives in arboreal nests, which typically take the shape of low, irregular earthen constructions in concave sections of tree trunks, between buttress roots or between leaves along the stem of spiny palms (Astrocaryum spp.). Alternatively, C. tuberosus may re-use abandoned nests of other arboreal termite species, such as Labiotermes labralis or Silvestritermes holmgreni. We sampled 47, 24, 3 and 21 nests of C. tuberosus in January 2012, September 2012, March 2014 and October 2014, respectively, from 15 sites located in two regions of French Guiana, the area of Petit Saut dam (sites A-H, J-O) and the Kaw-Roura National Nature Reserve (site I) (electronic supplementary material, table S1 and figure S1). These nests were brought to the laboratory and dissected to collect workers, soldiers and, when present, primary reproductives, alates and alate-destined fifth instar nymphs, neotenics and nymphs (from instars 1 to 4). This latter category included all short wing-budded immatures, among which future alates and future neotenics could generally not be distinguished. The sex of alates, neotenics and nymphs was determined according to the morphology of the last abdominal sternites [18]. Individuals were preserved in 100% ethanol until DNA extraction.
(b) Molecular analyses
Sixty-five nests were chosen for genetic analyses according to the presence of primary reproductives, neotenics and alates. Total DNA was extracted from termite heads using the Chelex-based method [19]. All collected primary reproductives, from 2 to 21 neotenics (mean ± s.d. = 10.52 ± 5.53), 1 to 38 female alates (10.73 ± 10.47), 7 to 35 male alates (12.56 ± 9.46), 571 nymphs of instars 1 to 5 (20.39 ± 10.57), 7 to 20 workers (9.44 ± 2.65) and 7 to 8 soldiers (7.84 ± 0.37), were genotyped at 17 polymorphic microsatellite loci (Ctub-21, Ctub-42, Ctub-43, Ctub-45, Ctub-60, Ctub-70, Ctub-72, Ctub-74, Ctub-77, Ctub-80, Ctub-84, Ctub-85, Ctub-86, Ctub-90, Ctub-91, Ctub-94 and Ctub-95 [21]). Polymerase chain reaction cycling conditions, reagent amounts and amplicon purification are fully described by Fournier et al. [20].
The degree of ploidy of a subset of 50 individuals (10 workers, 10 soldiers, 10 neotenics, 10 winged females and 10 winged males) was assessed by flow cytometry. For this purpose, heads were cut off and crushed each in 1 ml of a DNA-staining solution containing DAPI (4′,6-diamidino-2-phenylindole) as the DNA dye. The DNA content of each sample was analysed using a PA-I flow cytometer (PARTEC, Partec GmbH, Munster, Germany) with an optical arrangement as used by Cournault & Aron [21]. The testes of the winged males were also dissected and submitted to the same treatment.
(c) Statistical analyses
Allelic diversity, F-statistics and heterozygosities were calculated with GenAlEx v. 6.5 [22] and Fstat v. 2.9.3 [23]. Sibship reconstruction and identification of the most likely royal pairs (when missing) were conducted using the maximum-likelihood method implemented in the program Colony v. 2.0.5 [24,25]. Relatedness coefficients were estimated with the program Relatedness v. 5.08 [26] according to the algorithm described by Goodnight & Queller [27]. Hierarchical F-analyses were conducted with the program R v. 3.0.1 [28] using the hierfstat package [29]. Genetic variability was estimated over all levels, namely (i) individuals within nests, (ii) nests within sample site, (iii) sample sites within regions (i.e. area of Petit Saut dam and Kaw-Roura National Nature Reserve) and (iv) French Guiana; 95% confidence intervals (CIs) of the different F-statistics were obtained by bootstrapping Fst values over loci. Population structure among 61 nests collected in the area of Petit Saut dam was tested by using a Bayesian model-based clustering method. To estimate the number of population clusters (K), we used the InStruct algorithm [30], which models inbreeding and does not assume Hardy–Weinberg equilibrium. The model to infer population structure and inbreeding coefficients was run in five parallel chains with 200 000 Markov chain Monte Carlo repetitions and a burn-in of 100 000 iterations each. CLUMPP v. 1.1.2 [31] was used to eliminate label switching among clusters and obtain average Q values among chains (LargeKGreedy algorithm, 200 000 replicates). Results were graphically displayed using Distruct v. 1.1 [32]. Isolation by distance between nests within the two most representative sites (sites A and G) was tested by estimating the correlation between the matrix of genetic distances between pairs of nests (Fst/(1 − Fst)) and the matrix of geographical distances. The significance of the correlation was tested using a Mantel test with 10 000 permutations [33] as implemented in GenAlEx v. 6.5.
3. Results
(a) Nest composition
Primary queens and kings were collected in 39 and 40 nests, respectively (electronic supplementary material, figure S2a). Twenty-eight out of 95 nests comprised from 2 to 667 secondary reproductives (i.e. neotenics; mean ± s.d. = 86.32 ± 131.18), with all except four being female (electronic supplementary material, table S1). All neotenics were small and non-physogastric, but they displayed a characteristic ochre tinge (electronic supplementary material, figure S2b). In four nests, one male neotenic cohabited with 7–61 female neotenics. We found no occurrence of differentiated neotenics together with the same-sex primary, except for nest K_81, which hosted a primary queen with two neotenic females, and nest L_85, where a primary king cohabited with a male neotenic. Thirty-four nests contained future dispersers in the form of alates and/or alate-destined fifth instar nymphs. There was no significant relationship between the presence/absence of dispersers and the type of female reproductives (primary versus neotenics) in the nest (χ2 = 2.011, d.f. = 1, p = 0.156). The complete details of nest composition are given in electronic supplementary material, table S1.
(b) Genetic diversity
The mean number of alleles per locus varied across the 65 nests from 2 to 16 (mean ± s.e. = 6.24 ± 0.80). The mean number of effective alleles was lower (2.71 ± 0.33) and ranged from 1.38 to 5.83, indicating uneven allele frequencies at each locus. No nest (except nest N_96 at locus Ctub-91) contained more than four alleles at any of the 17 loci. Differences between observed and expected heterozygosities were noticed at all the loci. Combination of morphological observations and genotyping at 17 loci allowed the identification of a sex-linked microsatellite locus: at locus Ctub-94, female individuals (n = 484) were always homozygous (103/103), whereas male individuals (n = 170) were always heterozygous and bore the allele 103 at a frequency of 0.5 and alleles 128, 150 or 153 at frequencies of 0.129, 0.338 and 0.032, respectively. This observed pattern of exclusively homozygous females and heterozygous males can be explained by the location of the microsatellite Ctub-94 on the sex chromosomes.
(c) Mating system
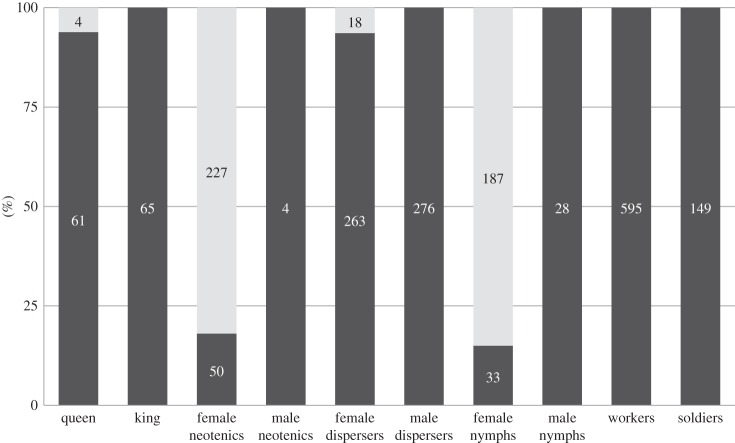
Genetic analyses revealed that 227 (81.95%) neotenics were homozygous at all 17 loci. None of them had any allele that could be unambiguously attributed to the king. All were homozygous for alleles present in the inferred queen. This suggests that a significant proportion of neotenic females are produced asexually by thelytokous parthenogenesis. The proportion of homozygotes among parthenogens was significantly different from that expected under apomixis or under automixis with central fusion or random fusion [34], but not from that expected under automictic parthenogenesis with terminal fusion or gamete duplication [34,35]. However, as parthenogens were consistently homozygous even at loci heterozygous in their mother, gamete duplication is the cytological mechanism most likely to be involved in the production of thelytokous offspring by queens of C. tuberosus [36]. Flow cytometry analysis on the subset of 10 parthenogenetic neotenics confirmed the restoration of diploidy (DNA content was double than that in males' sperm cells). For primary queens displaying a heterozygous genotype at a given locus (e.g. genotype ab at locus i), genotypes of parthenogenetically produced females were equally distributed (genotypes aa and bb occur at frequencies not different from 0.5) in all nests (binomial exact tests, p > 0.070) except one, the nest J_73. In this nest, all eight parthenogens showed a single homozygous genotype 134/134 at locus Ctub-43, whereas the primary queen had a heterozygous genotype 134/148 (binomial exact test, p = 0.008). Within nests, proportions of parthenogenetically produced neotenics varied from 0% to 100% (mean ± s.d. = 84 ± 28%; electronic supplementary material, table S1). The other neotenics (n = 54, among which were four male individuals; figure 1) were heterozygous at at least three loci and showed genotypes consistent with those expected under sexual reproduction between the primary reproductives.
Figure 1.
Proportion of sexually (dark grey) and parthenogenetically (light grey) produced individuals for primary reproductives, neotenics, dispersers (i.e. alates and alate-destined fifth instar nymphs), workers, soldiers and nymphs (from instars 1 to 4) of C. tuberosus. Numbers of individuals indicated within bars (no indication = 0).
Accordingly, the mean observed heterozygosity (Ho ± s.e.) in parthenogenetically produced neotenics was 0 (mean expected heterozygosity He = 0.530 ± 0.053), whereas it reached 0.483 ± 0.059 (He = 0.496 ± 0.055) for sexually produced female ones (table 1). Estimates of coefficients of relatedness among nest-mates were also consistent with the conditional use of sex in C. tuberosus (table 2). Parthenogenetically produced neotenics, which are half clones of their mother, were related to their mother primary queen (inferred from the workers and soldiers' genotypes; r ± s.e.jackknife = 0.592 ± 0.043), but were unrelated to the primary king (0.090 ± 0.027). By contrast, queens and kings were related to sexually produced neotenics as expected from parent–offspring relationships (r ± s.e.jackknife varied from 0.352 ± 0.032 to 0.771 ± 0.047, table 2; the relatedness coefficient between a king and the nest-mate female sexually produced neotenics reached 0.409 ± 0.036, when the sex-linked locus Ctub-94 was discarded from the analysis).
Table 1.
Mean values (±s.e.) of observed (Ho), expected (He) and unbiased expected (uHe) heterozygosities for kings, queens, neotenics, dispersers (i.e. alates and alate-destined fifth instar nymphs), nymphs (from instars 1 to 4), workers and soldiers of the humivorous termite C. tuberosus.
| sexually produced individuals |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| kings | queens | female neotenics | male neotenics | female dispersers | male dispersers | female nymphs | male nymphs | workers | soldiers | |
| Ho | 0.601 ± 0.051 | 0.503 ± 0.047 | 0.483 ± 0.059 | 0.525 ± 0.079 | 0.529 ± 0.056 | 0.588 ± 0.052 | 0.519 ± 0.062 | 0.582 ± 0.059 | 0.540 ± 0.034 | 0.494 ± 0.046 |
| He | 0.581 ± 0.038 | 0.546 ± 0.050 | 0.496 ± 0.055 | 0.425 ± 0.041 | 0.533 ± 0.051 | 0.569 ± 0.038 | 0.507 ± 0.056 | 0.549 ± 0.046 | 0.570 ± 0.037 | 0.523 ± 0.050 |
| uHe | 0.586 ± 0.039 | 0.551 ± 0.050 | 0.502 ± 0.056 | 0.544 ± 0.062 | 0.534 ± 0.051 | 0.571 ± 0.038 | 0.516 ± 0.057 | 0.561 ± 0.047 | 0.571 ± 0.037 | 0.526 ± 0.051 |
| parthenogenetically produced individuals |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| queens | female neotenics | female dispersers | female nymphs | |||||||
| Ho | 0 | 0 | 0 | 0 | ||||||
| He | 0.360 ± 0.055 | 0.530 ± 0.053 | 0.465 ± 0.056 | 0.510 ± 0.055 | ||||||
| uHe | 0.417 ± 0.063 | 0.531 ± 0.053 | 0.481 ± 0.058 | 0.511 ± 0.056 | ||||||
Table 2.
Coefficients of relatedness between queen or king and nest-mate neotenics, dispersers (i.e. alates and alate-destined fifth instar nymphs), nymphs (from instars 1 to 4), workers and soldiers of C. tuberosus. Mean ± s.e.jackknife and CI (in square brackets) are reported for sexually and parthenogenetically produced individuals.
| sexually produced individuals |
||||||||
|---|---|---|---|---|---|---|---|---|
| female neotenics | male neotenics | female dispersers | male dispersers | female nymphs | male nymphs | workers | soldiers | |
| queen versus | 0.689 ± 0.069 | 0.499 ± 0.091 | 0.565 ± 0.040 | 0.570 ± 0.033 | 0.518 ± 0.054 | 0.539 ± 0.062 | 0.594 ± 0.022 | 0.632 ± 0.047 |
| [0.531–0.848] | [0.211–0.787] | [0.483–0.648] | [0.502–0.639] | [0.399–0.638] | [0.386–0.692] | [0.549–0.638] | [0.533–0.731] | |
| king versus | 0.352 ± 0.032 | 0.771 ± 0.047 | 0.483 ± 0.034 | 0.523 ± 0.028 | 0.529 ± 0.031 | 0.519 ± 0.026 | 0.548 ± 0.014 | 0.577 ± 0.021 |
| [0.278–0.425] | [0.620–0.921] | [0.412–0.554] | [0.465–0.581] | [0.460–0.598] | [0.456–0.581] | [0.520–0.576] | [0.532–0.622] | |
| parthenogenetically produced individuals |
||||||||
|---|---|---|---|---|---|---|---|---|
| female neotenics | female dispersers | female nymphs | ||||||
| queen versus | 0.592 ± 0.043 | 0.671 ± 0.136 | 0.524 ± 0.028 | |||||
| [0.504–0.680] | [0.340–1.003] | [0.466–0.581] | ||||||
| king versus | 0.090 ± 0.027 | 0.049 ± 0.076 | 0.080 ± 0.031 | |||||
| [0.034–0.146] | [−0.138–0.235] | [0.017–0.143] | ||||||
Among queens, four were homozygous at all 17 loci. The probability that the mother and the father of queens transmit the same allele at each of the 17 loci is sufficiently low (p = 2.29 × 10−7) to consider these four queens as parthenogenetically produced. Likewise, 18 female alates and alate-destined fifth instar nymphs (out of 281), and 187 out of 220 female nymphs, exhibiting homozygosity at all loci, were considered as parthenogenetically produced (figure 1; electronic supplementary material, table S1). By contrast, workers, soldiers, male alates or alate-destined fifth instar nymphs, almost all female alates or alate-destined fifth instar nymphs (263 out of 281), 33 female nymphs (out of 220) and all 28 male nymphs were produced by sexual reproduction (i.e. they displayed genotypes consistent with recombination of king and queen genotypes; figure 1; electronic supplementary material, table S1). Heterozygosity of these sexually produced individuals was as expected for offspring produced by outcrossing of the primary queen and the primary king (table 1). Additionally, queens and kings were related to these sexual individuals as expected from parent–offspring relationships: relatedness estimates yielded values not different or slightly higher than the expected value of 0.5 (r ± s.e.jackknife varied from 0.483 ± 0.024 to 0.632 ± 0.047; table 2).
(d) Colony breeding structure
At a microgeographic scale, investigation of the social organization of nests revealed that 40 nests (62%) had genotypes and genotype frequencies consistent with the presence of a single pair of reproductives and were thus considered to be simple families. We also included in this category the nest N_96 for which a fifth allele was encountered at locus Ctub-91 in a single individual (over 50 genotyped). This fifth allele probably corresponds to a genotyping error or a mutation rather than a true allele inherited from a supplementary reproductive. Twenty-five nests (38%) had genotypes or genotype frequencies inconsistent with simple families, indicating the presence of multiple same-sex reproductives in an extended family. Intra-nest symmetric relatedness of workers (sexually produced individuals) and coefficients of inbreeding for individuals relative to their nests (Fis) for simple and extended families are in line with the values expected when a nest is initiated by a single female reproductive who mates with a single male (table 3). The coefficient of inbreeding for individuals relative to the total population (Fit) was near zero (0.060; 95% CI: 0.002–0.103). The levels of relatedness between the queens and their mates were slightly positive (mean ± s.e. = 0.095 ± 0.029; 95% CI: 0.036–0.153) and ranged from −0.383 ± 0.153 to 0.553 ± 0.146.
Table 3.
Intra-nest symmetric relatedness of workers (sexually produced individuals) and coefficients of inbreeding for individuals relative to their nests (Fis) as observed for C. tuberosus. Mean ± s.e.jackknife and CI (in square brackets) are reported for simple and extended families. Expected values are based on computer simulations [13].
| simple families |
extended families |
|||
|---|---|---|---|---|
| r | Fis | r | Fis | |
| observed values | 0.579 ± 0.020 | −0.356 ± 0.015 | 0.586 ± 0.030 | −0.341 ± 0.020 |
| [0.539–0.619] | [−0.385 to −0.330] | [0.525–0.647] | [−0.379 to −0.305] | |
| expected values | 0.5 | −0.34 | >0.5 | >−0.34 |
A close inspection of genotype distribution revealed that some extended families were the result of a succession of reproductives and that nests may have been headed by offspring of the primary reproductives. In the nest H_80, the eight neotenics had a sexual origin, whereas the queen (inferred from the neotenics and the king collected) had a thelytokous origin. Similarly, three out of the four neotenics of the nest L_85 were sexually produced. In the nests G_93, K_78, K_100 and M_90, parthenogenetic nymphs and female neotenics showed more than two classes of homozygous genotypes, respectively, at 3, 6, 3 and 1 loci. This suggests that in these nests, a first generation of sexually produced neotenics transmitted paternal alleles to a second generation of neotenics, produced by parthenogenesis. Alternatively, field observations revealed the presence of six small primary queens in one nest, which suggests that several genotype classes might result from an initial nest colonization by several primary reproductive pairs. Finally, male neotenics take part in the reproduction, except for the nest L_85. In this latter nest, sexually produced individuals were inconsistent with a mating between male and female neotenics. The primary king was still present and probably active, whereas the male neotenic showed unsclerotized cuticle, suggesting that he was not functional yet.
(e) Population genetic structure and dispersal strategies
At a macrogeographic scale, the hierarchical F-analysis detected structure at two levels: differentiation was found (i) at the region level, between specimens collected in the area of Petit Saut and those sampled in the Kaw-Roura National Nature Reserve (Fregion/tot = 0.124; 95% CI: 0.001–0.208), and (ii) at the nest level, between nests within sampled sites (Fnest/site = 0.292; 95% CI: 0.267–0.310). By contrast, the sites were not differentiated from each other (Fsite/region = 0.026; 95% CI: −0.001 to 0.060). The optimal number of clusters identified by InStruct was K = 21 (electronic supplementary material, figure S3). All clusters identified were estimated to have selfing rates between 0.051 and 0.063. These inferred clusters for the area of Petit Saut do not correspond to sampling sites or evident geographical and/or phenotypic subsets.
Genetic differentiation estimated from queens did not differ from that estimated from kings (Fst = 0.021 and 0.010; p > 0.501), which is consistent with a similar dispersal pattern for female and male alates. Moreover, genetic differentiation between nests within the two most representative sites was not correlated with geographical distance (site A: Mantel test, r = 0.002, p = 0.452; site G: r = 0.092, p = 0.306). Altogether, these results indicate that alates of both sexes disperse during large nuptial flights.
4. Discussion
The observation of C. tuberosus nests indicated that nests are headed by single pairs of primary reproductives or by neotenic reproductives together with one or both primary reproductives. The comparison of the F-statistics and relatedness coefficients under several simulated breeding systems [13] with the coefficients estimated for C. tuberosus indicated that nests are initiated by a single pair of outbred primary reproductives, and for nests in which mating occurs among primary king and multiple neotenics, neotenics descend from the primary queen. The lack of isolation by distance among nests suggests that new nests are initiated by winged queens and kings after large dispersal flights. Moreover, C. tuberosus displayed genetic differentiation among nests within sampled sites and between regions (i.e. area of Petit Saut and Kaw-Roura National Nature Reserve), but not between sites. This suggests that primary reproductives of C. tuberosus may disperse largely at distances greater than the distances separating sampled sites. For instance, Macrotermes michaelseni populations show low values of genetic differentiation across a large spatial scale so that populations may be regarded as panmictic on spatial scales of 25–50 km [37].
Our results demonstrate the common occurrence of AQS in C. tuberosus. With recent observations on E. neotenicus [15], the presence of AQS as a queen replacement strategy in the Termitidae is firmly established. Far from being restricted to temperate wood-feeding Rhinotermitidae of the genus Reticulitermes, AQS now involves two species which are tropical rainforest soil feeders [38,39] and represent distinct subfamilies of higher termites. In AQS systems, two contrasted dispersal and reproductive patterns occur simultaneously: sexually produced alates initiate new colonies after a long-distance dispersal, while asexually produced neotenics reproduce inside the mother-nest. These co-occurring patterns could be seen as resulting from different selective pressures on one single genome. High dispersal abilities should be counter-selected after the successful colonization of a new environment because individuals that continue to disperse widely will almost certainly be unsuccessful and thus be removed from the newly colonized environment, whereas the non-dispersers remain in the local deme [40]. On the other hand, sexual reproduction enhances the genetic variation (i.e. new combinations of traits) among sexually produced offspring that are more able to adapt to new or changing environmental conditions [41], whereas clonal reproduction may have short-term benefits to exploit stable environments [41–43].
Queen replacement in C. tuberosus is not accidental: the queen often goes missing while the primary king remains alive and active, and the parthenogenetic production of female neotenics constitutes evidence that the queen anticipates her succession. Parthenogenetic nymphs were indeed almost systematically present with physiologically reproductive primary queens. However, the dynamics of AQS in C. tuberosus presents two singularities: first, primary queens were found more frequently than neotenics in field-collected colonies (41% versus 29%), including in mature (i.e. disperser-producing) ones (62% versus 21%); second, all neotenics of C. tuberosus were small and non-physogastric. These features contrast with AQS in Reticulitermes spp. and E. neotenicus, whose queens appear to be systematically replaced early in the colony's life by neotenics which soon become highly physogastric [10,15]. This latter strategy is consistent with the hypothesis that AQS boosts the colony's growth rate, which would otherwise be limited by the maximum egg-laying capacity of the primary queen, while perpetuating her genes and avoiding costs of inbreeding. However, in C. tuberosus, the late occurrence of queen replacement and the small size and presumably low fecundity of neotenic females suggest that the enhanced colony growth rate is not the main benefit of AQS. Another hypothesis is that the queen's fecundity would be sufficient to sustain the growth of the colony during its early years, but would reach a limiting plateau or even collapse in mature colonies, making her replacement necessary. However, primary queens of C. tuberosus only develop moderate physogastry, and colonies of this species are clearly not among the most populous Termitidae. Intrinsic physiological limits to the queen's fecundity appear unlikely to require queen replacement. However, a rapid boost in the colony's growth rate may be favoured under special circumstances. For instance, populous C. tuberosus colonies sometimes occupy nests built by other species (e.g. L. labralis): the dynamics of such invasions is unknown, but they might provide opportunities for rapid colony development requiring high egg production rates, with high numbers of neotenics compensating for their small size. The actual egg-laying potential of primary and neotenic queens deserves to be evaluated.
Besides increased fecundity, trading a large queen for many small ones might also offer benefits in terms of safety or mobility. If a single large queen is present, her accidental loss might jeopardize the future of the colony, whereas the loss of a few neotenics out of many would be more easily overcome. Furthermore, C. tuberosus possesses mechanically defended soldiers, endowed with a phragmotic frons adapted for blocking passages between nest chambers matching precisely the size of their head. Keeping queens small may be necessary to allow them to move between nest chambers in the case of nest disturbance.
Neotenic parthenogens of C. tuberosus are completely homozygous, whereas those of Reticulitermes species are homozygous at most loci [9,11,12] and those of E. neotenicus are almost completely identical to their mother [15]. These patterns are typical of diploidy restoration by gamete duplication, automixis with terminal fusion and automixis with central fusion, respectively. Gamete duplication in which meiosis is followed by a diploidization of the haploid cell is thus the most likely mechanism involved in C. tuberosus parthenogenesis. It enforces homozygosity at all loci and therefore increases the probability of homozygosity for deleterious recessive alleles. However, gamete duplication also yields parthenogenetically reproducing lineages that are independently subject to natural selection/evolution [36,44]. These conditions, reminiscent of the evolutionary genetics of haplodiploids, imply an increase in the strength of natural selection against those deleterious alleles (i.e. a purge of the genetic load [45]). The AQS phase during which many homozygous neotenics are produced provides an opportunity for purging the gene pool from deleterious recessive alleles at a low cost, by the elimination and quick recycling of unfit neotenics. This purge process may be so efficient that full homozygotes might even be able to develop into dispersers and successfully found a new colony [9]. As in Reticulitermes species [10,11], a small proportion of fifth instar nymphs and alates of C. tuberosus were parthenogenetic. Here, we even documented four cases of functional primary queens of parthenogenetic origin, two of which were in mature colonies, which unambiguously demonstrates the ability of parthenogens to become successful foundresses.
In C. tuberosus, all parthenogenetically produced neotenics were homozygous for alleles present in the inferred queen and their genotypes were distributed at random (for a heterozygous primary queen ab, genotypes of neotenics are aa or bb at equal frequencies). Our data did not show significantly biased genotypic frequencies that could suggest the existence of strong meiotic driver genes. This contrasts with the unequal inheritance of primary queen alleles observed in R. speratus and R. virginicus [9], which can be due to a selfish genetic element biasing the meiotic division in parthenogenetic eggs developing into secondary queens, which in turn produce future dispersers.
AQS is now known in three phylogenetically distant genera of termites: the Rhinotermitidae Reticulitermes [10], and two Termitidae, Embiratermes (Syntermitinae) [15] and Cavitermes (Termitinae, this work), which are not closely related [18]. Reticulitermes is a temperate wood feeder, whereas both Termitidae are Neotropical soil feeders. AQS in these taxa differs by its mechanism of ploidy restoration and timing in the colony's life. Dedeine et al. [46] recently outlined that the three Reticulitermes species performing AQS do not form a monophyletic cluster and suggested that AQS evolved three times independently in this genus. However, one may also hypothesize that the essential novelty (i.e. the production of parthenogenetic neotenics) evolved once at the origin of Reticulitermes, but became a systematic strategy only in some particular species. If so, one could expect the discovery of casual parthenogenetic neotenics perhaps in emergency situations only, in other Reticulitermes species. In any case, as the mechanism of ploidy restoration is different in the two AQS-performing Termitidae, which are phylogenetically distant and ecologically remote from Reticulitermes, it is very likely that the process evolved independently in Embiratermes and Cavitermes. It may even be more widespread and have evolved more often. Female neotenic reproductives have been recorded from a broad range of species (reviewed in [7]), but few reports accurately describe who assumes the male function. Yet Emerson [47] mentions the occurrence of a primary king with female neotenics in Silvestritermes (formerly Armitermes) minutus (Syntermitinae) and Subulitermes parvellus (Nasutitermitinae) in Guyana, and Miller [48] reports two similar findings in the Australian Termitinae Cristatitermes pineaformis. Adultoids (i.e. alates shedding their wings and assuming reproduction in their home colony) are also widespread in Termitidae [49], but almost nothing is known of their origin and genetic constitution. There are thus still many candidates that could potentially broaden our knowledge and deepen our understanding of asexual processes in termite reproduction.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to P. Cerdan and the staff of the Laboratoire Environnement HYDRECO of Petit Saut (EDF-CNEH) for logistic support. We thank B. Host and K. Dolejšová for collecting and dissecting some of the nests used in this study, and the two anonymous referees for their constructive comments on the manuscript.
Data accessibility
The microsatellite dataset supporting the results of this study is available in the Dryad Data Repository: http://dx.doi.org/10.5061/dryad.v26gh.
Authors' contributions
D.F. and Y.R. designed the study. D.F. and S.H. carried out the molecular laboratory work and performed the genetic and statistical analyses. All authors collected the material and contributed significantly to the text of the manuscript and approved the final version.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by the Belgian National Fund for Scientific Research FRS-FNRS (FRFC grant nos. 2.4594.12 and PhD fellowship), the Czech Science Foundation (14-12774S) and the IOCB, ASCR (RVO: 61388963).
References
- 1.Bengtsson BO. 2009. Asex and evolution: a very large-scale overview. In Lost sex—the evolutionary biology of parthenogenesis (eds Schön I, Martens K, van Dijk P), pp. 1–19. Berlin, Germany: Springer. [Google Scholar]
- 2.Simon J-C, Rispe C, Sunnucks P. 2002. Ecology and evolution of sex in aphids. Trends Ecol. Evol. 17, 34–39. ( 10.1016/S0169-5347(01)02331-X) [DOI] [Google Scholar]
- 3.Decaestecker E, De Meester L, Mergeay J. 2009. Cyclical parthenogenesis in Daphnia: sexual versus asexual reproduction. In Lost sex—the evolutionary biology of parthenogenesis (eds Schön I, Martens K, van Dijk P), pp. 295–316. Berlin, Germany: Springer. [Google Scholar]
- 4.Fournier D, Aron S. 2009. No-male's land for an Amazonian ant. Curr. Biol. 19, R738–R740. ( 10.1016/j.cub.2009.07.021) [DOI] [PubMed] [Google Scholar]
- 5.Wenseleers T, Van Oystaeyen A. 2011. Unusual modes of reproduction in social insects: shedding light on the evolutionary paradox of sex. BioEssays 33, 927–937. ( 10.1002/bies.201100096) [DOI] [PubMed] [Google Scholar]
- 6.Rabeling C, Kronauer DJC. 2013. Thelytokous parthenogenesis in eusocial Hymenoptera. Annu. Rev. Entomol. 58, 273–292. ( 10.1146/annurev-ento-120811-153710) [DOI] [PubMed] [Google Scholar]
- 7.Myles TG. 1999. Review of secondary reproduction in termites (Insecta: Isoptera) with comments on its role in termite ecology and social evolution. Sociobiology 33, 1–94. [Google Scholar]
- 8.Roonwal ML. 1975. Sex ratio and sexual dimorphism in termites. J. Sci. Ind. Res. 34, 402–415. [Google Scholar]
- 9.Matsuura K. 2011. Sexual and asexual reproduction in termites. In Biology of termites: a modern synthesis (eds Bignell DE, Roisin Y, Lo N), pp. 255–277. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 10.Matsuura K, Vargo EL, Kawatsu K, Labadie PE, Nakano H, Yashiro T, Tsuji K. 2009. Queen succession through asexual reproduction in termites. Science 323, 1687 ( 10.1126/science.1169702) [DOI] [PubMed] [Google Scholar]
- 11.Vargo EL, Labadie PE, Matsuura K. 2012. Asexual queen succession in the subterranean termite Reticulitermes virginicus. Proc. R. Soc. B 279, 813–819. ( 10.1098/rspb.2011.1030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luchetti A, Velonà A, Mueller M, Mantovani B. 2013. Breeding systems and reproductive strategies in Italian Reticulitermes colonies (Isoptera: Rhinotermitidae). Insect. Soc. 60, 203–211. ( 10.1007/s00040-013-0284-8) [DOI] [Google Scholar]
- 13.Thorne BL, Traniello JFA, Adams ES, Bulmer M. 1999. Reproductive dynamics and colony structure of subterranean termites of the genus Reticulitermes (Isoptera Rhinotermitidae): a review of the evidence from behavioral, ecological, and genetic studies. Ethol. Ecol. Evol. 11, 149–169. ( 10.1080/08927014.1999.9522833) [DOI] [Google Scholar]
- 14.Grube S, Forschler BT. 2004. Census of monogyne and polygyne laboratory colonies illuminates dynamics of population growth in Reticulitermes flavipes (Isoptera: Rhinotermitidae). Annu. Entomol. Soc. Am. 97, 466–475. ( 10.1603/0013-8746(2004)097%5B0466:comapl%5D2.0.co;2) [DOI] [Google Scholar]
- 15.Fougeyrollas R, Dolejšová K, Sillam-Dussès D, Roy V, Poteaux C, Hanus R, Roisin Y. 2015. Asexual queen succession in the higher termite Embiratermes neotenicus. Proc. R. Soc. B 282, 20150260 ( 10.1098/rspb.2015.0260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmgren N. 1906. Studien über südamerikanische Termiten. Zool. Jahrb. Abt. Syst. Geogr. Biol. Tiere 23, 521–676. [Google Scholar]
- 17.Bourguignon T, et al. 2015. The evolutionary history of termites as inferred from 66 mitochondrial genomes. Mol. Biol. Evol. 32, 406–421. ( 10.1093/molbev/msu308) [DOI] [PubMed] [Google Scholar]
- 18.Zimet M, Stuart AM. 1982. Sexual dimorphism in the immature stages of the termite Reticulitermes flavipes (Isoptera: Rhinotermitidae). Sociobiology 7, 1–7. [Google Scholar]
- 19.Walsh PS, Metzger DA, Higuchi R. 1991. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10, 506–513. [PubMed] [Google Scholar]
- 20.Fournier D, Hanus R, Roisin Y. 2015. Development and characterization of microsatellite markers from the humivorous termite Cavitermes tuberosus (Isoptera: Termitinae) using pyrosequencing technology. Conserv. Genet. Resour. 7, 521–524. ( 10.1007/s12686-014-0411-5) [DOI] [Google Scholar]
- 21.Cournault L, Aron S. 2008. Rapid determination of sperm number in ant queens by flow cytometry. Insect. Soc. 55, 283–287. ( 10.1007/s00040-008-1003-8) [DOI] [Google Scholar]
- 22.Peakall R, Smouse PE. 2012. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28, 2537–2539. ( 10.1093/bioinformatics/bts460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goudet J. 1995. FSTAT (Version 1.2): a computer program to calculate F-statistics. J. Hered. 86, 485–486. [Google Scholar]
- 24.Wang J. 2004. Sibship reconstruction from genetic data with typing errors. Genetics 166, 1963–1979. ( 10.1534/genetics.166.4.1963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones OR, Wang J. 2010. COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol. Ecol. Resour. 10, 551–555. ( 10.1111/j.1755-0998.2009.02787.x) [DOI] [PubMed] [Google Scholar]
- 26.Goodnight KF, Queller DC. 2000. Relatedness 5.0.8. (5.0.8 ed). Houston, TX: Goodnight Software. [Google Scholar]
- 27.Queller DC, Goodnight KF. 1989. Estimating relatedness using genetic markers. Evolution 43, 258–275. ( 10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- 28.R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 29.Goudet J. 2005. HIERFSTAT, a package for R to compute and test hierarchical F-statistics. Mol. Ecol. Notes 5, 184–186. ( 10.1111/j.1471-8286.2004.00828.x) [DOI] [Google Scholar]
- 30.Gao H, Williamson S, Bustamante CD. 2007. A Markov chain Monte Carlo approach for joint inference of population structure and inbreeding rates from multilocus genotype data. Genetics 176, 1635–1651. ( 10.1534/genetics.107.072371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakobsson M, Rosenberg NA. 2007. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23, 1801–1806. ( 10.1093/bioinformatics/btm233) [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg NA. 2004. DISTRUCT: a program for the graphical display of population structure. Mol. Ecol. Notes 4, 137–138. ( 10.1046/j.1471-8286.2003.00566.x) [DOI] [Google Scholar]
- 33.Smouse PE, Long JC, Sokal RR. 1986. Multiple regression and correlation extensions of the Mantel test of matrix correspondance. Syst. Zool. 35, 627–632. ( 10.2307/2413122) [DOI] [Google Scholar]
- 34.Pearcy M, Hardy O, Aron S. 2006. Thelytokous parthenogenesis and its consequences on inbreeding in an ant. Heredity 96, 377–382. ( 10.1038/sj.hdy.6800813) [DOI] [PubMed] [Google Scholar]
- 35.Lamb RY, Willey RB. 1987. Cytological mechanisms of thelytokous parthenogenesis in insects. Génome 29, 367–369. ( 10.1139/g87-062) [DOI] [Google Scholar]
- 36.Stenberg P, Saura A. 2010. Cytology of asexual animals. In Lost sex—the evolutionary biology of parthenogenesis (eds Schön I, Martens K, van Dijk P), pp. 63–74. Berlin, Germany: Springer. [Google Scholar]
- 37.Brandl R, Hacker M, Epplen JT, Kaib M. 2005. High gene flow between populations of Macrotermes michaelseni (Isoptera, Termitidae). Insect. Soc. 52, 344–349. ( 10.1007/s00040-005-0820-2) [DOI] [Google Scholar]
- 38.Davies RG. 2002. Feeding group responses of a Neotropical termite assemblage to rain forest fragmentation. Oecologia 133, 233–242. ( 10.1007/s00442-002-1011-8) [DOI] [PubMed] [Google Scholar]
- 39.Bourguignon T, Šobotník J, Lepoint G, Martin J-M, Hardy OJ, Dejean A, Roisin Y. 2011. Feeding ecology and phylogenetic structure of a complex neotropical termite assemblage, revealed by nitrogen stable isotope ratios. Ecol. Entomol. 36, 261–269. ( 10.1111/j.1365-2311.2011.01265.x) [DOI] [Google Scholar]
- 40.Gillespie RG, Baldwin BG, Waters JM, Fraser CI, Nikula R, Roderick GK. 2015. Long-distance dispersal: a framework for hypothesis testing. Trends Ecol. Evol. 27, 47–56. ( 10.1016/j.tree.2011.08.009) [DOI] [PubMed] [Google Scholar]
- 41.Lehtonen J, Jennions MD, Kokko H. 2012. The many costs of sex. Trends Ecol. Evol. 27, 172–178. ( 10.1016/j.tree.2011.09.016) [DOI] [PubMed] [Google Scholar]
- 42.Foucaud J, Jourdan H, Le Breton J, Loiseau A, Konghouleux D, Estoup A. 2006. Rare sexual reproduction events in the clonal reproduction system of introduced populations of the little fire ant. Evolution 60, 1646–1657. ( 10.1554/06-099.1) [DOI] [PubMed] [Google Scholar]
- 43.Foucaud J, Orivel J, Fournier D, Delabie JHC, Loiseau A, Le Breton J, Cerdan P, Estoup A. 2009. Reproductive system, social organization, human disturbance and ecological dominance in native populations of the little fire ant, Wasmannia auropunctata. Mol. Ecol. 18, 5059–5073. ( 10.1111/j.1365-294X.2009.04440.x) [DOI] [PubMed] [Google Scholar]
- 44.Suomalainen E, Saura A, Lokki J. 1987. Cytology and evolution in parthenogenesis. Boca Raton, FL: CRC Press. [Google Scholar]
- 45.Crow JF. 1970. Genetic loads and the cost of natural selection. In Mathematical topics in population genetics (ed. Kojima K-i.), pp. 128–177. Berlin, Germany: Springer. [Google Scholar]
- 46.Dedeine F, Dupont S, Guyot S, Matsuura K, Wang C, Habibpour B, Bagnères A-G, Mantovani B, Luchetti A. 2016. Historical biogeography of Reticulitermes termites (Isoptera: Rhinotermitidae) inferred from analyses of mitochondrial and nuclear loci. Mol. Phylogenet. Evol. 94, 778–790. ( 10.1016/j.ympev.2015.10.020) [DOI] [PubMed] [Google Scholar]
- 47.Emerson AE. 1933. Conditioned behavior among termites (Isoptera). Psyche 40, 125–129. ( 10.1155/1933/72040) [DOI] [Google Scholar]
- 48.Miller LR. 1991. A revision of the Termes-Capritermes branch of the Termitinae in Australia (Isoptera: Rhinotermitidae). Invertebr. Taxon. 4, 1147–1282. ( 10.1071/IT9901147) [DOI] [Google Scholar]
- 49.Noirot C. 1985. Differentiation of reproductives in higher termites. In Caste differentiation in social insects (eds Watson AL, Okot-Kotber BM, Noirot C), pp. 177–186. Oxford, UK: Pergamon Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The microsatellite dataset supporting the results of this study is available in the Dryad Data Repository: http://dx.doi.org/10.5061/dryad.v26gh.