Abstract
Sex differences in morphology, physiology, and behaviour are caused by sex-linked genes, as well as by circulating sex-steroid levels. Thus, a shift from genotypic to environmental sex determination may create an organism that exhibits a mixture of male-like and female-like traits. We studied a lizard species (Central Bearded Dragon, Pogona vitticeps), in which the high-temperature incubation of eggs transforms genetically male individuals into functional females. Although they are reproductively female, sex-reversed dragons (individuals with ZZ genotype reversed to female phenotype) resemble genetic males rather than females in morphology (relative tail length), general behaviour (boldness and activity level), and thermoregulatory tactics. Indeed, sex-reversed ‘females’ are more male-like in some behavioural traits than are genetic males. This novel phenotype may impose strong selection on the frequency of sex reversal within natural populations, facilitating rapid shifts in sex-determining systems. A single period of high incubation temperatures (generating thermally induced sex reversal) can produce functionally female individuals with male-like (or novel) traits that enhance individual fitness, allowing the new temperature-dependent sex-determining system to rapidly replace the previous genetically based one.
Keywords: Pogona vitticeps, sex reversal, concordant male, concordant female, sex-reversed female, behavioural consequences
1. Introduction
Research continues to reveal plasticity in many traits once regarded as fixed, species-specific characteristics [1]. One striking example of that paradigm shift involves sex-determining systems in vertebrates, once classified into the dichotomy of genetically determined versus environmentally mediated [2–4]. We now know that an individual's sex can be determined by multiple factors, including an interaction between heritable (genetic) traits, maternal effects, transgenerational epigenetic factors, and local environmental conditions [5–8]. Phylogenetic analyses confirm frequent shifts between alternative modes of sex determination in groups such as squamate reptiles (lizards and snakes) [9].
Although theoreticians have generally assumed that such shifts are likely to occur slowly, a recent experimental study of Central Bearded Dragons (Pogona vitticeps) revealed a different scenario. This species exhibits female heterogamety (males are ZZ and females are ZW), with genotypically determined sex over a wide range of incubation temperatures [10]. However, the incubation of eggs at high temperatures (greater than or equal to 32°C) induces sex reversal in genetically male embryos [10,11]. These animals develop into functional females, whose annual output of eggs exceeds that of ‘normal’ (genetically concordant) females [11]. With the female (W) chromosome lost from the progeny of mating between such a female and a normal (ZZ) male, sex of the next generation is determined by incubation temperature rather than genetic factors [11]. At least in the laboratory, then, the system can shift from genotypic sex determination (GSD) to environmental sex determination (ESD) within a single generation.
Therefore, there are actually three ‘sexes’ in this species: concordant males (genetic sex is concordant with phenotypic sex: ZZm), concordant females (ZWf), and sex-reversed females (ZZf) arising from ZZ sex reversal (individuals with ZZ genotype reversed to female phenotype). Importantly, sex-reversed individuals have been found in the field as well as in the laboratory; and the frequency of sex reversal appears to be increasing [11]. However, apart from Fisher's frequency-dependent selection [11], the selective forces acting on a transition between GSD and ESD remain unclear, and hinge on the phenotypes created by sex reversal. At one extreme, sexually dimorphic traits (such as size, shape, and behaviour) might be determined by steroids associated with gonadal sex. In this scenario, the phenotype of a female lizard will be the same regardless of whether its sex is determined by genes or incubation temperature. Once sex has been fixed, a cascade of developmental processes will generate species-typical ‘female’ traits. At the other extreme, genes with sex-specific effects are tightly linked to the sex-determining locus (in the non-recombining region of the sex chromosomes). That linkage (rather than a physiological cascade) is responsible for typically female traits being manifested in genetically female individuals. Those two alternatives would create very different phenotypes in a sex-reversed dragon. Under the former scenario, we expect sex-reversed females to resemble normal females in morphology, physiology, and behaviour. As a result, the relative fitness of normal and sex-reversed females should be similar, except for incubation-induced effects. Under the second scenario, however, sex-reversed females would combine female reproductive function with male-like phenotypes in traits such as shape and behaviour. If that novel phenotype had a different fitness than normal females, we might see rapid selection-driven shifts either towards a higher frequency of ESD or back towards the ancestral condition of GSD.
Do sex-reversed dragons broadly resemble conspecific normal females in their size, shape, and behaviour, or do they instead resemble normal males in aspects other than reproductive biology? Alternatively, sex-reversed individuals might exhibit a novel constellation of traits, different from those in either males or females with GSD. To test among those scenarios, we measured a range of phenotypic traits of captive lizards. As well as quantifying size and shape, we also examined the possibility that a lizard's ‘sex’ affects its behaviour.
2. Material and methods
(a). Study species and husbandry
The Central Bearded Dragon (P. vitticeps) is a large (to 515 mm total length) agamid lizard that occurs over a wide range of arid to semiarid regions in eastern Australia [12]. The University of Canberra (UC) maintains a breeding colony of these lizards, and we exploited that resource to gather behavioural data in March 2015, on 61 juvenile lizards (ranging from 354 to 519 days old, from eggs incubated at constant temperatures of 26°C, 28°C, 30°C, 32°C, and 34°C in the summer of 2013–2014) and 54 hatchlings (ranging from 29 to 147 days old, from eggs incubated at constant temperatures of 28°C, 30°C, 32°C, 34°C, and 36°C in 2014–2015). In total, we gathered behavioural data on 20 sex-reversed females (ZZf) (seven hatchlings and 13 juveniles), 55 concordant males (ZZm) (26 hatchlings and 29 juveniles), and 40 concordant females (ZWf) (21 hatchlings and 19 juveniles). An additional 80 lizards (some of them older) provided morphological data, but were not tested behaviourally (see electronic supplementary material for data). Lizards were fed with vitamin-dusted crickets (Acheta domesticus) or cockroaches (Periplaneta australasiae), plus vegetables or commercial reptile food, and had constant access to water. All of the lizards' home enclosures were subject to a light cycle set from 08.00 to 18.00 h, and a constant ambient air temperature of 23 ± 1°C. A basking bulb (switched on from 09.30 to 17.00 h, mimicking field conditions) at one end of each home enclosure created thermal gradients that allowed lizards to thermoregulate behaviourally. Lizards were kept in group cages (3–10 per cage) and size-sorted to minimize any risks of injury from agonistic interactions.
(b). Collection of morphological data
Juvenile lizards were marked using pit-tags (Hongteng Barcode Technology Co. Ltd., Guangzhou, China), and hatchlings (because of their small size) were marked using a non-toxic paint pen to write a number on the back of each animal. Prior to behavioural trials, we weighed each lizard (±0.1 g) and measured snout-vent length (SVL), tail length, and head length (along the upper jaw, from the tip of the snout to the rear where the upper and lower jaw meet) with a transparent plastic ruler and calipers (±0.01 cm).
(c). Trials to quantify behaviour
We conducted two kinds of tests on each lizard (activity levels and time to emergence from a shelter) in order to assess repeatability of behaviour in different trial contexts. Two of the activity trials (the first and the last) were designed to assess exploration (in a simple arena containing an empty perforated transparent plastic container); the second trial for each lizard was designed to assess neophobia (by adding a novel stimulus—a moving plastic fishing lure with waving arms triggered by a small motor—inside the perforated transparent plastic container). The third trial was designed to measure sociality (a conspecific was present in the perforated transparent plastic container). We also ran additional trials to record the duration of time before a lizard emerged from a shelter into an open arena (often used as a measure of boldness) [13–15]. Each lizard was tested in the same order (exploration trial 1, neophobia trial, boldness trial, sociality trial, and exploration trial 2) over five consecutive days. For the neophobia, sociality, and boldness trials, we divided all the lizards into three groups, and conducted trials in the same order for each group (one-third of the total lizards) each day during the three consecutive days. Therefore, each lizard was tested at the same time each day. Preliminary analyses showed that time of testing did not affect the behavioural traits that we measured (i.e. no consistent differences between lizards tested in mornings versus afternoons).
Prior to trials, all lizards had the opportunity to thermoregulate under heat lamps for at least 1 h. Thirty minutes prior to behavioural testing, the lizards were transferred (in groups of up to 10, all of approximately the same body size) to the trial room (without access to heat lamps) in order to acclimate to the test temperature (26°C). This acclimation period reduced but did not eliminate thermal differentials among lizards, so we recorded each lizard's body temperature using an infrared thermometer (Digitech, Jaycar Electronics, Sydney, Australia) immediately before trials.
For each behavioural trial, a lizard was placed in a standard position (7 cm from one wall) in an open arena (690 × 470 × 385 mm). A transparent plastic container (500 ml) was positioned 14 cm from one of the other walls, except in the ‘boldness’ trials (in which the lizard was first placed within an inverted cup with a single exit, rather than simply being put down in the open arena). Video cameras recorded each trial for 15 min, after which time the lizards were returned to their home enclosure. The videos were analysed with automated image-based tracking software (Ethovision XT 10.0, Noldus Information Technology, The Netherlands). From the videos of activity trials, we scored four behavioural traits (distance moved, mean velocity, cumulative duration of movement, and frequency of head rotation).
(d). Phenotypic sexing
The phenotypic sex for all captive bred animals was established by hemipene eversion upon hatching [16], hemipenal transillumination at one to four months of age [17], and by gross external morphology at four to nine months of age.
(e). Molecular detection of sex reversal
The genotypic sex of all individuals was determined using a polymerase chain reaction (PCR)-based molecular sex test that amplifies a W-chromosome-specific size polymorphism [11]. PCR conditions followed Holleley et al. [11], using primers H2 and F [18]. Two bands amplify in ZW individuals (identifying the presence of the W chromosome), whereas a single control band amplifies in ZZ individuals. Animals showing genotype–phenotype discordance were classified as sex-reversed. All putative sex-reversed animals were independently genotyped at least twice, to account for a low but quantifiable genotyping error rate (2.5% of PCR tests; n = 196). All molecular sex tests were conducted with the investigator blinded to the identity and phenotypic sex of the samples.
(f). Statistical analysis
We used ANOVA to compare the three sexes of dragons in terms of mean ages and body sizes (ln-transformed values of head length, tail length, and body mass). We used analysis of covariance (ANCOVA) to compare the sexes in terms of body proportions (ln head length and ln mass relative to ln SVL, using sex as the factor and ln SVL as the covariate).
Data on behavioural traits were non-normally distributed. To achieve normality and reduce the numbers of correlated variables, we conducted principal component analysis on the four activity measures (distance moved, mean velocity, cumulative duration of movement, and frequency of head rotation) from all four of the activity trials. The first principal component axis (PC1) explained 79% of the total variation in the dataset and was positively correlated with all four of the activity-related traits we scored (in all of these cases, r > 0.80), suggesting that PC1 provides a robust index of a lizard's overall activity level. Also, PC1 was normally distributed. We thus used PC1 as an index of activity level and investigated sex differences in activity levels with ANCOVA using PC1 as the dependent variable, sex and trial type as the factors, and age (days since hatching) and before trial body temperature as covariates. Animal ID was included as a random variable, to control for pseudoreplication. Data on boldness (time to emerge from a shelter) were examined using ANOVA, with sex as the factor and time to emerge as the dependent variable. We used ANOVA with lizard age category (hatchling versus juvenile) and sex as factors to compare mean body temperatures of lizards (averaged over the five trials).
Where significant, analyses were followed by post hoc Tukey's honestly significant difference (HSD) tests to locate meaningful differences. Data analyses were performed using JMP Pro v. 11.0 (SAS Institute, Cary, NC, USA). All values in figures are presented as mean ± standard error (s.e.), and the significance level was set at α = 0.05. The electronic supplementary material (tables S1 and S2) also provides Pearson product–moment correlation coefficients, to quantify the nature and magnitude of relationships (i) between morphological data and behavioural scores and (ii) between an individual's behaviour in different types of trials (e.g. ‘boldness trials’ versus neophobia, sociality, and exploration trials). The levels of statistical significance of such tests are questionable because multiple testing can lead to artefactually ‘significant’ results; authorities disagree on whether or not such p-values should be ‘corrected’ [19,20]. However, the r- and p-values provide useful information about patterns of covariation among traits.
Sex-reversed females are produced only at incubation temperatures greater than or equal to 32°C in this species, with few phenotypic males from incubation temperatures above this level [11]. Thus, the effect of a lizard's sex is confounded with the incubation temperature that it experienced during embryogenesis. All of the sex effects we documented in the overall dataset were still evident (and statistically significant) if we restricted the dataset to animals incubated at 32°C or above (see the electronic supplementary material). Because animals in the field would emerge from eggs that have incubated under a range of thermal regimes, the text below reports analyses based on the entire sample (i.e. from all incubation conditions).
3. Results
(a). Morphological differences
Concordant males (ZZm), concordant females (ZWf), and sex-reversed females (ZZf) in our sample of laboratory-reared lizards did not differ significantly in mean ages or body sizes (age, F2,112 = 1.90, p = 0.15; SVL, F2,112 = 2.27, p = 0.11; ln mass, F2,112 = 2.17, p = 0.12), nor in head length relative to SVL (ANCOVA interaction sex × ln SVL, F2,195 = 2.09, p = 0.13; main effect of sex, F2,192 = 0.91, p = 0.40). Concordant females had shorter tails than did individuals of the other two sexes (intact tails only; ANCOVA interaction sex × ln SVL, F2,156 = 4.03, p = 0.02; figure 1a), but sex-reversed females and concordant females had higher body condition than did concordant males (ANCOVA interaction sex × ln SVL, F2,190 = 2.99, p = 0.053; main effect of sex, F2,192 = 4.86, p < 0.009; figure 1b).
Figure 1.
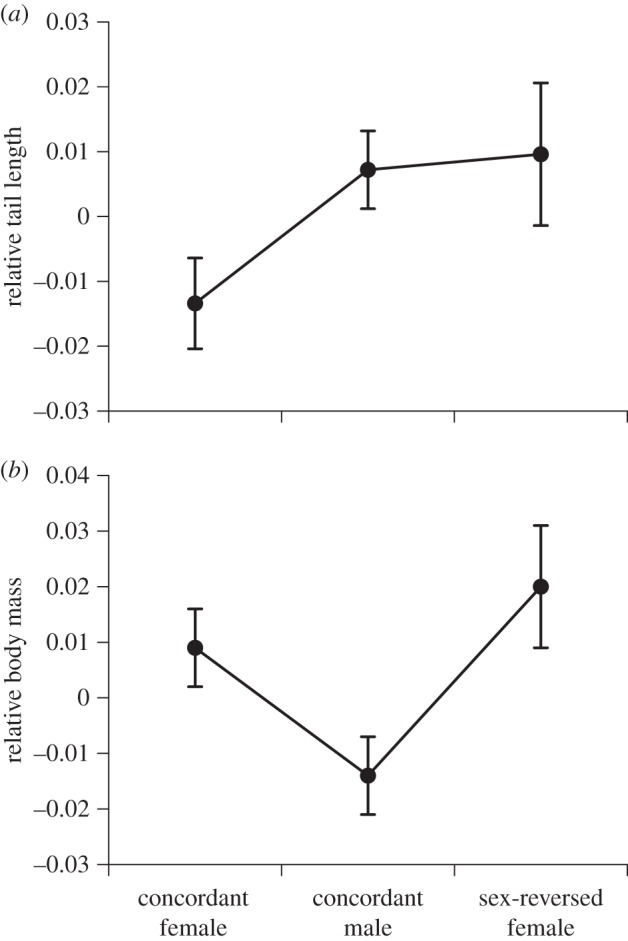
Morphological differences among sexes of Central Bearded Dragons (Pogona vitticeps). Data are shown for three sexes: males in which genetic and phenotypic sex are concordant (ZZm), females in which genetic and phenotypic sex are concordant (ZWf), and sex-reversed females which are genetically male, but with functional female gonads (ZZf). Data for morphological traits are shown by residual values from the linear regression of ln trait (tail length or body mass) against ln snout-vent length (SVL), for ease of interpretation, but statistical tests in the text are based on ANCOVA. (a) Relative tail length and (b) relative body mass.
(b). Activity level
Behaviour scores were neither significantly correlated with any morphological traits (all p > 0.07), nor with body temperature prior to trials (all p > 0.12). Pearson product–moment correlation coefficients between our descriptors of lizard behaviour in different activity trials were all positive, and between activity level and emergence time were all negative (see the electronic supplementary material, tables S1 and S2), suggesting that an individual who was highly active in one type of trial was also active in others. Sex-reversed females were more active than concordant individuals of either sex (figure 2). Using PC1 as an index of activity level, ANOVA revealed strong differences between the sex-reversed females versus either concordant males or concordant females (means ± s.e. = 0.94 ± 0.21, 0.004 ± 0.13, −0.49 ± 0.15, respectively; F2,256 = 16.00, p < 0.0001). Post hoc Tukey's HSD tests revealed significant differences between all three groups (sex-reversed females, concordant males, and concordant females). The higher activity levels of sex-reversed females were more evident in the neophobia trial and the second exploration trial than in the sociality or first exploration trials (thus, interaction F3,390.2 = 5.93, p < 0.001), but the main effect of sex remains interpretable because the interaction primarily affected the degree not the direction of sex differences in activity (figure 2).
Figure 2.
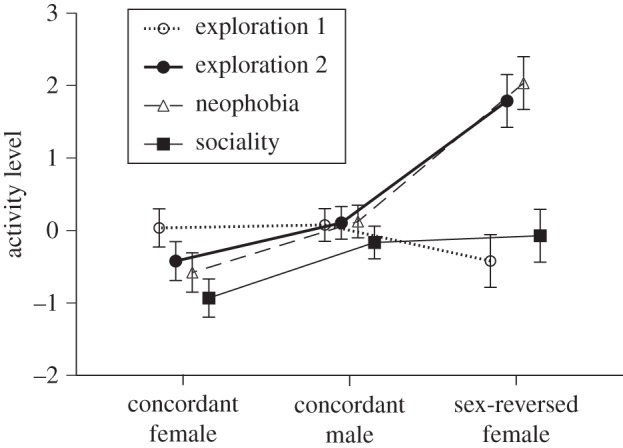
Activity levels of Central Bearded Dragons (Pogona vitticeps) in standardized trials. Data are shown for three sexes: males in which genetic and phenotypic sex are concordant (ZZm), females in which genetic and phenotypic sex are concordant (ZWf), and sex-reversed females which are genetically male, but with functional female gonads (ZZf). Each lizard was tested in four trials, in a consistent order, to assess exploration, neophobia, sociality, and then exploration again. Y-axis (activity level) is the first axis from a principal component analysis based on total distance moved, mean velocity, cumulative duration of movement, and frequency of head rotation during each 15 min behaviour trial. The graph shows mean values and associated standard errors.
(c). Boldness test
The duration of time before a lizard emerged from a shelter into the open arena showed the same pattern as overall activity level (above); sex-reversed females emerged sooner than did concordant individuals of either sex (table 1 and figure 3). A lizard's body temperature prior to the trials did not affect its time to emergence (all p > 0.69).
Table 1.
The effects of sexual category (concordant male, concordant female, and sex-reversed female) and age (days since hatching) on the behaviour of Central Bearded Dragons (Pogona vitticeps) as quantified by the time taken to emerge from a shelter. These probability values have not been corrected for multiple tests, but all remain significant at p < 0.05 even after Holm–Bonferroni corrections are applied. Values are from two-way ANOVAs. ZZf, sex-reversed female; ZZm, concordant male; ZWf, concordant female. Categories with identical 'a' or 'b' did not differ significantly from each other in post hoc tests.
| behaviour scores | sex | age | sex × age interaction | post hoc comparison |
|---|---|---|---|---|
| time for head out |
F2,112 = 6.24 p = 0.003 |
n.s. | n.s. | Sex: ZWfa, ZZmab, ZZfb |
| time for back leg out |
F2,112 = 6.43 p = 0.002 |
n.s. | n.s. | Sex: ZWfa, ZZma, ZZfb |
| time for full body out |
F2,112 = 7.88 p < 0.001 |
n.s. | n.s. | Sex: ZWfa, ZZma, ZZfb |
Figure 3.
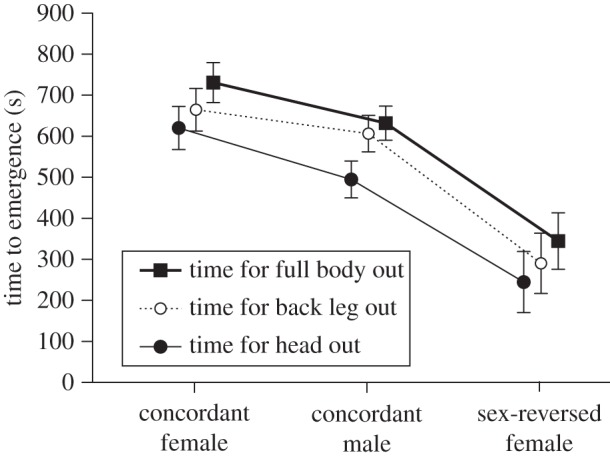
Time taken for Central Bearded Dragons (Pogona vitticeps) to emerge from a shelter in standardized trials to assess their boldness. Data are shown for three sexes: males in which genetic and phenotypic sex are concordant (ZZm), females in which genetic and phenotypic sex are concordant (ZWf), and sex-reversed females which are genetically male, but with functional female gonads (ZZf). Each hatchling was video-recorded to quantify the time taken for it to protrude its head, body, and then tail, from a shelter. The graph shows mean values and associated standard errors.
(d). Body temperature
A lizard's sex and age affected its mean body temperature (as measured immediately before trials). Juveniles were hotter than hatchlings, and sex-reversed females and concordant males were warmer than concordant females (sex effect, F2,110 = 10.52, p < 0.001, post hoc, sex-reversed female = concordant male > concordant female; age effect, F1,110 = 67.40, p < 0.001, post hoc, juvenile > hatchling; sex × age interaction, F2,110 = 1.01, p = 0.37; figure 4). The sex effects on behaviour (see above) remained significant when we included lizard body temperature as an additional covariate.
Figure 4.
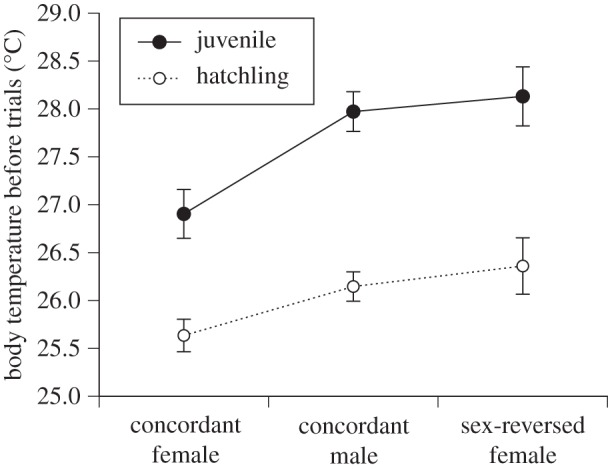
Mean body temperatures of hatchling and juvenile Central Bearded Dragons (Pogona vitticeps) shown separately for sex-reversed females (ZZf), concordant males (ZZm), and concordant females (ZWf). Body temperatures were averaged over five trials, as measured immediately before each trial. Hatchlings (from 29 to 147 days old) averaged 61.80 mm 'snout-vent length (SVL), whereas juveniles (from 354 to 519 days old) averaged 126.98 mm snout-vent length. The graph shows mean values and associated standard errors.
4. Discussion
Sex-reversed Central Bearded Dragons show a combination of phenotypic traits not seen in concordant individuals of either sex. Although functionally female (i.e. capable of producing viable eggs), the only phenotypic trait in which sex-reversed females resembled normal females was in body condition. Males were thinner than either normal or sex-reversed females (figure 1b). For all other traits, sex-reversed female dragons either resembled males, or were even more different from concordant females (i.e. more active, bolder) than were concordant males (figures 2 and 3). That similarity between male and sex-reversed female lizards extended to body temperatures (figure 4). In summary, sex reversal (by egg incubation at high temperatures) changes not only a lizard's gonadal sex, but also some of its behavioural and morphological traits. Some of those changes result in these sex-reversed ZZ females being even more male like than concordant males; others generate a mixture of traits not seen in concordant individuals of either sex.
Remarkably, then, high-temperature incubation changes an animal's functional sex as well as some (but not all) aspects of its phenotype. By contrast, studies on another reptile species (the leopard gecko, Eublepharis macularius) show that incubation temperature determines not only gonadal sex, but also reproductive behaviour and physiology [8]. These geckos lack heteromorphic sex chromosomes, so we cannot evaluate the relative role of genetic versus incubation temperature effects on lizard phenotypes. The pattern seen in our dragons suggests that many of the sexually dimorphic features of this species (especially in terms of general activity level and boldness) are driven by genetic factors rather than the cascade of endocrine changes initiated by sex determination.
Extensive research has documented the mechanistic underpinnings of sexual dimorphism in anatomical, physiological, and behavioural traits in vertebrates [8,21]. Although some sex-based differences are direct consequences of hormonal levels [22], sex-specific genetic architecture and gene expression also influence organismal phenotypes [23–25]. In the present study, sex reversal of chromosomally male ZZ individuals to phenotypic females changed gonadal function but not some other sexually dimorphic traits, including morphology, physiology, and behaviour. The discordant ZZ females arising from sex reversal resembled concordant males rather than concordant females in having relatively longer tails, higher body temperatures, and being more active and bolder. Similarly, studies in mice have shown that gene(s) on the sex chromosomes (other than the testis-determining gene Sry) affect sex differences in brain function and aggressive behaviour [26]. Studies on inbred and wild mice that vary in intensity of male–male aggression have also suggested that aggressive behaviour may be modified by genes on the Y chromosome [27]. We have no data on hormone levels, but the high reproductive output of sex-reversed lizards [11] suggests that their endocrine profiles are similar to (or higher than) those of concordant females.
The pattern of the activity levels among the three sexes of bearded dragons is mirrored by the divergence in body temperatures (concordant males and sex-reversed females are hotter as well as more active than concordant females). A reptile's body temperature can affect the rate at which it acquires and expends energy, and its ability to avoid predators [28]. The maintenance of a high body temperature between bouts of activity may allow the animal to rapidly exploit future opportunities for foraging or mating. A link between thermal preferenda and other behavioural traits may occur at several levels in reptiles. For example, Belliure et al. [29] compared thermal preference and foraging mode in two Mediterranean lacertid lizards (Acanthodactylus erythrurus and Psammodromus algirus), showing that the more active species (P. algirus) heated faster, cooled more slowly, and basked more often but for shorter periods and at warmer patches than the less active species (A. erythrurus).
One puzzling result from our studies was that the higher activity level of sex-reversed female dragons tended to be more evident in the neophobia trials than in other trials (figure 2). That pattern may reflect a greater boldness of the sex-reversed females, as also indicated by their shorter time to emergence in other trials (figure 3). Bolder individuals with higher body temperatures may be able to explore their surroundings more rapidly, indicative of a proactive behavioural type [30,31].
The consistency and magnitude of behavioural differences between sex-reversed and concordant females suggest that such animals might differ in fitness—and hence, such differentials might drive transitions between alternative modes of sex determination. Sex-reversed females were more active (especially when confronted with a novel object, in the neophobia trials), and emerged from shelter sooner, than did concordant females. That boldness might enhance feeding rates (and hence reproductive output), explaining the higher rates of egg production in sex-reversed than concordant female dragons in this laboratory population [11]. Under field conditions, where lizards may be vulnerable to several types of predators, it is less clear that bolder behaviour will increase rather than decrease individual fitness [32].
Regardless, the dramatic behavioural differences between concordant ZW females and sex-reversed ZZ females are likely to affect the relative frequency of these two types of individuals within a population. In a habitat where bolder behaviour enhances feeding rates without incurring a high mortality cost via predation, the frequency of sex reversal likely would increase because of the higher fitness of sex-reversed females. Such a shift might be strong enough to drive a local population rapidly from genetic to environmental sex determination, with the complete loss of the female heteromorphic sex chromosome (the W). Alternatively, a predator-rich environment might rapidly eliminate the novel sex-reversed behavioural phenotype, maintaining GSD within the population. Field studies to measure rates of survival and reproduction of these alternative sexes would be of great interest, and might substantially clarify the process by which evolution moulds sex-determining systems. One critical question, not addressed in our study, is whether or not the behavioural characteristics of juvenile dragons —and especially, the differences between sex-reversed females and concordant females—persist into adult life? The higher fecundity of sex-reversed ZZ females compared with concordant ZW females [11] hints at long-lasting effects, but empirical data on adult behaviour would be of great interest.
In summary, our results suggest that thermally induced sex reversal in Central Bearded Dragons creates a truly novel phenotype that combines female gonadal function with ‘male-like’ morphology, thermal preference, and behaviour. That combination of traits likely affects individual fitness, and hence may play a major role in driving or preventing rapid shifts in sex-determining systems in the wild.
Supplementary Material
Acknowledgements
We thank Wendy Ruscoe for assistance with animal husbandry and data curation. The Institute for Applied Ecology at the University of Canberra supported the Reptile Housing Facility that made this project possible.
Ethics
All research was conducted under the University of Canberra animal ethics protocol CEAE 15-01 and the University of Sydney animal ethics protocol 2015/789.
Data accessibility
Data are available from Dryad (http://dx.doi.org/10.5061/dryad.55174).
Authors' contributions
R.S. and H.L. conceived and designed the experiments. H.L. performed the experiments. C.H. conducted molecular sex testing. H.L. and R.S. analysed the data. All authors contributed to writing the paper.
Competing interests
The authors declare no competing interests.
Funding
H.L. thanks the Jiangsu Overseas Research & Training Program for its support in the form of a University Prominent Young & Middle-Aged Teacher's Fellowship; H.L. was funded by the National Natural Science Foundation of China (31400341), R.S. was funded by the Australian Research Council Laureate Fellowship FL12010007; A.G. and C.E.H. were funded by the Australian Research Council Discovery grant no. DP110104377.
References
- 1.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. 2005. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685–692. ( 10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 2.Shine R, Elphick MJ, Donnellan S. 2002. Co-occurrence of multiple, supposedly incompatible modes of sex determination in a lizard population. Ecol. Lett. 5, 486–489. ( 10.1046/j.1461-0248.2002.00351.x) [DOI] [Google Scholar]
- 3.Sarre SD, Georges A, Quinn A. 2004. The ends of a continuum: genetic and temperature-dependent sex determination in reptiles. Bioessays 26, 639–645. ( 10.1002/bies.20050) [DOI] [PubMed] [Google Scholar]
- 4.Uller T. 2011. From the origin of sex-determining factors to the evolution of sex-determining systems. Q. Rev. Biol. 86, 163–180. ( 10.1086/661118) [DOI] [PubMed] [Google Scholar]
- 5.Radder RS, Quinn AE, Georges A, Sarre SD, Shine R. 2008. Genetic evidence for co-occurrence of chromosomal and thermal sex-determining systems in a lizard. Biol. Lett. 4, 176–178. ( 10.1098/rsbl.2007.0583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warner DA, Shine R. 2008. The adaptive significance of temperature-dependent sex determination in a reptile. Nature 451, 566–568. ( 10.1038/nature06519) [DOI] [PubMed] [Google Scholar]
- 7.Kato Y, Kobayashi K, Watanabe H, Iguchi T. 2011. Environmental sex determination in the branchiopod crustacean Daphnia magna: deep conservation of a doublesex gene in the sex-determining pathway. PLoS Genet. 7, e1001345 ( 10.1371/journal.pgen.1001345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutzke WHN, Crews D. 1988. Embryonic temperature determines adult sexuality in a reptile. Nature 332, 832–834. ( 10.1038/332832a0) [DOI] [PubMed] [Google Scholar]
- 9.Sarre SD, Ezaz T, Georges A. 2011. Transitions between sex-determining systems in reptiles and amphibians. Annu. Rev. Genomics Hum. Genet. 12, 391–406. ( 10.1146/annurev-genom-082410-101518) [DOI] [PubMed] [Google Scholar]
- 10.Quinn AE, Georges A, Sarre SD, Guarino F, Ezaz T, Marshall Graves JA. 2007. Temperature sex reversal implies sex gene dosage in a reptile. Science 316, 411 ( 10.1126/science.1135925) [DOI] [PubMed] [Google Scholar]
- 11.Holleley CE, O'Meally D, Sarre SD, Marshall Graves JA, Ezaz T, Matsubara K, Azad B, Zhang X, Georges A. 2015. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature 523, 79–82. ( 10.1038/nature14574) [DOI] [PubMed] [Google Scholar]
- 12.Cogger H. 2014. The reptiles and amphibians of Australia. Sydney, Australia: Reed Books. [Google Scholar]
- 13.Brown C, Jones F, Braithwaite V. 2005. In situ examination of boldness-shyness traits in the tropical poeciliid, Brachyraphis episcopi. Anim. Behav. 70, 1003–1009. ( 10.1016/j.anbehav.2004.12.022) [DOI] [Google Scholar]
- 14.Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A. 2010. Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc. R. Soc. B 277, 1571–1579. ( 10.1098/rspb.2009.2128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biro PA, Stamps JA. 2015. Using repeatability to study physiological and behavioural traits: ignore time-related change at your peril. Anim. Behav. 105, 223–230. ( 10.1016/j.anbehav.2015.04.008) [DOI] [Google Scholar]
- 16.Harlow PS. 1996. A harmless technique for sexing hatchling lizards. Herpetol. Rev. 27, 71–72. [Google Scholar]
- 17.Brown D. 2009. Hemipenal transillumination as a sexing technique in Varanids. Biawak 3, 26–29. [Google Scholar]
- 18.Quinn AE, Ezaz T, Sarre SD, Marshall Graves JA, Georges A. 2010. Extension, single-locus conversion and physical mapping of sex chromosome sequences identify the Z microchromosome and pseudo-autosomal region in a dragon lizard, Pogona vitticeps. Heredity 104, 410–417. ( 10.1038/hdy.2009.133) [DOI] [PubMed] [Google Scholar]
- 19.Schwager SJ. 1984. Bonferroni sometimes loses. Am. Stat. 38, 192–197. [Google Scholar]
- 20.Nakagawa S. 2004. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav. Ecol. 15, 1044–1045. ( 10.1093/beheco/arh107) [DOI] [Google Scholar]
- 21.Ober C, Loisel DA, Gilad Y. 2008. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 9, 911–922. ( 10.1038/nrg2415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackburn DG, Bernardo VA. 1998. Sexual dimorphism and testosterone responsiveness in hypaxial muscles of the northern leopard frog, Rana pipiens. Amphibia-Reptilia 19, 269–279. ( 10.1163/156853898X00179) [DOI] [Google Scholar]
- 23.De Vries GJ, et al. 2002. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 22, 9005–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. 2006. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J. Neurosci. 26, 2335–2342. ( 10.1523/JNEUROSCI.3743-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ. 2006. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 16, 995–1004. ( 10.1101/gr.5217506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Oortmerssen GA, Sluyter F. 1994. Studies on wild house mice. V. Aggression in lines selected for attack latency and their Y-chromosomal congenics. Behav. Genet. 24, 73–78. ( 10.1007/BF01067930) [DOI] [PubMed] [Google Scholar]
- 27.Sluyter F, Van Oortmerssen GA, de Ruiter AJ, Koolhaas JM. 1996. Aggression in wild house mice: current state of affairs. Behav. Genet. 26, 489–496. ( 10.1007/BF02359753) [DOI] [PubMed] [Google Scholar]
- 28.Heinrich B. 1977. Why have some animals evolved to regulate a high body temperature? Am. Nat. 111, 623–640. ( 10.1086/283196) [DOI] [Google Scholar]
- 29.Belliure J, Carrascal LM, Díaz JA. 1996. Covariation of thermal biology and foraging mode in two Mediterranean lacertid lizards. Ecology 77, 1163–1173. ( 10.2307/2265585) [DOI] [Google Scholar]
- 30.Sih A, Bell A, Johnson JC. 2004. Behavioural syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 31.Careau V, Bininda-Emonds ORP, Thomas WD, Réale D, Humphries MM. 2009. Exploration strategies map along fast-slow metabolic and life-history continua in muroid rodents. Funct. Ecol. 23, 150–156. ( 10.1111/j.1365-2435.2008.01468.x) [DOI] [Google Scholar]
- 32.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368. ( 10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from Dryad (http://dx.doi.org/10.5061/dryad.55174).


