Abstract
Seawater pH and the availability of carbonate ions are decreasing due to anthropogenic carbon dioxide emissions, posing challenges for calcifying marine species. Marine mussels are of particular concern given their role as foundation species worldwide. Here, we document shell growth and calcification patterns in Mytilus californianus, the California mussel, over millennial and decadal scales. By comparing shell thickness across the largest modern shells, the largest mussels collected in the 1960s–1970s and shells from two Native American midden sites (∼1000–2420 years BP), we found that modern shells are thinner overall, thinner per age category and thinner per unit length. Thus, the largest individuals of this species are calcifying less now than in the past. Comparisons of shell thickness in smaller individuals over the past 10–40 years, however, do not show significant shell thinning. Given our sampling strategy, these results are unlikely to simply reflect within-site variability or preservation effects. Review of environmental and biotic drivers known to affect shell calcification suggests declining ocean pH as a likely explanation for the observed shell thinning. Further future decreases in shell thickness could have significant negative impacts on M. californianus survival and, in turn, negatively impact the species-rich complex that occupies mussel beds.
Keywords: ocean acidification, California mussel, California current large marine ecosystem, ocean pH, shell thickness, Mytilus californianus
1. Introduction
As fossil fuel-sourced carbon dioxide continues to enter the surface ocean, ocean pH is decreasing, along with the concentration of carbonate ions available to calcifying organisms [1]. As a result, many recent studies of marine species have focused on how such changes affect the abilities of organisms to calcify (reviewed in [2,3]). Our current knowledge of how marine calcifiers are likely to respond to changing ocean pH is based, to a large extent, on laboratory experiments. An increasing number of studies have placed a variety of different organisms in seawater with pCO2 levels ranging from approximately 380 to more than 3000 µatm and carefully monitored the responses. The majority of these experiments are short-term, ranging from multiple days to a few weeks (e.g. [2–5]). Across all studies, a complex picture emerges. Calcification in some species decreased, while those of others increased with increasing pCO2; several showed highest rates of calcification at intermediate pCO2 levels, and some species showed no significant change even under very high pCO2 (e.g. [6]; see [3,7] for reviews).
In the case of shells, calcification is also affected by parameters other than seawater carbonate saturation state—notably temperature [8,9], wave exposure (e.g. [10]), food availability [11] and predation pressure [12,13], some of which can interact, either counteracting the effects of changing seawater saturation state [11] or exacerbating them [3]. All of these factors probably contribute to how a species modulates calcification internally (e.g. [14]). In general, available data suggest that considerable variation exists within and among taxa in their ability to calcify and/or survive under changing CO2 regimes, but the causes of that variation remain poorly understood.
Experiments have the advantage of being able to manipulate the saturation state of seawater and permit accurate measurements of the responses of the calcifiers, but the majority of the experiments so far have been short-term. Several exceptional long-term experiments show the existence of transgenerational effects on calcification [15], and the potential for adaptation [16] and evolutionary change [17,18]. However, such experiments are difficult for species with longer generation times or complex life cycles, and the responses of long-lived benthic marine species to ocean acidification (OA) remains poorly known [7]. Of particular importance is the role of intra-specific variation in calcification responses. Among population variation that is due to plasticity, standing genetic variation or local adaptation is likely to be important in determining the long-term consequences for many species [7,19,20], including macrobenthic species that have key functions in marine ecosystems. Thus, a more complete understanding of species responses to anthropogenic OA requires integrating experimental insights with information about how calcification rates vary in real populations over time, reflecting net responses to different selective forces, data that are currently scarce. Here, we quantify temporal trends in shell calcification of an intertidal mussel, Mytilus californianus, using field surveys in conjunction with archaeological and historical information. Specifically, we investigate how shell calcification in this species has changed over decadal to millennial scales and also discuss the implication of such changes for the future of this species.
Marine mussels in the genus Mytilus are found across sharp environmental gradients in intertidal and subtidal areas. Many Mytilus species are locally abundant and can play a foundational role in coastal environments by filtering great amounts of seawater and creating habitats that harbour a diverse assemblage of algal and invertebrate species [21–24]. Marine mussels are also extensively harvested for human consumption and are an important component of commercial aquaculture. Because of their importance as a food item and easy accessibility, intertidal mussels have been the target of subsistence harvesting over millennia and Mytilus shells are often common in archaeological middens. Owing to both their global importance and findings that suggest they are vulnerable to a changing ocean carbonate system [25,26], an assessment of changes to shell calcification in marine mussels over time is needed on a time scale relevant to population and evolutionary processes as well as changes in ocean chemistry. Here, we address this issue by testing whether shell calcification in M. californianus has changed in the northeast Pacific Ocean over decadal and millennial time scales. Inferring the cause(s) of any observed long-term trend in shell calcification can be challenging, given the diverse set of processes that influence calcification, many of which vary with the microhabitats a species experiences. We thus consider the role of diverse environmental drivers, including testing the effect of variation along intertidal gradients. Despite the potential for differences in shell thickness through time to be muted by confounding factors, we find strong differences across decadal and millennial scales.
2. Material and methods
We compared individual measurements from living populations of M. californianus with those from archival shell collections and previous studies at two different sites in the northeast Pacific: Tatoosh Island, WA (48.32° N, 127.74° W) and Sand Point, WA (48.126° N, 124.702° W) in the Olympic National Park, USA (electronic supplementary material, table S1). Tatoosh Island has been used by the Makah tribe for millennia [27,28] and has been the site of extensive ecological investigation of intertidal communities for five decades [25,29–32]. Sand Point is 30 km south of Tatoosh Island and also has a documented history of human shellfish harvesting for millennia [27]. Both sites are relatively remote and free of impacts such as point-source pollution and agricultural runoff. Based on the availability of historical information, we present analyses at two different temporal scales—millennial and decadal.
(a). Millennial-scale comparisons
We used archival shell collections to test whether shell thickness of mussels (a proxy for calcification) has changed through time by measuring the thickness of the calcitic inner prismatic layer (e.g. [33]) of modern shells collected from 2009–2011 and that of shells from the archaeological middens. Measuring the thickness after sectioning the shell along the axis of maximum growth allowed us to estimate thickness in a uniform way, even for partially preserved shells. At Tatoosh Island, we collected live M. californianus approximately one decade old in 2009 (n = 31 among three sites; electronic supplementary material, table S1). We compared these large, modern shells to Native American midden shells radiocarbon dated from 1000–1340 years BP in 2010 (AD 663–1008, analysed at the National Ocean Sciences Accelerator Mass Spectrometry facility, www.whoi.edu/nosams; n = 11; estimated lengths 80–117 mm) provided by the Makah Cultural and Research Center in 2009. At Sand Point, we obtained midden shells that ranged from 55 to 112 mm in estimated length (n = 18) and subsampled 10 shells to obtain radiocarbon dates from 2150–2420 years BP in 2015. Modern shells were collected at two locales closest to the midden site at approximately 1.0 m above mean lower low water (MLLW), with six collected 1 km north of the midden site by C.A.P. (Sand Point North, 48.135° N, 124.415° W, measured lengths = 49–62 mm, July 2010) and 10 collected by JTW at an adjacent rocky point 2 km south (Yellow Banks, 48.104° N, 124.670° W, measured lengths = 98–117 mm, August 2010). After collection, we removed the soft tissue and any epibionts, dried the shells to a constant mass, and measured the length and mass of a single valve. Because of post-mortem breakage of midden shells, we measured the fragment size and then estimated the total length of the individual using extrapolations based on the living mussels. Of the 18 midden shells we obtained from Sand Point, three could not be aged due to poor preservation of annual bands.
Annual and total shell growth was estimated by measuring the calcitic inner prismatic layer of the shell in a perpendicular direction to the main anterior–posterior axis of the shell, after preparing the shells using the methodology described by Pfister et al. [34]. Although there are many regions in cross-section where annual growth bands can be seen, we measured them in the thickest area of calcite in the shell distal to the umbo region. Measurements were taken from digital images using ImageJ (http://imagej.nih.gov/ij). Years were matched to each shell layer by assigning the first layer near the mantle as the most recent year of growth prior to collection. Successively older annual bands were then counted outward. The year of recruitment (1999–2000) for a cohort of the modern shells from Tatoosh Island was known [35], and thus confirmed the accuracy of our identification of annual bands. Although annual banding has been confirmed in many molluscs and is often attributed to winter cessation of growth [36], the precise cause and role, if any, of dissolution remains unknown. In cases where distinct annual bands were difficult to determine, the total thickness of the calcite was still measured, but the shell was not used when analysing thickness related to age. To control for age differences among shells, we restricted our analyses to shells of similar lifespans in each time bin and thus focused on shells greater than 5 years of age. The shell material that persisted in the middens was from larger/older mussels and thus our modern samples also focused on longer-lived shells.
The age of the midden shells thus did not differ significantly from modern ones (mean of 7.07 years versus 6.44, F1,29 = 0.690, p = 0.413), but our samples of living individuals (in 2010) from two localities surrounding Sand Point differed in the length-to-age relationship: shells from Yellow Banks were longer per year of age than those from Sand Point North. We combined the two sites in order to bracket the greatest range of relationships that we might see in the midden shells and make the most conservative comparison, but our conclusions are qualitatively the same when we use only the larger modern shells from Yellow Banks.
(b). Decadal-scale comparisons
On Tatoosh Island, we compared the thickness of shell calcite layers of the 2009 population to shells collected by T.H.S. at the Glacier (GL) site and archived (n = 6). Other historical measurements from the GL site (1974–1976 versus 2011), as well as the North Island (NI) site (2001 versus 2011–2012), were made for smaller individuals. For Tatoosh GL, we used the measurements reported by Paine [29], supplemented by those of Suchanek [37], because the shells were measured in the 1970s and no longer exist. In cases where the original data from the published studies were not available, we digitized the x and y coordinates of each point in the published figures using ImageJ. These 1970s mussel measurements from the GL site were the smallest measurable individuals (13–45 mm in length) and reside at the upper margin of the mussel bed. We made a corresponding collection of 130 small individuals less than 46 mm SL at GL in 2011–2012 for comparisons to these earlier studies by T.H.S. and R.T.P. The collection of the smallest GL individuals was at the upper edge of the mussel bed (approx. 2.0 m above MLLW) where R.T.P. and T.H.S. had noted that they collected. We made further comparisons at the Tatoosh NI locales of Sweet 16 and NW Point using live shells collected in 2001 and 2011–2012. We compared live-collected shells with those collected in the middle tide heights (approx. 1.0 m above MLLW) of the mussel bed by E.S. in 2001 (n = 42) [38]. For comparison, at the two NI sites, C.A.P. collected 111 living individuals from the mid intertidal zone in 2011 and 2012, and targeted the same range of lengths (56–72 mm). After collection, we removed the soft tissue and any epibionts, dried the shells to a constant mass, and measured length and mass.
(c). Habitat-related variation
Because intertidal areas over which many Mytilus species range represent a strong gradient of emersion and access to food, we tested the effect of intertidal height on mass-to-length relationships in M. californianus. In 2011 and 2012, C.A.P. supplemented the 111 mid intertidal mussels at the NI sites, with 45 shells in the highest part of the mussel bed (approx. 2.0 m above MLLW) and 59 in the lowest band of the mussel bed (approx. 0.0 m) for a total of 215 M. californianus sampled from Tatoosh Island ranging from 39.8 to 87.6 mm in length. Mytilus californianus spans a large vertical gradient at these locales, because they face into a prominent swell direction, allowing us to look for submersion effects over a pronounced gradient.
3. Results
At Tatoosh Island, the total thickness of midden shells of M. californianus older than 5 years of age and living between AD 663 and 1008 was on average 27.6% greater than that of a modern counterpart, while archival shells from the 1970s were similar to midden shells and 32.2% thicker than modern shells (figure 1a). When shell thickness was normalized to either shell length or to the age of the individual, the midden shells remained thicker per unit length (42.1%) and thicker per unit age (32.9%) compared with modern shells (figure 1b,c). Archival shells, when normalized to shell length, were thicker than modern shells (figure 1b), but significantly thinner per unit length than midden shells (figure 1b).
Figure 1.
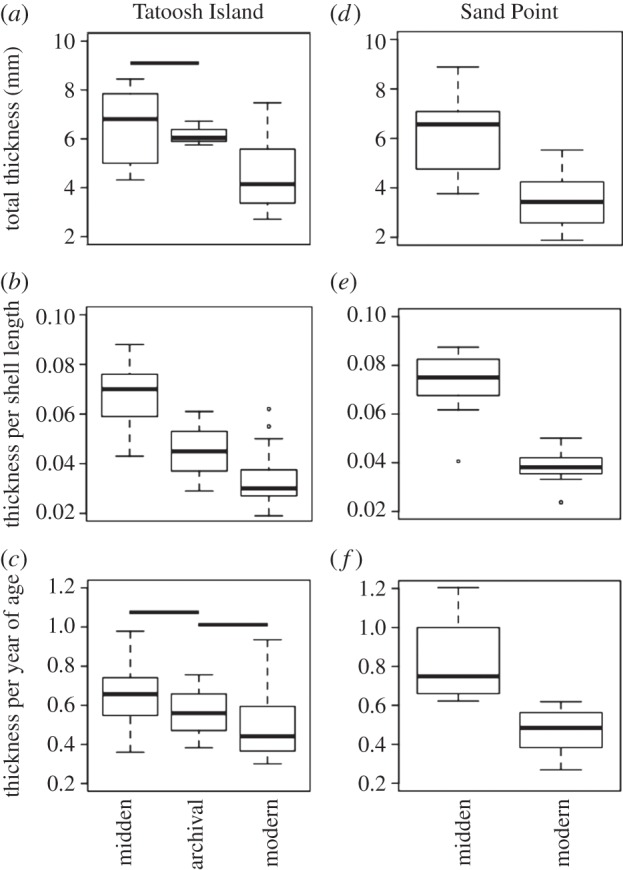
A comparison of the relative thickness of modern, archival and midden Mytilus californianus at two sites in Washington state: Tatoosh Island (a–c) and Sand Point (d–f); the total thickness (a,d), the thickness per shell length (b,e) and the thickness per year of age (c,f) were greater in the past at both sites. Boxplots show medians and lower and upper quantiles. ANOVA and Tukey HSD indicated significant differences among Tatoosh Island time periods, excepting those denoted with shared horizontal lines (n = 11 midden, n = 5 archival, n = 31 modern shells). Sand Point midden shells (n = 12–15) always differed significantly from their modern counterparts (n = 11) ((d) t = 6.071, d.f. = 32, p < 0.001; (e) t = 9.896, d.f. = 32, p < 0.001; (f) t = 6.487, d.f. = 30, p < 0.001). Only shells older than 5 years of age based on growth bands were included.
At Sand Point, WA, midden shells dated from 2150 to 2420 years BP were also thicker (93.9%) than modern shells of comparable size (figure 1d). The thickness per unit length (figure 1e) or thickness per year of age (figure 1f) of these midden shells was almost twice that of their modern counterparts.
Because shells from modern populations were always thinner than shells from the last century or earlier, we asked whether this thinning showed a trend with age in modern individuals. A linear mixed-effects model found no effect of year in the annual shell thickness from 1997 to 2010 at Tatoosh Island (individual shell was a random effect, year was a fixed effect; coeff. = 0.0053, p = 0.313, number of measurements = 225, number of shells = 24). When we tested whether the annual thickness as a function of age differed between modern and midden shells at Tatoosh Island, we found no difference in the intercept (p = 0.209), slope (p = 0.137) or interaction term (p = 0.608, number of observations = 328, n = 34). Thus, there was no systematic change in the thickness of the annual bands of Tatoosh Island mussels during the lifespan of each individual, regardless of whether they lived approximately 1000 years ago or in the past two decades. Similarly, the Sand Point M. californianus shells showed no age-related trend, regardless of whether they were midden or modern shells (year coeff. = −0.016, p = 0.211; interaction coeff. = 0.022, p = 0.238). The intercept for midden shells, however, was significantly greater than that of modern shells (0.892 versus 0.431, p = 0.0002, number of measurements = 175, number of shells = 23). Thus, although overall shell thickness of M. californianus has decreased significantly over time at both of our sites, the width of annual increments of shell growth has not changed systematically over the past decades.
The shell mass per unit length of the smallest M. californianus showed no significant change from the 1970s compared with 2011–2012 on Tatoosh Island (figure 2). Model II regression (R package ‘lmodel2’; www.r-project.org) indicated that the confidence intervals for both the intercepts and the slopes overlapped for both archival and modern shells at the GL site. Similarly, at the NI site, shell thickness per length also did not differ in 2011–2012 versus 2001 for mussels occupying the middle of the mussel bed and ranging in length from 56 mm to 72 mm (figure 3).
Figure 2.
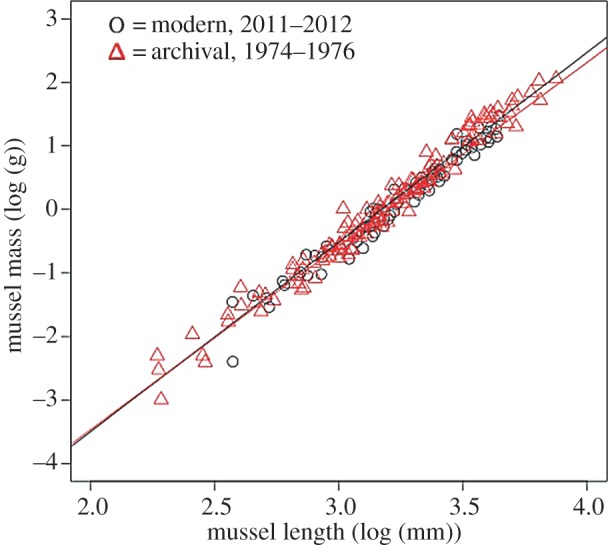
The mass-to-length relationship among 130 mussels on Tatoosh Island at the GL site 2011–2012, compared with shells from 1974–1976 [37] (R.T.P., unpublished; n = 86; 13–45 mm length) at the highest zone of the intertidal. Log mass (g) versus log length (mm) of both valves is plotted; confidence intervals for the slope and the intercept overlapped between the two groups with Model II regression. The modern mussels had a slope of 3.076 and an intercept of −9.752 (r2 = 0.967), while the archival shells had a slope of 2.978 and an intercept of −9.528 (r2 = 0.968).
Figure 3.
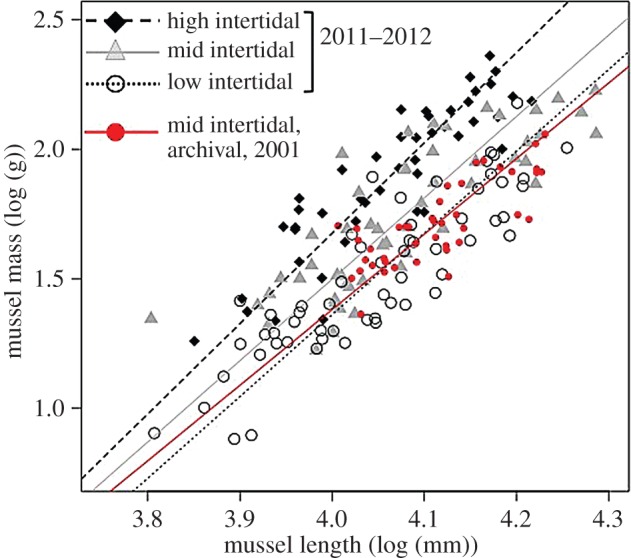
The mass-to-length relationship among 215 mussels at the Sweet 16 and NW Point sites on Tatoosh Island in 2011–2012, demonstrating that at the same locale, mussels that spend more time emergent are thicker per unit size. There was no statistical interaction between tide height and shell length as a predictor of shell mass (ANOVA, F3,207 = 0.892, p = 0.446), indicating the slopes did not differ for high = 3.488 (dashed line), mid = 3.142 (grey line) and low = 3.156 (dotted line). However, the investment of shell mass : length did differ and shells in the high zone were thicker than those in either the mid or low zones (ANCOVA, F3,207 = 37.713, p < 0.001, high =−12.277, mid =−11.067, low =−11.263). The red points are 42 Mytilus californianus collected at the same site in 2001, at the mid intertidal height. These shells showed a statistically similar mass-to-length relationship as the mid intertidal mussels from 2011–2012 (slope = 2.917, intercept =−10.287, r2 = 0.612). Best-fit lines from a Model II regression (major axis) are shown. Log mass (g) versus log length (mm) of a single valve is plotted.
Our collection of individuals across three specific tidal heights (highest zone, mid zone and lower zone) showed no statistical interaction between tide height and shell length as a predictor of shell mass (ANOVA, F3,207 = 0.892, p = 0.446), indicating the slopes did not differ (figure 3). However, the investment of shell mass per unit length did differ and shells in the high zone were significantly thicker than those in either the mid or low zones (F3,207 = 37.713, p < 0.001), highlighting plasticity in shell thickness.
4. Discussion
Our results show that shells of M. californianus in Washington State are significantly thinner today compared with conspecific individuals in middens dating from 1000 to 2500 years BP. Although our extrapolation of shell dimensions in midden shells assumed that shell shape did not change, we note that this would not have changed our finding that these midden shells surpass in thickness or in thickness per unit age any modern shell we have ever observed in the vicinity of these midden locales. When we compared mussels in the largest sizes on Tatoosh Island over recent decades, total thickness and thickness per shell length were significantly lower in modern shells compared with archived shells from the 1970s; however, thickness per shell age did not exhibit a significant relationship. For mussels in the smallest sizes, we found no significant difference in the mass per unit size of mussels from the 1970s until the present (figure 2) or over the past decade (figure 3). For both sets of shells, the mass per unit shell length was unchanged through time.
The relationship of shell mass to length was a function of tidal height, with the most calcium carbonate per unit length seen in the highest part of the tidal range of M. californianus (figure 3). These tidal height patterns highlight the importance of comparing shells through time from areas of similar intertidal height. Owing to the dispersal distance of California mussel larvae, their propensity to dislodge and move as adults, and the lack of substantial genetic differentiation across the geographical range of M. californianus [39], could be the result of strong local selection or a phenotypic response to local conditions. Although increased submergence time and access to food is known to increase linear growth rates in M. californianus [40,41], it may also result in increased allocation to the thickness of the shell material with increasing tidal height, as demonstrated at other locales for Mytilus species. For example, M. edulis in north Wales showed increased shell mass to area at greater intertidal heights, a pattern attributed to increased protection from predators [42], but may also be due to decreased food [22]. Regardless of the cause, shell thickness differences across the intertidal zone provide a cautionary tale about latitudinal or temporal comparisons among archival shells and motivated our careful comparison among studies. For the midden versus modern comparisons, there is no possibility that intertidal height plays a role in explaining the differences, as we never found modern mussels on Tatoosh Island that rivalled those of the midden shell in thickness. For the NI shells collection in figure 3, we compared the shell at the middle level based on collection notes (by E.S.).
The discrepancy between a decline in our measure of shell calcite thickness (figure 1) while shell mass-to-length ratio was unaffected (figures 2 and 3) is somewhat at odds with the result that the two variables were positively related among 48 live-collected individuals of M. californianus (correlation coefficient = 0.764), though there was considerable scatter around the relationship (r2 = 0.58). The decrease through time in shell calcite thickness may indicate that the thickness of the shell calcite layer is more sensitive to changes in environmental conditions than total shell mass. A second reason that shell calcite thickness showed a decline when shell mass : length did not may be related to the observation that the thickness of the calcite layer (per unit length) is a less variable metric by half than the total shell mass (per unit length) (CV = 28.2 versus 56.4), perhaps indicating that detecting relatively small population-level changes in shell mass per unit length would require sample sizes larger than the ones used here or necessitate more precise metrics of size than only length. However, because the proximal and thickest region of the shell is generally preserved in archival specimens, calcite thickness may serve as an accurate metric of shell growth through time.
The long-term decline in shell thickness over the past millennia (figure 1) has several potential explanations, including a decrease in food availability, changes in seawater temperature, changes in predator-induced mortality and reduced calcification due to increasing pCO2 of the coastal ocean (figure 4). Shell calcification is affected by a multitude of biotic and abiotic factors. Because many of these parameters are changing in response to anthropogenic activities, understanding how calcifiers will respond to changing ocean carbon chemistry requires models that account for all direct and indirect influences on calcification and energetic trade-offs within the animal.
Figure 4.
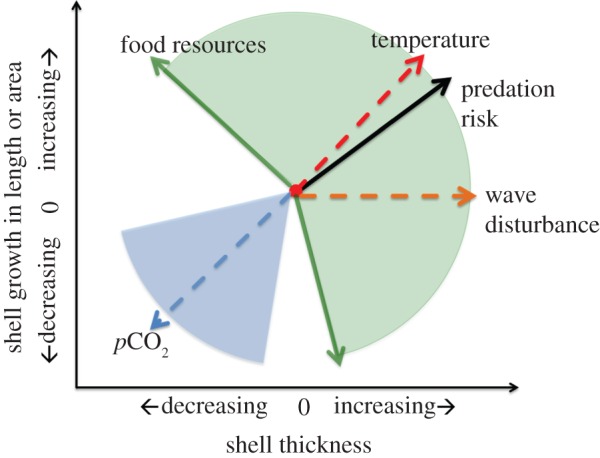
Possible shell length (or area) and thickness responses of a mussel shell to various biotic (solid) and abiotic factors (dashed) represented as vectors. The figure highlights the range of possible shell growth responses to environmental variables, and we suggest that coincident, opposing and interacting [43] environmental variables are possible. The green area designates the range of possible responses to increased food resources, including increases in both length and thickness, while the blue area shows possible negative responses to ocean acidification (e.g. [44]).
In considering an integrated picture of animal investment to shell growth (figure 4), we placed shell area and shell thickness on different response axes to illustrate that environmental factors can have independent effects on each. We recognize, however, that environmental factors and shell responses can interact. Some molluscs also invest in increasing size for a period of time prior to investment in thickness (e.g. limpets in the genus Patella [45]). Thus, studies need to be attentive to the natural ontogenetic changes in shell growth to identify the appropriate response variable; short-term responses may not indicate the organism's response over its lifespan. Because we consider that museum specimens and the fossil record provide excellent archival material over the lifespan of the individual, we must look critically at which environmental drivers affect shell material investment in overall dimensions of area (size) versus thickness per unit area of shell. Further, investment in shell dimensions related to overall size versus investment to thickness can have distinct ecological outcomes for shells in their ecosystems and can imply different constraints [39].
The direct effect of increasing pCO2 has been shown to produce a range of calcification and minerology responses in molluscs, both in the laboratory and in nature. Decreased size and thickness of mussels has resulted from elevated pCO2, including during larval stages [26]. There are also an increasing number of studies that show decreased growth in shell area with decreasing pH (see review in [15]), sometimes as a result of net dissolution [46] and reduced shell density [47]. Melzner et al. [48] recorded dissolution internally in a congener (M. edulis) of our focal species, though we note that M. edulis has an aragonitic inner layer that is more susceptible to dissolution. Dissolution may also contribute to the annual banding pattern, as shown for other bivalves where winter conditions and low oxygen conditions promote dissolution and the formation of a darker organic-rich band [36]. Because the potential role of dissolution is untested here, we assumed that shell thickness reflected total calcification. Some species have been shown to maintain overall size dimensions while reducing shell thickness, as in the juveniles of M. galloprovincialis [49]. Shell composition can also change with pH. The mussel M. galloprovincialis showed a decrease in the amount of aragonitic nacreous material in the shell as pH decreased in proximity to CO2-emitting vents [50]. Further, material properties of shells can be affected, with increased pCO2 increasing the stiffness of the outer shell material while increasing the softness of the interior shell in M. edulis [9].
While food availability can potentially affect shell calcification [11,51] and long-term global declines in phytoplankton have recently been reported [52], measurements of open ocean chlorophyll closest to our sites show no such decline over this period [34]. Nonetheless, in the absence of a millennial-scale database on phytoplankton abundance, changing food availability remains a possible explanation for the changes seen here. Though shell calcification rates in Mytilus correlate positively with temperature and salinity [53,54], analyses of the stable isotopes of oxygen in M. californianus through time provided no evidence of significant changes in seawater temperature over the past millennium in our focal region [34] and thus provide little support for warmer sea surface temperatures favouring thicker shells during the period 1000–2500 years BP.
Predators are another factor that influence the thickness of many marine molluscs [13], with a reduction in shell thickness reflecting a decline in predation pressure. Predation is a process that may have changed through time to change the selection pressures on mussel shell construction. Sea otter (Enhydra lutris) abundance in the Pacific Northwest is known to have decreased markedly over time [55], and there is evidence that they were used and hunted by Native Americans in this region, although their remains are nearly absent in the Sand Point midden [56]. Shell thickness may offer little protection against predation by sea otters, which prey on an entire patch of mussels at once [57]. More importantly, sea otters were successfully reintroduced south of Sand Point in 1970, and have experienced rates of population growth between 10% and 20% during the 1980s and 1990s, respectively [58]. This predicts that if shell thickness confers any protection against sea otters, it should be increasing at both Tatoosh Island and Sand Point, opposite of the trend we document. The black oystercatcher (Haematopus bachmani) is another predator of M. californianus, but oystercatcher abundance has been nearly constant for the past two decades at Tatoosh Island (J.T.W. 1974, unpublished data). Although their numbers prior to that are unknown, they are not crushing predators and are thus also unlikely to exert strong selection on shell thickness. The ability of sea stars to consume M. californianus is likely to be more dependent on the strength of the adductor muscle, not shell thickness, and this predator was common at all of our sites until 2015 [59]. Drilling predators such as whelks, on the other hand, are likely to select for increased shell thickness because a thicker, larger shell offers protection from such predation. Whelks were abundant in Native American middens in Washington state [27], and one common species (Nucella canaliculata) attains densities of 67.95 per m2 at Tatoosh Island sites where our modern mussels were collected [32], suggesting that whelks have been a persistent selection pressure. The interaction between M. californianus and N. canaliculata varies geographically as a function of alternative prey [60,61]; the interaction is strong where alternative prey are rare (southern part of the range of N. canaliculata in central and northern California) and the snail drills individuals in excess of 100 mm, and weak where alternative prey such as barnacles are common (Oregon and Washington), where M. californianus is rarely drilled above a size of 30 mm [62,63]. In sum, the available information about spatial and temporal changes in predator communities does not provide any compelling evidence that predation pressure has had any significant influence on the concordant long-term declines in shell calcification documented here. Indeed, if decreased shell mass is occurring even as predator numbers are relatively high, as is suggested by our study, then modern mussels may be poorly matched with the predator mortality they will experience.
A difference in thickness between the midden shells and modern populations could also emerge if Native Americans were consistently harvesting shells larger than those in our modern samples. A bias towards large size is a common characteristic of marine invertebrates in archaeological middens because selective harvesting of larger individuals for human consumption is a common practice [64]. However, our thickness estimates for modern shells from Tatoosh Island (figure 1) are also biased towards large sizes because we specifically collected the largest/oldest shells for comparison with midden specimens. Additionally, we controlled for shell size and age in our analyses. Thus, it is highly unlikely that the observed trend of reduced thickness over time reflects a size-related sampling bias.
Finally, shell thickness of mussels may also be affected by wave exposure, as demonstrated in other molluscs [65], but available evidence suggests an increase in wave height over past decades [66], not the decrease expected to produce the observed decrease in shell mass shown here.
Available evidence suggests that the long-term decline in shell calcification in M. californianus may represent a response to OA. The decreases in pH documented at Tatoosh Island since 2000 [25,67] and the low pH and aragonite saturation states seen in California [68,69] suggest that carbon cycle changes in the ocean are producing an environment where aragonite and calcite are more difficult for organisms to accrete.
More importantly, regardless of the exact cause(s), the fact that shells of M. californianus are substantially thinner now compared with decades or centuries ago raises concerns about whether this species can retain its current role as a foundation species if shell thickness further declines and these thickness decreases lead to increased vulnerability to predation and disturbance (e.g. [13,43,70]). Similarly, if mussel byssal thread function is weakened with OA [71], and the viability of M. californianus at its upper intertidal limit is compromised by increased ambient temperatures [72], the net effect may be a decreased niche and a smaller climate envelope within which the California mussel can persist, altering the species interactions under which it evolved. If continued reductions in shell thickness significantly increase mortality risk for M. californianus, the integrity of their structurally complex mussel beds that support over 300 species of associated organisms [21,24] could be compromised, potentially reducing associated intertidal species throughout the range of the California mussel.
There is a clear need for careful monitoring of seawater chemistry, for further studies of shell calcification of this foundation species, and for increased understanding of the physiological, mineralogical and genetic aspects of shell calcification to aid in the development of predictive models of species response to OA. Mussels not only provide an opportunity to understand the link between seawater chemistry and calcification, but their ecosystem importance and strong links to the carbon cycle make this imperative [73].
Supplementary Material
Acknowledgements
We are grateful to the Makah Tribal Nation for the access to Tatoosh and midden shells, with special thanks to J. Ledford, G. Wessen and the Makah Cultural and Research Center. The Olympic National Park loaned Sand Point material (Permit OLYM-2010-SCI-0056); thanks to D. Conca and G. Hunter. We thank C. Belanger, A. Barner, B. Colson and G. Siegmund for help with measurements, and D. Boyce for chlorophyll data. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Authors' contributions
C.A.P., K.R. and S.J.M. initiated the research. C.A.P., S.J.M., R.T.P., T.H.S., E.S. and J.T.W. collected data and provided samples. C.A.P., K.R. and S.J.M. performed analyses. All authors contributed to data interpretation and to writing the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Funding was provided by The SeaDoc Foundation, NSF grants OCE01-17801 (J.T.W. and C.A.P.), OCE09-28232 (C.A.P.), DEB09-19420 (J.T.W.), OCE-75-20958 (T.H.S.) and DoD NDSEG, and NSF GRFP Fellowships (S.J.M.).
References
- 1.Orr JC, et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686. ( 10.1038/nature04095) [DOI] [PubMed] [Google Scholar]
- 2.Kroeker KJ, Kordas RL, Crim RN, Singh GG. 2010. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434. ( 10.1111/j.1461-0248.2010.01518.x) [DOI] [PubMed] [Google Scholar]
- 3.Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso J-P. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896. ( 10.1111/gcb.12179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gazeau F, Parker LM, Comeau S, Gattuso J-P, O'Connor WA, Martin S, Pörtner H-O, Ross PM. 2013. Impacts of ocean acidification on marine shelled molluscs. Mar. Biol. 160, 2207–2245. ( 10.1007/s00227-013-2219-3) [DOI] [Google Scholar]
- 5.Sanford E, Gaylord B, Hettinger A, Lenz EA, Meyer K, Hill TM. 2014. Ocean acidification increases the vulnerability of native oysters to predation by invasive snails. Proc. R. Soc. B 281, 20132681 ( 10.1098/rspb.2013.2681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ries JB, Cohen AL, McCorkle DC. 2009. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134. ( 10.1130/G30210A.1) [DOI] [Google Scholar]
- 7.Hofmann GE, Barry JP, Edmunds PJ, Gates RD, Hutchins DA, Klinger T, Sewell MA. 2010. The effect of ocean acidification on calcifying organisms in marine ecosystems: an organism-to-ecosystem perspective. Annu. Rev. Ecol. Evol. Syst. 41, 127–147. ( 10.1146/annurev.ecolsys.110308.120227) [DOI] [Google Scholar]
- 8.Rodolfo-Metalpa R, et al. 2011. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Change 1, 308–312. ( 10.1038/nclimate1200) [DOI] [Google Scholar]
- 9.Fitzer SC, Zhu W, Tanner KE, Phoenix VR, Kamenos NA, Cusack M. 2015. Ocean acidification alters the material properties of Mytilus edulis shells. J. R. Soc. Interface 12, 20141227 ( 10.1098/rsif.2014.1227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox DL, Coe WR. 1943. Biology of the California sea-mussel (Mytilus californianus). II. Nutrition, metabolism, growth and calcium deposition. J. Exp. Zool. 93, 205–249. ( 10.1002/jez.1400930204) [DOI] [Google Scholar]
- 11.Thomsen J, Casties I, Pansch C, Körtzinger A, Melzner F. 2013. Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments. Glob. Change Biol. 19, 1017–1027. ( 10.1111/gcb.12109) [DOI] [PubMed] [Google Scholar]
- 12.Vermeij GJ. 1976. Interoceanic differences in vulnerability of shelled prey to crab predation. Nature 260, 135–136. ( 10.1038/260135a0) [DOI] [Google Scholar]
- 13.Seeley RH. 1986. Intense natural selection caused a rapid morphological transition in a living marine snail. Proc. Natl Acad. Sci. USA 83, 6897–6901. ( 10.1073/pnas.83.18.6897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goffredo S, et al. 2014. Biomineralization control related to population density under ocean acidification. Nat. Clim. Change 4, 593–597. ( 10.1038/nclimate2241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker LM, Ross PM, O'Connor WA, Pörtner HO, Scanes E, Wright JM. 2013. Predicting the response of molluscs to the impact of ocean acidification. Biology 2, 651–692. ( 10.3390/biology2020651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohbeck KT, Riebesell U, Reusch TBH. 2012. Adaptive evolution of a key phytoplankton species to ocean acidification. Nat. Geosci. 5, 346–351. ( 10.1038/ngeo1441) [DOI] [Google Scholar]
- 17.Collins S, Rost B, Rynearson TA. 2014. Evolutionary potential of marine phytoplankton under ocean acidification. Evol. Appl. 7, 140–155. ( 10.1111/eva.12120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez-Romero A, Jarrold MD, Massamba-N'Siala G, Spicer JI, Calosi P. In press. Multi-generational responses of a marine polychaete to a rapid change in seawater pCO2. Evol. Appl. ( 10.1111/eva.12344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly MW, Padilla-Gamiño JL, Hofmann GE. 2013. Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Glob. Change Biol. 19, 2536–2546. ( 10.1111/gcb.12251) [DOI] [PubMed] [Google Scholar]
- 20.Pespeni MH, et al. 2013. Evolutionary change during experimental ocean acidification. Proc. Natl Acad. Sci. USA 110, 6937–6942. ( 10.1073/pnas.1220673110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suchanek TH. 1985. Chapter VI. Mussels and their role in structuring rocky shore communities. In The ecology of rocky coasts (eds Moore PG, Seed R), pp. 70–96. London, UK: Hodder and Stoughton. [Google Scholar]
- 22.Seed R, Suchanek TH. 1992. Population and community ecology of Mytilus. In The mussel Mytilus (ed. Gosling EM.), pp. 87–169. Amsterdam, The Netherlands: Elsevier Science Publishers. [Google Scholar]
- 23.Smith JR, Fong P, Ambrose RF. 2006. Dramatic declines in mussel bed community diversity: response to climate change? Ecology 87, 1153–1161. ( 10.1890/0012-9658(2006)87%5B1153:DDIMBC%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 24.Lafferty KD, Suchanek TH. In press Revisiting Paine's 1966 sea star removal experiment, the most-cited empirical paper in The American Naturalist. Am. Nat . [DOI] [PubMed] [Google Scholar]
- 25.Wootton JT, Pfister CA, Forester JD. 2008. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc. Natl Acad. Sci. USA 105, 18 848–18 853. ( 10.1073/pnas.0810079105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaylord B, Hill TM, Sanford E, Lenz EA, Jacobs LA, Sato KN, Russell AD, Hettinger A. 2011. Functional impacts of ocean acidification in an ecologically critical foundation species. J. Exp. Biol. 214, 2586–2594. ( 10.1242/jeb.055939) [DOI] [PubMed] [Google Scholar]
- 27.Wessen G. 1988. The use of shellfish resources on the Northwest Coast: the view from Ozette. In Research in economic anthropology, supplement 3: prehistoric economies of The Pacific Northwest Coast, pp. 179–207. Greenwich, UK: JAI Press. [Google Scholar]
- 28.McMillan AD. 1999. Since the time of the transformers: the ancient heritage of the Nuu-Chah-Nulth, Ditidaht and Makah. Illustrated edition Vancouver, Canada: University of British Columbia Pr. [Google Scholar]
- 29.Paine RT. 1974. Intertidal community structure: experimental studies on the relationship between a dominant competitor and its principal predator. Oecologia 15, 93–120. ( 10.1007/BF00345739) [DOI] [PubMed] [Google Scholar]
- 30.Paine RT, Levin SA. 1981. Intertidal landscapes: disturbance and the dynamics of pattern. Ecol. Monogr. 51, 145–178. ( 10.2307/2937261) [DOI] [Google Scholar]
- 31.Paine RT, Wootton JT, Pfister CA. 2010. A sense of place—Tatoosh. In The ecology of place: contributions of place-based research to ecological and evolutionary understanding (eds I Billick, MV Price), pp. 229–250. Chicago, IL: University of Chicago Press. [Google Scholar]
- 32.Wootton JT. 1994. Predicting direct and indirect effects: an integrated approach using experiments and path analysis. Ecology 75, 151–165. ( 10.2307/1939391) [DOI] [Google Scholar]
- 33.Dodd JR. 1964. Environmentally controlled variation in the shell structure of a Pelecypod species. J. Paleontol. 38, 1065–1071. [Google Scholar]
- 34.Pfister CA, McCoy SJ, Wootton JT, Martin PA, Colman AS, Archer D. 2011. Rapid environmental change over the past decade revealed by isotopic analysis of the California Mussel in the Northeast Pacific. PLoS ONE 6, e25766 ( 10.1371/journal.pone.0025766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paine RT, Trimble AC. 2004. Abrupt community change on a rocky shore–biological mechanisms contributing to the potential formation of an alternative state. Ecol. Lett. 7, 441–445. ( 10.1111/j.1461-0248.2004.00601.x) [DOI] [Google Scholar]
- 36.Lutz RA, Rhoads DC. 1977. Anaerobiosis and a theory of growth line formation. Science 198, 1222–1227. ( 10.1126/science.198.4323.1222) [DOI] [PubMed] [Google Scholar]
- 37.Suchanek TH. 1981. The role of disturbance in the evolution of life history strategies in the intertidal mussels Mytilus edulis and Mytilus californianus. Oecologia 50, 143–152. ( 10.1007/BF00348028) [DOI] [PubMed] [Google Scholar]
- 38.Sanford E, Roth MS, Johns GC, Wares JP, Somero GN. 2003. Local selection and latitudinal variation in a marine predator-prey interaction. Science 300, 1135–1137. ( 10.1126/science.1083437) [DOI] [PubMed] [Google Scholar]
- 39.Palmer AR. 1981. Do carbonate skeletons limit the rate of body growth? Nature 292, 150–152. ( 10.1038/292150a0) [DOI] [Google Scholar]
- 40.Dehnel PA. 1956. Growth rates in latitudinally and vertically separated populations of Mytilus californianus. Biol. Bull. 110, 43–53. ( 10.2307/1538891) [DOI] [Google Scholar]
- 41.Blanchette CA, Helmuth B, Gaines SD. 2007. Spatial patterns of growth in the mussel, Mytilus californianus, across a major oceanographic and biogeographic boundary at Point Conception, California, USA. J. Exp. Mar. Biol. Ecol. 340, 126–148. ( 10.1016/j.jembe.2006.09.022) [DOI] [Google Scholar]
- 42.Beadman HA, Caldow RWG, Kaiser MJ, Willows R. 2003. How to toughen up your mussels: using mussel shell morphological plasticity to reduce predation losses. Mar. Biol. 142, 487–494. ( 10.1007/s00227-002-0977-4) [DOI] [Google Scholar]
- 43.Kroeker KJ, et al. In press. Interacting environmental mosaics drive geographic variation in mussel performance and predation vulnerability. Ecol. Lett. ( 10.1111/ele.12613) [DOI] [PubMed] [Google Scholar]
- 44.Kroeker KJ, Sanford E, Jellison BM, Gaylord B. 2014. Predicting the effects of ocean acidification on predator-prey interactions: a conceptual framework based on coastal molluscs. Biol. Bull. 226, 211–222. [DOI] [PubMed] [Google Scholar]
- 45.Branch GM. 1974. The ecology of Patella linnaeus from the Cape Peninsula, South Africa. 3. Growth rates. Trans. R. Soc. South Afr. 41, 161–193. ( 10.1080/00359197409520069) [DOI] [Google Scholar]
- 46.Nienhuis S, Palmer AR, Harley CD G. 2010. Elevated CO2 affects shell dissolution rate but not calcification rate in a marine snail. Proc. R. Soc. B 277, 20100206 ( 10.1098/rspb.2010.0206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Queirós AM, et al. 2015. Scaling up experimental ocean acidification and warming research: from individuals to the ecosystem. Glob. Change Biol. 21, 130–143. ( 10.1111/gcb.12675) [DOI] [PubMed] [Google Scholar]
- 48.Melzner F, Stange P, Trübenbach K, Thomsen J, Casties I, Panknin U, Gorb SN, Gutowska MA. 2011. Food supply and seawater pCO2 impact calcification and internal shell dissolution in the blue mussel Mytilus edulis. PLoS ONE 6, e24223 ( 10.1371/journal.pone.0024223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Range P, Piló D, Ben-Hamadou R, Chícharo MA, Matias D, Joaquim S, Oliveira AP, Chícharo L. 2012. Seawater acidification by CO2 in a coastal lagoon environment: Effects on life history traits of juvenile mussels Mytilus galloprovincialis. J. Exp. Mar. Biol. Ecol. 424–425, 89–98. ( 10.1016/j.jembe.2012.05.010) [DOI] [Google Scholar]
- 50.Hahn S, Rodolfo-Metalpa R, Griesshaber E, Schmahl WW, Buhl D, Hall-Spencer JM, Baggini C, Fehr KT, Immenhauser A. 2012. Marine bivalve shell geochemistry and ultrastructure from modern low pH environments: environmental effect versus experimental bias. Biogeosciences 9, 1897–1914. ( 10.5194/bg-9-1897-2012) [DOI] [Google Scholar]
- 51.Palmer AR. 1992. Calcification in marine molluscs: how costly is it? Proc. Natl Acad. Sci. USA 89, 1379–1382. ( 10.1073/pnas.89.4.1379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boyce DG, Lewis MR, Worm B. 2010. Global phytoplankton decline over the past century. Nature 466, 591–596. ( 10.1038/nature09268) [DOI] [PubMed] [Google Scholar]
- 53.Coe WR, Fox DL. 1942. Biology of the California sea-mussel (Mytilus californianus). I. Influence of temperature, food supply, sex and age on the rate of growth. J. Exp. Zool. 90, 1–30. ( 10.1002/jez.1400900102) [DOI] [Google Scholar]
- 54.Dodd JR. 1963. Paleoecological implications of shell mineralogy in two Pelecypod species. J. Geol. 71, 1–11. ( 10.1086/626872) [DOI] [Google Scholar]
- 55.Estes JA, Palmisano JF. 1974. Sea Otters: their role in structuring nearshore communities. Science 185, 1058–1060. ( 10.1126/science.185.4156.1058) [DOI] [PubMed] [Google Scholar]
- 56.Wessen G, Suttles W. 1990. The archaeology of the ocean coast of Washington. In Handbook of North American Indians, vol. 7 (ed. W Suttles), pp. 412–421. Washington, DC: Smithsonian Press. [Google Scholar]
- 57.Vanblaricom GR. 2001. Sea otters. Stillwater, MN: Voyageur Press. [Google Scholar]
- 58.Laidre KL, Jameson RJ. 2006. Foraging patterns and prey selection in an increasing and expanding sea otter population. J. Mammal. 87, 799–807. ( 10.1644/05-MAMM-A-244R2.1) [DOI] [Google Scholar]
- 59.Pfister CA, Paine RT, Wootton JT. In press The iconic keystone predator has a pathogen. Front. Ecol. Environ. 14, 285–286. [Google Scholar]
- 60.Sanford E, Worth DJ. 2009. Genetic differences among populations of a marine snail drive geographic variation in predation. Ecology 90, 3108–3118. ( 10.1890/08-2055.1) [DOI] [PubMed] [Google Scholar]
- 61.Sanford E, Worth DJ. 2010. Local adaptation along a continuous coastline: prey recruitment drives differentiation in a predatory snail. Ecology 91, 891–901. ( 10.1890/09-0536.1) [DOI] [PubMed] [Google Scholar]
- 62.Dayton PK. 1971. Competition, disturbance, and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecol. Monogr. 41, 351–389. ( 10.2307/1948498) [DOI] [Google Scholar]
- 63.Wootton JT. 2002. Mechanisms of successional dynamics: consumers and the rise and fall of species dominance. Ecol. Res. 17, 249–260. ( 10.1046/j.1440-1703.2002.00484.x) [DOI] [Google Scholar]
- 64.Fenberg PB, Roy K. 2008. Ecological and evolutionary consequences of size-selective harvesting: how much do we know? Mol. Ecol. 17, 209–220. ( 10.1111/j.1365-294X.2007.03522.x) [DOI] [PubMed] [Google Scholar]
- 65.Ward J. 1967. Distribution and growth of the keyhole limpet Fissurella barbadensis Gmelin. Bull. Mar. Sci. 17, 299–318. [Google Scholar]
- 66.Ruggiero P, Komar PD, Allan JC. 2010. Increasing wave heights and extreme value projections: the wave climate of the US Pacific Northwest. Coast. Eng. 57, 539–552. ( 10.1016/j.coastaleng.2009.12.005) [DOI] [Google Scholar]
- 67.Wootton JT, Pfister CA. 2012. Carbon system measurements and potential climatic drivers at a site of rapidly declining ocean pH. PLoS ONE 7, e53396 ( 10.1371/journal.pone.0053396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hofmann GE, et al. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983 ( 10.1371/journal.pone.0028983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frieder CA, Nam SH, Martz TR, Levin LA. 2012. High temporal and spatial variability of dissolved oxygen and pH in a nearshore California kelp forest. Biogeosciences 9, 3917–3930. ( 10.5194/bg-9-3917-2012) [DOI] [Google Scholar]
- 70.Bourdeau PE, Butlin RK, Brönmark C, Edgell TC, Hoverman JT, Hollander J. 2015. What can aquatic gastropods tell us about phenotypic plasticity? A review and meta-analysis. Heredity 115, 312–321. ( 10.1038/hdy.2015.58) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Donnell MJ, George MN, Carrington E. 2013. Mussel byssus attachment weakened by ocean acidification. Nat. Clim. Change 3, 587–590. ( 10.1038/nclimate1846) [DOI] [Google Scholar]
- 72.Harley CDG. 2011. Climate change, keystone predation, and biodiversity loss. Science 334, 1124–1127. ( 10.1126/science.1210199) [DOI] [PubMed] [Google Scholar]
- 73.Gutiérrez JL, Jones CG, Strayer DL, Iribarne OO. 2003. Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos 101, 79–90. ( 10.1034/j.1600-0706.2003.12322.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


