Abstract
Highly migratory marine species can travel long distances and across entire ocean basins to reach foraging and breeding grounds, yet gaps persist in our knowledge of oceanic dispersal and habitat use. This is especially true for sea turtles, whose complex life history and lengthy pelagic stage present unique conservation challenges. Few studies have explored how these young at-sea turtles navigate their environment, but advancements in satellite technology and numerical models have shown that active and passive movements are used in relation to open ocean features. Here, we provide the first study, to the best of our knowledge, to simultaneously combine a high-resolution physical forcing ocean circulation model with long-term multi-year tracking data of young, trans-oceanic North Pacific loggerhead sea turtles during their ‘lost years’ at sea. From 2010 to 2014, we compare simulated trajectories of passive transport with empirical data of 1–3 year old turtles released off Japan (29.7–37.5 straight carapace length cm). After several years, the at-sea distribution of simulated current-driven trajectories significantly differed from that of the observed turtle tracks. These results underscore current theories on active dispersal by young oceanic-stage sea turtles and give further weight to hypotheses of juvenile foraging strategies for this species. Such information can also provide critical geographical information for spatially explicit conservation approaches to this endangered population.
Keywords: loggerhead sea turtle, ocean circulation model, migration, foraging, distribution
1. Introduction
Understanding animal movement and distribution has been inherently difficult in the open ocean. Animals with complex life histories can undergo long-distance migrations and use a variety of habitats throughout various stages [1]. Extended time in the pelagic environment can make understanding their navigational behaviour and habitat selection along migratory routes difficult. This is especially true for sea turtles, which disperse into the ocean upon hatching and remain relatively undetectable for several years [2,3]. During this time, the gaps in knowledge of where they disperse, what habitats they use and the length of time they exploit these habitats, are characterized as the ‘lost years’ [2]. With their small size, positive buoyancy and limited locomotion, it was long assumed that young oceanic-stage sea turtles passively drift through the large-scale gyres transported along their migratory pathways by surface ocean currents, spending years undergoing a ‘long-term, unidirectional gyre-based developmental migration’ [1,2].
However, developments in electronic tracking and biologging capabilities, coupled with laboratory experiments, have begun to shed light on the predominant hypotheses of entirely passive at-sea behaviour and distribution [3–6]. It is now known that oceanic dispersal is a product of passive and active movements [7–10]. Emerging studies have begun to recognize that even early-stage turtles rely on some level of active swimming to achieve success with long-distance ocean transport [3,5]. In combination with well-known geomagnetic navigational cues [4], small amounts of directional swimming can exert a strong effect on migratory routes and endpoints within ocean circulation [3,10,11].
Because the ocean is a complex and dynamic environment, inferences about the directed movement of an individual animal within the open ocean require attention to the underlying physical processes that shape migratory pathways [8,12,13]. This is especially true for ocean currents, which have been shown to play key roles in sea turtle ecology [7,8]. Biophysical analyses that incorporate high-resolution hydrodynamic models into particle-tracking algorithms can be useful tools to study supposed patterns of passively drifting particles [14]. Such simulations serve as ‘null models’ to test hypotheses on dispersal and distribution [10]. Studies using numerical simulation models to compare long-term drift trajectories with sea turtle tracking data have enhanced the understanding of how sea turtles successfully manage the long-distance journey across entire ocean basins [5,6,8,11]. Specifically, they have helped reconstruct timelines between empirical observations of adults with distant reproductive and foraging grounds [15]. Such studies have also given rise to imprint hypotheses (see [5]), which tie the role of passive dispersal in the evolution of active migration routes and habitat preference in later life-history stages [5,9,16].
For loggerhead sea turtles (Caretta caretta), current knowledge of post-hatchling navigation and dispersal has been based, almost exclusively, on studies of the North Atlantic population [4,6]. However, growth rates, developmental migrations and foraging strategies may differ among populations and ocean basins [17,18]. Even within a population, considerable variation may exist between migratory trajectories and the length of time necessary for hatchlings from the same cohort to reach foraging locations, not to mention the locations themselves (i.e. alternative foraging strategies; [1,19–21]). Thus, understanding scenarios of how these animals spend this life-history stage is critical, as the success of the population critically depends on the survival of juveniles [22]. In the Atlantic, studies now suggest that hatchling and juvenile sea turtles are distributed around the ocean basin to a certain extent by prevailing currents, but that active swimming may augment movement patterns such that young sea turtles may significantly differ in spatio-temporal distribution from distributions that would be expected for passively drifting objects [10].
In the North Pacific, focus on the loggerhead sea turtle has increased as the conservation status of this population has recently been changed to endangered status [23]. Nesting exclusively in Japan, hatchlings from this population undertake developmental migrations that span the entire North Pacific basin [24–27]. Animals from this subpopulation are genetically distinct from other loggerhead subpopulations [28]. Foraging hotspots for juveniles have been identified in the central North Pacific [29,30] and in the eastern Pacific off the Baja California Peninsula (BCP), Mexico [27,31,32]. Upon maturity, turtles migrate back to their natal beaches of Japan and remain in the western Pacific as adults [25,33].
Knowledge of this population's juvenile stage stems from exceptional insights into the movement ecology and environmental correlates within pelagic habitat [29,30,34–38]. However, connectivity between their oceanic dispersal and foraging grounds has remained poorly understood. From an ecological perspective, much can still be learned as to how physical forcing from ocean circulation influences the dispersal pathways and spatio-temporal dispersal of young juveniles from this population. From a conservation standpoint, current United States fisheries management strategies in the central North Pacific Ocean are specifically geared towards the interaction with at-sea loggerheads [39], PIFSC TurtleWatch Programme (http://www.pifsc.noaa.gov/eod/turtlewatch.php). This may still leave potentially important foraging areas within the high seas unprotected. For this reason, understanding transport and behavioural responses during this stage is crucial.
Here, we combine high-resolution ocean circulation models with long-term, previously unpublished 20–36 month old loggerhead sea turtle tracks from the North Pacific Ocean basin. The trajectories of passively drifting particles are compared with those of observed turtles released from the same date and nesting location in the western North Pacific, thereby testing the passive migration hypothesis for juvenile loggerhead sea turtles in the North Pacific Ocean. Such insights will help assess the role of long-distance transport from currents and provide useful long-term information on small juveniles during a period where little is known [40]. This knowledge can also provide critical geographical information for conservation management strategies in areas that may host high concentrations of young individuals from this population.
2. Material and methods
(a). Sea turtle tracks
During 2010–2011, Argos-linked satellite transmitters were attached to the carapaces of 44 juvenile loggerhead sea turtles, aged 1–3 years (29.7–37.5 SCL cm), following the procedures recommended in Balazs et al. [41] and Parker et al. [37]. These tracks represent new, never before published data. Turtles were hatched and raised in the Port of Nagoya Public Aquarium in Minato-ku, Japan. All loggerhead turtles were outfitted with Telonics (Mesa, AZ) model TAM-2619 (n = 4) TAM-2639 (n = 4) or Wildlife Computers (Redmond, WA) SPOT5 (n = 36) electronic transmitters.
Turtles were released from ships in two batches, offshore of Japan. Seventeen turtles were released in April 2010 at 29° N 130° E, and 27 turtles were released in July 2011, at 36° N 141° E (figure 1a and electronic supplementary material, table S1). Locations were collected by ARGOS-CLS. All raw surface locations were filtered using a Bayesian state space switching model [42], which regularized track positions at daily 24 h intervals. Such methodologies have been used in sea turtle movement studies to estimate animal location while accounting for satellite positioning errors [18].
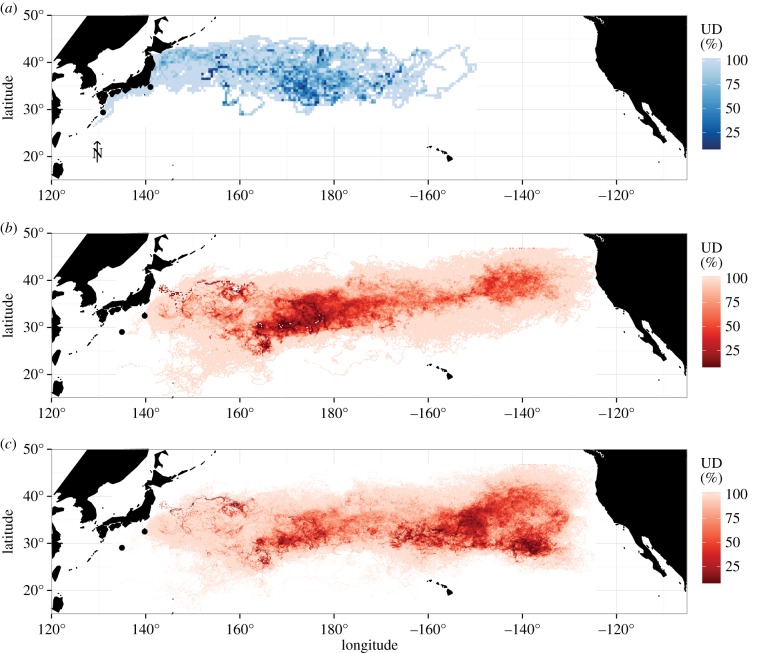
Figure 1.
(a) Spatial distribution of 44 satellite deployed loggerhead tracks from 2010 to 2013. Probability of an animal within a given cell (utilization distribution, UD) ranges from 0 to 100. The two main deploy locations are shown with black dots. Spatial distribution (UD) of 6000 simulated particles, released at the same date per region as observed turtles. Release dates centred around actual turtle deploys. Simulations ran for (b) 0–865 days (equal to the maximum number of turtle tracking days) and for long-term comparison, (c) 0–1460 days (4 years). Release locations are shown with grey dots.
(b). Particle simulation
Particle dispersal was simulated using the particle-tracking program ICHTHYOP (v. 3.2) [43]. Surface current velocity fields were extracted from the global hybrid coordinate ocean model (HYCOM) 1/12-degree analysis (http://hycom.org). HYCOM is forced using wind speed, wind stress, precipitation and heat flux [11]. The resulting data product uses satellite altimetry, sea surface height and in situ measurements of temperature and salinity. The standard global HYCOM output has a 1 day temporal resolution and 0.08° spatial resolution. The HYCOM product can resolve mesoscale features known to be related to sea turtle dispersal and movement (e.g. fronts [19,34,36,44], currents [7,8] and eddies [29,45]).
Particle-tracking start dates were centred around the deployments of actual satellite-tagged turtles. Between April 2010 and July 2011, 3000 particles were released per year, for a total of 6000 tracked particles. For each year, 1000 particles were released on the exact date coinciding with sea turtle deployment. Another 1000 were released the day before and after sea turtle deployment date. Separate release dates will experience a wider range of physical oceanographic conditions and provide a more representative view of dispersal scenarios [46]. In order to minimize the influence of coastal transport and retention unable to be quantified by HYCOM [14], particles were released approximately 50 km offshore within a zone that corresponded to the main deployment locations for 2010 and 2011. The movement of individual particles through HYCOM velocity fields was simulated using Lagrangian advection with a fourth-order Runge–Kutta time-stepping method [43], similar to Putman et al. [6]. Transport of particles was calculated every half an hour and recorded at 24 h intervals.
For each release date, particles were tracked in the model for (i) 865 days and (ii) 4 years. The first scenario corresponded to the maximum number of days an animal transmitted (electronic supplementary material, table S1). The second scenario was the longest possible uninterrupted time series of HYCOM data that corresponded to the earliest tag deployment date. This length also fell within the window of time a hatchling turtle could conceivably undergo a trans-Pacific migration to reach eastern boundary foraging grounds [47]. Simulations starting in 2010 ran through to 2014. Simulations starting in 2011 ran through 2014 and then ‘looped’ back over to resume in 2010 for a total of 4 years. The methodology of ‘looping’ has been used for previous particle-tracking simulations for sea turtles (see [6,14]) and for oceanographic simulations [48]. Such a technique helps to diminish the impact of anomalous ocean current conditions on dispersal outcomes from just a single year [6].
Given the varied duration of satellite tag transmissions, tracks were normalized and time weighted using a threshold scheme [49,50]. Briefly, each location estimate was weighted by the inverse number of individuals with locations on the same day of transmission. Beyond an 85th percentile threshold, animal locations received equal weighting. Locations were then summed within 0.1° × 0.1° grid cells. From these values, time-integrated utilization distributions (UDs) were calculated to show the probability of an animal being found within a grid cell [51]. Similar grid summation and UD calculations were performed for simulated particle densities; however, particle trajectories were not weighted as all trajectories ran for exactly the same length of time during both scenarios.
As they traverse the North Pacific, loggerhead sea turtles are thought to move from the stronger eastward currents of the North Pacific Subtropical Gyre's (NPSG) Kuroshio Extension Current (KEC), west of 155° E and Bifurcation Region (KEBR), defined as the prevailing current system from 155° E to 180° longitude [29], to the weaker flows of the North Pacific Current (NPC), east of 180° longitude. Currents are further weakened by 160° W [52]. The percentage of sea turtles and particles that passed through the KEBR (155° E), NPC (180° E) and eastern North Pacific (160° W) was recorded. In order to highlight where animal movement may differ from the prevailing current (particles), we compared the average velocity and directional heading of sea turtle movement and particle drift along their eastward trajectories. Speed (km h−1) and directional headings were calculated along each individual sea turtle and particle location. Mean speed was then averaged by degree longitude. Mean directional headings were calculated as the 14 day average heading to minimize the variable accuracy estimates from ARGOS [53,54]. Directional headings of sea turtles and particles were then summarized at the boundaries of the prevailing currents (155° E, 180° E and 160° W; table 1). A first-order Rayleigh's z-test [55] was employed to determine the existence of: (i) a prevailing direction of current flow (particles), and (ii) directional uniformity among individual turtle tracks. Mardia–Watson–Wheeler tests of homogeneity were used to test for significant differences between sea turtle and particle mean headings, in effect highlighting the presence of active movement against prevailing current drift. As the number of turtles moving east of 180° diminished substantially (e.g. below 10% by 160°W), subsequent longitudes were not included in further statistical analyses. All statistical analyses were carried out using the Circular package in R [56].
Table 1.
Percentage of turtle tracks and particles transported eastward across the North Pacific and their average directional heading (degrees). (Asterisk denotes statistically significant (p < 0.05) directionality (Rayleigh's z-test) along longitudinal dispersal.)
| east of 155° E | east of 180° | east of 160° W | east of 140° W (%) | |
|---|---|---|---|---|
| turtles | 100% (93.4°)* | 64.4% (112.7°)* | 6.7% (56.3°) (n.s.) | 0 |
| particles | 95.4% (65.5°) (n.s.) | 66.7% (65.4°) (n.s.) | 66.58% (64.7°) (n.s.) | 47.18 |
3. Results
(a). Dispersal and spatial extent
Young oceanic loggerheads dispersed over a wide area, with the highest abundance of turtles found between 175°E and 175° W (figure 1a). Track durations ranged from 173 to 865 days, with a mean value of 468.8 days (±164.2 days s.d.; electronic supplementary material, table S1). The average distance travelled by a turtle was 11 290 km (±3067.9 km s.d.). The average easternmost longitude by 44 turtles was 175.5° W. The maximum eastward longitude reached by an individual animal was 150.0° W.
Under both simulation scenarios (865 days and 4 years), dispersal routes encompassed nearly the width of the entire North Pacific Ocean, with high abundances near 180° and in the eastern North Pacific (figure 1b,c). After 865 days, particles dispersed into the eastern North Pacific, with an average easternmost longitude of 173.5° W and a maximum of longitude of 125° W. After 4 years, the mean distance travelled by particles was 28 642 km (±18 417.2 s.d.). The average easternmost longitude was 130.2° W with a maximum of 124.4° W. Neither simulated trajectories nor sea turtles had reached the coastal waters of BCP, Mexico (figure 1a,c).
(b). Active versus passive movements
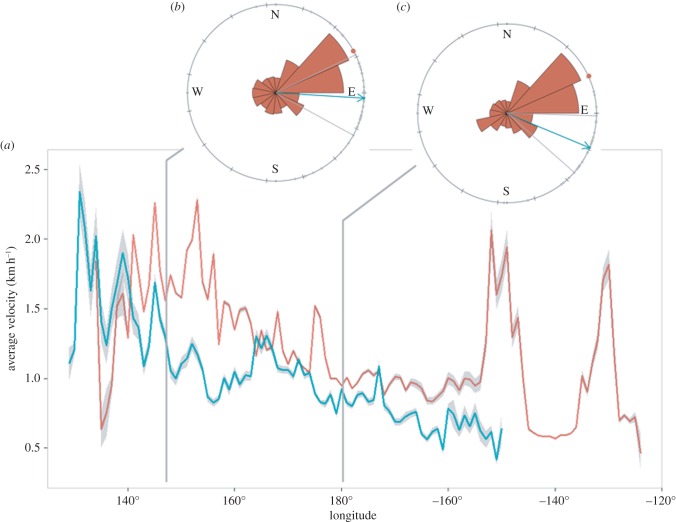
Sea turtle and particle velocities decreased with eastward location; however, particle drift velocities did increase again near 150° W. While sea turtle velocities were faster than particle velocities near 140° E, 165° E and 175° W, (figure 2a), average eastward velocities were significantly slower for turtles (1.0 ± 0.4 km h−1) than for particles (1.2 ± 0.4 km h−1; Mann–Whitney U-test, U103 = 3254, p = 0.007). The KEC is a fast-moving western boundary current, and as expected, the greatest velocities for both turtles (2.3 ± 0.9 km h−1) and particles (2.3 ± 6.5 km h−1) were found west of 155° E, within the bounds of the KEC (figure 2a).
Figure 2.
(a) Average speed by degree longitude for turtle tracks (blue) and particles (red) and 95% confidence intervals (grey). Directional diagrams of animal movement and particle drift upon entering (b) the Kuroshio Extension Bifurcation Region (KEBR) at 155°E and (c) transitioning to the North Pacific Current at 180°. Average directional heading of turtles (grey dots) with grand mean average (blue arrow) are compared with the average directional heading of particles transported by currents (red wind rose) with grand mean average (red dot).
One hundred per cent of turtles and 95% of all particles moved through the KEC and into the KEBR (155° E; table 1). By 180°, more than half of all tracks and particles continued in an eastward direction (64.4% and 66.7%, respectively). Crossing both locations (155° E and 180°), particles consistently travelled in a northeastward direction, whereas sea turtles headed in an east/southeast direction (figure 2b). Sea turtles moving into both the KEBR at 155° E and the NPC at 180° E showed a statistically significant directional orientation (Rayleigh's z p = 2.8 × 10−13 and p = 1.7 × 10−8, respectively; table 1). Results of Mardia–Watson–Wheeler tests showed a significant difference between turtle orientation and particles at both 155° E and 180° (W155 = 38.9, d.f. = 2, p = 3.6 × 10−9 and W180 = 38.4, d.f. = 2, p = 4.5 × 10−9, respectively). There were too few data points to perform these tests for turtles east of 160° W.
4. Discussion
In just a few years after leaving the coastal waters of Japan, young oceanic loggerheads disperse over a large area of the North Pacific Ocean. Our results provide the first evidence, to the best of our knowledge, for the Pacific Ocean that young (1–3 year old) loggerhead sea turtles are not passively distributed by current but exhibit some active swimming along migratory routes. These results, coupled with sample trajectories of the four longest transmitting animal tracks (greater than 2 years; electronic supplementary material, figure S1), show that sea turtles demonstrated oriented swimming in both stronger current regimes (KEBR) and weaker currents of the CNP. The KEBR is a possible foraging hotspot for oceanic North Pacific loggerheads and shows a high residency for small-sized captive and larger wild-caught juveniles [29]. Such movements may allow turtles to use the biologically rich waters of the KEBR while staying out of the Kuroshio countercurrent (KCC). Indeed, the spatial overlap of a separate dataset of turtles (n = 43) tracked from 2000 to 2004 [29] with the data presented here (n = 44, 2010–2013) supports the hypothesis that this area may be an important and persistent foraging location for juveniles of this population [27].
The high concentration of passively drifting particles and increase in particle velocity within the eastern North Pacific (figure 1b,c) are consistent with the large-scale circulation patterns in the NPSG. The NPSG causes surface convergence and retention of biomass and debris [52]. Various studies have described this feature, often called the ‘great North Pacific Garbage Patch’ or ‘Eastern Garbage Patch’ [57]. However, the data still present a fragmented understanding of basin-wide connectivity of this population. The fact that the easternmost area used by young juvenile North Pacific loggerhead sea turtles (figure 1a) significantly differed from that of the passively drifting particles (figure 1b) adds to the current gaps in knowledge of this population. As neither turtles nor particles reached Baja California, the migration strategies and known relationship to a highly populated foraging ground in the eastern North Pacific remain unknown.
Regardless, young juvenile loggerheads are found in these eastern foraging grounds. Most recently, Tomaszewicz et al. [47] provided the first ever empirical age estimates for juvenile North Pacific loggerhead sea turtles along the BCP. Their results found juvenile loggerheads in Baja California ranged from 3 to 24 years of age. Juvenile loggerheads are believed to undergo an ontogenetic shift from oceanic to coastal waters upon reaching a straight carapace length (SCL, of 42–59 cm [20,58]. The SCL of the youngest and smallest juveniles found off BCP (aged 3–6 years; 27.7–42.3 cm SCL) [47] overlap with the range of measured sizes of deployed captive-reared turtles in this study (SCL of 29.7–37.5 cm). This suggests that after 1 year of transmission after deployment, sea turtles from this study might be old enough and of comparable size to undergo a similar shift to neritic waters, but at present, no such tracking data confirm this. Four of the 44 sea turtles transmitted for more than 2 years, yet none displayed a trans-Pacific migration to Baja California (electronic supplementary material, figure S1). Like many of the observed tracks, the longest transmitting tracks exhibited oriented behaviour in marked contrast to particle simulation scenarios (electronic supplementary material, figure S1). Given the time of year (summer–autumn), perhaps these eastward moving animals reversed direction to time a return that would allow them to exploit the highest seasonally productive waters of the KEBR–KEC and subsequently the TZCF in the autumn [29]. This timing may also enable an overwintering strategy [59] in order to remain in productive and thermally optimal ocean conditions. Indeed, limitations still exist in the length of time these animals can be monitored.
(a). Application to conservation and spatially explicit threats
Basin-level differences in environmental and oceanographic conditions can affect population dynamics, access to resources, growth rates and age at maturity [52]. Bailey [18] reinforced this basin-level comparison for leatherbacks (Dermochelys coriacea), suggesting that the processes which drive foraging success may strongly shape the conservation status of conspecific populations. Despite great advancements in satellite telemetry and biologging capabilities, limitations still exist in the ability to track individuals throughout their life-history stages. Combining tracking dispersal with existing empirical information on young stage juveniles can provide useful information where such gaps in knowledge still exist [40]. The use of state-of-the-art numerical simulation-ocean circulation models has shed light on previous knowledge of at-sea orientation strategies, suggesting that even the ‘general’ distribution of turtles might not be well predicted by ocean currents alone [46]. As a first approximation, dispersal studies can provide us with useful scenarios of at-sea behaviour for young oceanic sea turtles [10,60]. However, these predictions will not depict exactly how an animal travels at sea [10]. They might provide a useful ‘null model’ for examining the role of ocean currents on the ecology of marine species [5,46,61]. Therefore, there are certain caveats to drawing direct movement comparisons between simulations and observed turtles in this study. Foremost, simulations assumed entirely passive drift that is typically representative of hatchlings, whereas observed turtle tracks represent stronger, more capable 1–3 year old turtles [29]. Second, these data rely primarily on the behaviour of captive-reared animals; however, previous studies by Polovina et al. [29] and Abecassis et al. [30] note that there are no apparent differences in the post-release behaviours of sea turtles that have been in captivity and wild turtles. Narazaki et al. [59] noted spatial overlap between similar-sized wild-caught and head-started juveniles in the western Pacific. Nonetheless, results can provide a long-term window of the at-sea behaviour and movements of ‘lost years’ juveniles. Therefore, comparison of observed animal movement with simulations over a variety of ocean conditions can provide useful information during an otherwise cryptic life-history stage [6,8,11].
In summary, we have shown that the orientation and distribution of juvenile loggerhead sea turtles can differ from predominant currents in the North Pacific Ocean. This information can have a profound influence on traditional hypotheses of oceanic transport and basin-scale connectivity. The time that it takes to traverse the open ocean and the fact that these highly migratory animals spend years to decades in this pelagic habitat leaves sea turtles vulnerable to anthropogenic threats, in particular bycatch in fisheries [39]. Knowledge of dispersal patterns, the areas highly frequented by vulnerable sea turtles and the corridors of movement between life stages is a priority for sea turtle conservation [40,46]. Such information can be used for conservation management strategies that ensure these populations persist with time.
Supplementary Material
Supplementary Material
Acknowledgements
The authors acknowledge the personnel at Port of Nagoya Public Aquarium and the two anonymous referees that improved the quality of this manuscript.
Ethics
Animal care and permitting needs for this study were fulfilled by the Port of Nagoya Public Aquarium. All treatments were humane and in full compliance with the requirements and approval of the government of Japan of which the Aquarium is an entity. Permission to tag and release turtles were completed by Port of Nagoya Public Aquarium of Japan.
Authors' contributions
M.K., T.S. and H.O. conducted fieldwork including animal care and tag deployment. D.K.B., G.H.B., J.J.P., M.K., T.S., H.O. and M.R. conceived and designed experiments. D.M.P., G.H.B., D.K.B. acquired and analysed data. D.K.B. performed modelling analysis. D.M.P., G.H.B., J.J.P., L.B.C., M.K., T.S., H.O. and M.R. contributed to theoretical concepts and research materials. D.K.B. drafted the paper. D.M.P., G.H.B., J.J.P. and L.B.C. contributed to paper revisions for important intellectual content. All authors approve of the final version to be published.
Competing interests
We have no competing interests.
Funding
Funding for D.K.B. was provided by the Crowder Laboratory at Hopkins Marine Station Stanford University.
References
- 1.Bolten AB. 2003. Variation in sea turtle life history patterns: neritic vs. oceanic developmental stages. Biol. Sea Turtles 2, 243–257. [Google Scholar]
- 2.Carr A. 1987. New perspectives on the pelagic stage of sea turtle development. Conserv. Biol. 1, 103–121. ( 10.1111/j.1523-1739.1987.tb00020.x) [DOI] [Google Scholar]
- 3.Mansfield KL, Wyneken J, Porter WP, Luo J. 2014. First satellite tracks of neonate sea turtles redefine the ‘lost years’ oceanic niche. Proc. R. Soc. B 281, 20133039 ( 10.1098/rspb.2013.3039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohmann KJ, Cain SD, Dodge SA, Lohmann CM. 2001. Regional magnetic fields as navigational markers for sea turtles. Science 294, 364–366. ( 10.1126/science.1064557) [DOI] [PubMed] [Google Scholar]
- 5.Hays GC, Fossette S, Katselidis KA, Mariani P, Schofield G. 2010. Ontogenetic development of migration: Lagrangian drift trajectories suggest a new paradigm for sea turtles. J. R. Soc. Interface 7, 1319–1327. ( 10.1098/rsif.2010.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Putman NF, Verley P, Shay TJ, Lohmann KJ. 2012. Simulating transoceanic migrations of young loggerhead sea turtles: merging magnetic navigation behavior with an ocean circulation model. J. Exp. Biol. 215, 1863–1870. ( 10.1242/jeb.067587) [DOI] [PubMed] [Google Scholar]
- 7.Luschi P, Hays GC, Papi F. 2003. A review of long-distance movements by marine turtles and the possible role of ocean currents. Oikos 103, 293–302. ( 10.1034/j.1600-0706.2003.12123.x) [DOI] [Google Scholar]
- 8.Gaspar P, Georges J-Y, Fossette S, Lenoble A, Ferraroli S, Le Maho Y. 2006. Marine animal behaviour: neglecting ocean currents can lead us up the wrong track. Proc. R. Soc. B 273, 2697–2702. ( 10.1098/rspb.2006.3623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott R, Hays GC. 2014. Ontogeny of long distance migration. Ecology 95, 2840–2850. ( 10.1890/13-2164.1) [DOI] [Google Scholar]
- 10.Putman NF, Mansfield KL. 2015. Direct evidence of swimming demonstrates active dispersal in the sea turtle ‘lost years’. Curr. Biol. 25, 1221–1227. ( 10.1016/j.cub.2015.03.014) [DOI] [PubMed] [Google Scholar]
- 11.Putman NF, Scott R, Verley P, Marsh R, Hays GC. 2012. Natal site and offshore swimming influence fitness and long-distance ocean transport in young sea turtles. Mar. Biol. 159, 2117–2126. ( 10.1007/s00227-012-1995-5) [DOI] [Google Scholar]
- 12.Fossette S, Putman NF, Lohmann KJ, Marsh R, Hays GC. 2012. A biologist's guide to assessing ocean currents: a review. Mar. Ecol. Prog. Ser. 457, 285–301. ( 10.3354/meps09581) [DOI] [Google Scholar]
- 13.Van Houtan KS, Francke DL, Alessi S, Jones TT, Martin SL, Kurpita L, King CS, Baird RW. 2016. The developmental biogeography of hawksbill sea turtles in the North Pacific. Ecol. Evol. 6, 2378–2389. ( 10.1002/ece3.2034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putman NF, He R. 2013. Tracking the long-distance dispersal of marine organisms: sensitivity to ocean model resolution. J. R. Soc. Interface 10, 20120979 ( 10.1098/rsif.2012.0979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luschi P, Casale P. 2014. Movement patterns of marine turtles in the Mediterranean Sea: a review. Ital. J. Zool. 81, 478–495. ( 10.1080/11250003.2014.963714) [DOI] [Google Scholar]
- 16.Gaspar P, Benson SR, Dutton PH, Réveillère A, Jacob G, Meetoo C, Dehecq A, Fossette S. 2012. Oceanic dispersal of juvenile leatherback turtles: going beyond passive drift modeling. Mar. Ecol. Prog. Ser. 457, 265 ( 10.3354/meps09689) [DOI] [Google Scholar]
- 17.Zug GR, Balazs GH, Wetherall JA. 1995. Growth in juvenile loggerhead seaturtles (Caretta caretta) in the north Pacific pelagic habitat. Copeia 1995, 484–487. ( 10.2307/1446917) [DOI] [Google Scholar]
- 18.Bailey H, et al. 2012. Movement patterns for a critically endangered species, the leatherback turtle (Dermochelys coriacea), linked to foraging success and population status. PLoS ONE 7, e36401 ( 10.1371/journal.pone.0036401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkes LA, Broderick AC, Coyne MS, Godfrey MH, Lopez-Jurado L-F, Lopez-Suarez P, Merino SE, Varo-Cruz N, Godley BJ. 2006. Phenotypically linked dichotomy in sea turtle foraging requires multiple conservation approaches. Curr. Biol. 16, 990–995. ( 10.1016/j.cub.2006.03.063) [DOI] [PubMed] [Google Scholar]
- 20.McClellan CM, Read AJ. 2007. Complexity and variation in loggerhead sea turtle life history. Biol. Lett. 3, 592–594. ( 10.1098/rsbl.2007.0355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansfield KL, Saba VS, Keinath JA, Musick JA. 2009. Satellite tracking reveals a dichotomy in migration strategies among juvenile loggerhead turtles in the Northwest Atlantic. Mar. Biol. 156, 2555–2570. ( 10.1007/s00227-009-1279-x) [DOI] [Google Scholar]
- 22.Crouse DT, Crowder LB, Caswell H. 1987. A stage-based population model for loggerhead sea turtles and implications for conservation. Ecology 68, 1412–1423. ( 10.2307/1939225) [DOI] [Google Scholar]
- 23.(NMFS and USFWS) National Marine Fisheries Service US Fish and Wildlife Service. 2011. Endangered and threatened species; determination of nine distinct population segments of loggerhead sea turtles as endangered. Fed. Regist. 76, 58868. [Google Scholar]
- 24.Bowen B, Abreu-Grobois F, Balazs G, Kamezaki N, Limpus C, Ferl R. 1995. Trans-Pacific migrations of the loggerhead turtle (Caretta caretta) demonstrated with mitochondrial DNA markers. Proc. Natl Acad. Sci. USA 92, 3731–3734. ( 10.1073/pnas.92.9.3731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols W, Resendiz A, Seminoff J, Resendiz B. 2001. Transpacific migration of a loggerhead turtle monitored by satellite telemetry. Bull. Mar. Sci. 67, 937–947. [Google Scholar]
- 26.Peckham SH, Maldonado Diaz D, Koch V, Mancini A, Gaos A, Tinker MT, Nichols WJ. 2008. High mortality of loggerhead turtles due to bycatch, human consumption and strandings at Baja California Sur, Mexico, 2003 to 2007. Endangered Species Res. 5, 171–183. ( 10.3354/esr00123) [DOI] [Google Scholar]
- 27.Seminoff JA, Eguchi T, Carretta J, Allen CD, Prosperi D, Rangel R, Gilpatrick JW, Forney K, Peckham SH. 2014. Loggerhead sea turtle abundance at a foraging hotspot in the eastern Pacific Ocean: implications for at-sea conservation. Endangered Species Res. 24, 207–220. ( 10.3354/esr00601) [DOI] [Google Scholar]
- 28.Bowen B, Karl S. 2007. Population genetics and phylogeography of sea turtles. Mol. Ecol. 16, 4886–4907. ( 10.1111/j.1365-294X.2007.03542.x) [DOI] [PubMed] [Google Scholar]
- 29.Polovina J, Uchida I, Balazs G, Howell EA, Parker D, Dutton P. 2006. The Kuroshio extension bifurcation region: a pelagic hotspot for juvenile loggerhead sea turtles. Deep Sea Res. II 53, 326–339. ( 10.1016/j.dsr2.2006.01.006) [DOI] [Google Scholar]
- 30.Abecassis M, Senina I, Lehodey P, Gaspar P, Parker D, Balazs G, Polovina J. 2013. A model of loggerhead sea turtle (Caretta caretta) habitat and movement in the oceanic North Pacific. PLoS ONE 8, e73274 ( 10.1371/journal.pone.0073274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peckham SH, Maldonado-Diaz D, Tremblay Y, Ochoa R, Polovina J, Balazs G, Dutton PH, Nichols WJ. 2011. Demographic implications of alternative foraging strategies in juvenile loggerhead turtles Caretta caretta of the North Pacific Ocean. Mar. Ecol. Prog. Ser. 425, 269–280. ( 10.3354/meps08995) [DOI] [Google Scholar]
- 32.Wingfield DK, Peckham SH, Foley DG, Palacios DM, Lavaniegos BE, Durazo R, Nichols WJ, Croll DA, Bograd SJ. 2011. The making of a productivity hotspot in the coastal ocean. PLoS ONE 6, e27874 ( 10.1371/journal.pone.0027874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatase H, et al. 2002. Population structure of loggerhead turtles, Caretta caretta, nesting in Japan: bottlenecks on the Pacific population. Mar. Biol. 141, 299–305. ( 10.1007/s00227-002-0819-4) [DOI] [Google Scholar]
- 34.Polovina JJ, Kobayashi DR, Parker DM, Seki MP, Balazs GH. 2000. Turtles on the edge: movement of loggerhead turtles (Caretta caretta) along oceanic fronts, spanning longline fishing grounds in the central North Pacific, 1997–1998. Fish. Oceanogr. 9, 71–82. ( 10.1046/j.1365-2419.2000.00123.x) [DOI] [Google Scholar]
- 35.Parker DM, Cooke WJ, Balazs GH. 2005. Diet of oceanic loggerhead sea turtles (Caretta caretta) in the central North Pacific. Fish. Bull. 103, 142–152. [Google Scholar]
- 36.Polovina JJ, Balazs GH, Howell EA, Parker DM, Seki MP, Dutton PH. 2004. Forage and migration habitat of loggerhead (Caretta caretta) and olive Ridley (Lepidochelys olivacea) sea turtles in the central North Pacific Ocean. Fish. Oceanogr. 13, 36–51. ( 10.1046/j.1365-2419.2003.00270.x) [DOI] [Google Scholar]
- 37.Parker D, Balazs G, Rice M, Tomkeiwicz S. 2014. Variability in reception duration of dual satellite tags on sea turtles tracked in the Pacific Ocean. Micronesica 3, 1–8. [Google Scholar]
- 38.Howell EA, Dutton PH, Polovina JJ, Bailey H, Parker DM, Balazs GH. 2010. Oceanographic influences on the dive behavior of juvenile loggerhead turtles (Caretta caretta) in the North Pacific Ocean. Mar. Biol. 157, 1011–1026. ( 10.1007/s00227-009-1381-0) [DOI] [Google Scholar]
- 39.Howell EA, Kobayashi DR, Parker DM, Balazs GH, Polovina A. 2008. TurtleWatch: a tool to aid in the bycatch reduction of loggerhead turtles Caretta caretta in the Hawaii-based pelagic longline fishery. Endangered Species Res. 5, 267–278. ( 10.3354/esr00096) [DOI] [Google Scholar]
- 40.Casale P, Mariani P. 2014. The first ‘lost year’ of Mediterranean sea turtles: dispersal patterns indicate subregional management units for conservation. Mar. Ecol. Prog. Ser. 498, 263–274. ( 10.3354/meps10640) [DOI] [Google Scholar]
- 41.Balazs GH, Miya RK, Beavers S. 1996. Procedures to attach a satellite transmitter to the carapace of an adult green turtle, Chelonia mydas. NOAA Tech. Memorandum NMFS-SEFSC 387, 21–26. [Google Scholar]
- 42.Jonsen ID, Myers RA, James MC. 2007. Identifying leatherback turtle foraging behaviour from satellite telemetry using a switching state-space model. Mar. Ecol. Prog. Ser. 337, 255–264. ( 10.3354/meps337255) [DOI] [Google Scholar]
- 43.Lett C, Verley P, Mullon C, Parada C, Brochier T, Penven P, Blanke B. 2008. A Lagrangian tool for modelling ichthyoplankton dynamics. Environ. Model. Softw. 23, 1210–1214. ( 10.1016/j.envsoft.2008.02.005) [DOI] [Google Scholar]
- 44.Scales KL, Miller PI, Varo-Cruz N, Hodgson DJ, Hawkes LA, Godley BJ. 2015. Oceanic loggerhead turtles Caretta caretta associate with thermal fronts: evidence from the Canary Current Large Marine Ecosystem. Mar. Ecol. Prog. Ser. 519, 195–207. ( 10.3354/meps11075) [DOI] [Google Scholar]
- 45.Revelles M, Isern-Fontanet J, Cardona L, San Félix M, Carreras C, Aguilar A. 2007. Mesoscale eddies, surface circulation and the scale of habitat selection by immature loggerhead sea turtles. J. Exp. Mar. Biol. Ecol. 347, 41–57. ( 10.1016/j.jembe.2007.03.013) [DOI] [Google Scholar]
- 46.Putman NF, et al. 2014. Numerical dispersal simulations and genetics help explain the origin of hawksbill sea turtles in Ascension Island. J. Exp. Mar. Biol. Ecol. 450, 98–108. ( 10.1016/j.jembe.2013.10.026) [DOI] [Google Scholar]
- 47.Tomaszewicz CN, Seminoff JA, Avens L, Goshe LR, Peckham SH, Rguez-Baron JM, Bickerman K, Kurle CM. 2015. Age and residency duration of loggerhead turtles at a North Pacific bycatch hotspot using skeletochronology. Biol. Conserv. 186, 134–142. ( 10.1016/j.biocon.2015.03.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brochier T, Lett C, Tam J, Fréon P, Colas F, Ayón P. 2008. An individual-based model study of anchovy early life history in the northern Humboldt current system. Prog. Oceanogr. 79, 313–325. ( 10.1016/j.pocean.2008.10.004) [DOI] [Google Scholar]
- 49.Block BA, et al. 2011. Tracking apex marine predator movements in a dynamic ocean. Nature 475, 86–90. ( 10.1038/nature10082) [DOI] [PubMed] [Google Scholar]
- 50.Maxwell SM, et al. 2013. Cumulative human impacts on marine predators. Nat. Commun. 4, 1–9. ( 10.1038/ncomms3688) [DOI] [PubMed] [Google Scholar]
- 51.Kernohan B, Gitzen R. 2001. Analysis of animal space use and movements. In Radio tracking and animal populations (eds Millspaugh JJ, Marzluff JM), pp. 125–166. San Diego, CA: Academic Press. [Google Scholar]
- 52.Van Houtan KS, Halley JM. 2011. Long-term climate forcing in loggerhead sea turtle nesting. PLoS ONE 6, e19043 ( 10.1371/journal.pone.0019043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Witt MJ, et al. 2011. Tracking leatherback turtles from the world's largest rookery: assessing threats across the South Atlantic. Proc. R. Soc. B 278, 2338–2347. ( 10.1098/rspb.2010.2467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witt M, et al. 2010. Assessing accuracy and utility of satellite-tracking data using Argos-linked Fastloc-GPS. Anim. Behav. 80, 571–581. ( 10.1016/j.anbehav.2010.05.022) [DOI] [Google Scholar]
- 55.Zar JH. 1999. Biostatistical analysis. Delhi, India: Pearson Education India. [Google Scholar]
- 56.Agostinelli C, Lund U.2013. R package ‘circular’: circular statistics (version 0.4-7). See https://cran.r-project.org/web/packages/circular .
- 57.Howell EA, Bograd SJ, Morishige C, Seki MP, Polovina JJ. 2012. On North Pacific circulation and associated marine debris concentration. Mar. Pollut. Bull. 65, 16–22. ( 10.1016/j.marpolbul.2011.04.034) [DOI] [PubMed] [Google Scholar]
- 58.Bjorndal KA, Bolten AB, Martins HR. 2000. Somatic growth model of juvenile loggerhead sea turtles Caretta caretta: duration of pelagic stage. Mar. Ecol. Prog. Ser. 202, 265–272. ( 10.3354/meps202265) [DOI] [Google Scholar]
- 59.Narazaki T, Sato K, Miyazaki N. 2015. Summer migration to temperate foraging habitats and active winter diving of juvenile loggerhead turtles Caretta caretta in the western North Pacific. Mar. Biol. 162, 1251–1263. ( 10.1007/s00227-015-2666-0) [DOI] [Google Scholar]
- 60.Luschi P. 2013. Long-distance animal migrations in the oceanic environment: orientation and navigation correlates. ISRN Zool. 2013, 1–23. ( 10.1155/2013/631839) [DOI] [Google Scholar]
- 61.Shillinger GL, Di Lorenzo E, Luo H, Bograd SJ, Hazen EL, Bailey H, Spotila JR. 2012. On the dispersal of leatherback turtle hatchlings from Mesoamerican nesting beaches. Proc. R. Soc. B 279, 2391–2395. ( 10.1098/rspb.2011.2348) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.