Abstract
Purpose
Osteoporosis occurs in both women and men, but most of what we know about the condition comes from studies in females. The present study examined bone structure and function over an 18-month period in male C57BL/6 mice maintained on either a rodent chow diet (AIN76A) or a high-fat, Western-style diet (HFWD). Effects of mineral supplementation were assessed in both diets.
Methods
Trabecular and cortical bone structure in femora and vertebrae were assessed by micro-CT analysis. Following this, bone stiffness and strength measurements were made. Finally, bone levels of several cationic trace elements were quantified, and serum biomarkers of bone metabolism evaluated.
Results
Bone loss occurred over time in both diets but was more rapid and extensive in mice on the HFWD. Dietary mineral supplementation reduced bone loss in both diets and increased bone stiffness in the femora and bone stiffness and strength in the vertebrae. Bone content of strontium was increased in response to mineral supplementation in both diets.
Conclusions
Bone loss was more severe in mice on the HFWD and mineral supplementation mitigated the effects of the HFWD. In comparison to previous findings with female C57BL/6 mice, the present studies indicate that males are more sensitive to diet and benefited from a healthy diet (AIN76A), while females lost as much bone on the healthy diet as on the HFWD. Male mice benefited from mineral supplementation, just as females did in the previous study.
Abbreviations: 2D, two-dimensional; 3D, three-dimensional; AIN76A, American Institute of Nutrition 76A; ANOVA, analysis of variance; AQ, Aquamin®; BMD, bone mineral density; BV/TV, bone volume/tissue volume; C8, Caudal 8 (vertebra); GRAS, generally regarded as safe; HFWD, high-fat Western-style diet; ICP-OES, Inductively Coupled Plasma-Optical Emission Spectrometry; Micro-CT, micro-computed tomography; ROI, regions of interest
Keywords: Bone, Calcium, Cationic minerals, Strontium, Male mice, Osteoporosis
Highlights
-
•
Male mice experienced more bone loss on a high-fat Western diet than on a chow diet.
-
•
Bone loss was mitigated with a combination of calcium and trace minerals.
-
•
Bone structure and function improved in both femora and vertebrae.
-
•
Bone strontium was increased in parallel with improvement.
1. Introduction
Osteoporosis is commonly thought of as a disease of post-menopausal women, but approximately 20% of cases and 30% of the osteoporosis-related bone fractures in Western societies occur in men (Guggenbuhl, 2009, Gielen et al., 2011, Adler, 2014). Additionally, osteoporosis and its consequences are rising rapidly in men, and while men suffer fewer osteoporosis-related fractures than women, several studies have confirmed that the consequences of fragility-related bone fractures tend to be more severe in men than women (De Laet et al., 1998, Center et al., 1999, Johnell and Kanis, 2006). In spite of the profound consequences of osteoporosis in men, our understanding of the disease in men is based, largely, on extrapolation of findings in women.
Genetic and environmental factors influence bone growth and loss in both women and men. Among environmental factors, diet is critical. Past studies have demonstrated that a high-fat diet promotes bone mineral loss and increases bone fragility (von Muhlen et al., 2007, Prentice, 2004, Rosen and Klibanski, 2009). The Western-style diet may be especially permissive because in addition to its high content of saturated fat and processed carbohydrate, the Western diet is also low in calcium (US Department of Agriculture, 2015, Peterlik and Cross, 2005) and other minerals that are nutritionally associated with calcium. Calcium is the major cationic mineral in bone, and an adequate supply of dietary calcium throughout life is critical to the formation and maintenance of healthy bone tissue (Heaney and Weaver, 2005, Peacock et al., 2000). What role other cationic minerals play is not as well understood, but adequate levels of several trace elements including boron, copper, chromium, magnesium, manganese, selenium, silicon, strontium and zinc are considered important for bone health (Odabasi et al., 2008, Lowe et al., 2002, Strause et al., 1994, Jugdaohsingh, 2007, Marie et al., 2001, Cashman and Flynn, 1998, Yamaguchi, 1998).
Rodents have been used to study various aspects of bone formation and loss. In rodents, as in humans, bone formation occurs rapidly during early post-natal development (in mice, through at least 2 months of age). Thereafter, trabecular bone is gradually lost. Net cortical bone formation continues through 5–7 months, after which formation and loss remain relatively stable (Boskey & Coleman, 2010). As true for humans, past studies have demonstrated that a high-fat diet contributes to bone mineral loss and increases bone fragility (i.e., promotes the osteoporotic phenotype) (Zernicke et al., 1995, Pelton et al., 2012, Parhaml et al., 2001, Glatt et al., 2007, Ward et al., 2003). Our own past studies carried out with female C57BL/6 mice demonstrated that trabecular bone loss occurred rapidly (within 5 months) in animals fed a Western-style diet and continued over an 18-month period (Aslam et al., 2010a, Aslam et al., 2013). Surprisingly, however, rapid bone loss was also seen in mice fed a low-fat rodent chow diet. When either diet was supplemented with a calcium-rich, multi-mineral natural product, Aquamin® (AQ), bone loss was substantially mitigated. The present study was carried out to assess effects of diet and mineral supplementation on bone structure/function in male C57BL/6 mice maintained on the same Western-style diet or on the same rodent chow diet as used previously with female mice. Findings from the present study with male mice are significantly different from results obtained previously with females (Aslam et al., 2013). Whereas female mice lost bone mass and bone strength on either diet, in male mice, bone structure and function were largely preserved over an 18-month time-period on the healthy, rodent chow diet (AIN76A). In males, bone loss was seen, primarily, in animals on the HFWD. In spite of this difference, mineral supplementation mitigated bone loss in males, just as it did in females.
2. Materials and methods
2.1. Mineral supplement
Aquamin® (AQ) was used to provide mineral supplementation. The mineral supplement is obtained from the skeletal remains of the red marine algae, Lithothamnion sp. (Aslam et al., 2010a, Aslam et al., 2013). AQ is approximately 15% calcium and 1.5% magnesium by weight, and also contains measurable levels of 72 additional trace minerals - essentially all of the minerals that the algae are able to gather from the marine water. AQ (GRAS 000028) is used in various products for human consumption in Europe, Asia, Australia, and North America [Marigot Ltd., Cork, IR]. The complete mineral composition of AQ (as assessed by Advanced Laboratories, Inc., Salt Lake City, UT) can be found in Supplement Table 1.
2.2. Mice and diet groups
In this study, 140 male C57BL/6 mice (Charles River, Portage, MI) were started at 3-weeks of age on either a standard rodent chow diet (AIN76A) or on a high-fat diet Western diet (HFWD) prepared according to the formulation of Newmark (Newmark et al., 2009). This variant of AIN76A is designed to mimic food consumption patterns common in Western society (US Department of Agriculture, 2015, Peterlik and Cross, 2005). The HFWD contains 20% fat from corn oil as compared to 5% in AIN76A. The percentage of calories from fat in this diet is 37.8% as compared to 11.5% in the AIN76A diet. Although sucrose is reduced relative to the amount in AIN76A, the overall calories provided in the HFWD is 4764 kcal% versus 3902 kcal% in AIN76A. In addition to its high fat content, the HFWD has additional modifications. Methionine is replaced with cysteine, amounts of fiber, folic acid and choline are reduced, and the total calcium level is reduced from approximately 5.25 g per kg to 0.41 g per kg. Both AIN76A and its high-fat variant contain a mix of essential trace elements including potassium, magnesium, manganese, chromium, copper, iron, selenium, sodium and zinc as part of the AIN76A formulation. For half of the animals on either diet, the calcium-rich, multi-mineral supplement (AQ) was included at 62 g per 1.062 kg of diet. At this concentration of AQ, the dietary calcium provided to animals on the HFWD is approximately 6.9 g per kg. The slightly increased calcium (relative to the amount in AIN76A) is based on the premise that mice on high fat diet will consume less food than animals on the corresponding lower fat diet (AIN76A).
From each diet group, 10 mice were euthanized after 5 months and 12 months, and 15 mice were euthanized after 18 months. A group of 5 mice were euthanized at the start of the study to provide baseline values. Diets were formulated and provided by Research Diets Incorporated (New Brunswick, NJ). The complete composition of each diet is presented in Supplement Table 2. Food and water were provided ad libitum. Animals were monitored at 2-day intervals throughout the in-life phase and were weighed at 2-week intervals. All of the procedures were reviewed and approved by the University Committee on Use and Care of Animals (UCUCA) at the University of Michigan.
2.3. Preparation of skeletal tissue and micro-computed tomography (micro-CT)
At euthanasia, the left femora and the C8 caudal vertebrae were carefully dissected free of associated connective tissue, immediately placed in sealed containers with lactated Ringer's solution, and frozen at -20 °C until use. Three-dimensional images of regions of interest (ROI) were obtained using a micro-CT system (eXplore Locus SP, GE Healthcare Pre-Clinical Imaging, London, Ontario, Canada) as previously described and validated (Meganck et al., 2009, Bouxsein et al., 2010). In the present study, we followed the same procedure as used in our previous studies with female mice (Aslam et al., 2013). A complete description of the micro-CT imaging procedure can be found in the Supplement under Methodology.
2.4. Biomechanical testing
Mechanical properties of the femora were determined by loading the left femora to failure in 4-point bending, exactly as described previously with female mice (Aslam et al., 2013). Three parameters – stiffness, post-yield displacement ratio (i.e., displacement at failure – displacement at yield) and maximum load were determined as described previously (Turner, 2006, Peterlik et al., 2006). Stiffness was defined as the slope of the linear region of the pre-yield load-displacement curve, and the yield point was defined as the point where the load-displacement curve intersected with a regression line that was 10% lower than that used to define stiffness. Maximum load was defined as the load at which the bone catastrophically failed.
Mechanical properties of intact caudal vertebrae were measured by compressing the vertebral body with a 3 mm diameter platen attached to a servohydraulic materials-testing machine as described in our earlier study (Salem et al., 1993, Tommasini et al., 2008). In the compression tests, the cranial and caudal endplates of the caudal vertebrae were not altered prior to testing. Parameters assessed included stiffness and maximum load. Stiffness was defined as the slope of the linear region of the pre-yield load-displacement curve and maximum load was defined as the highest load preceding a rapid decrease in the measured load (i.e., catastrophic failure).
2.5. Levels of individual trace elements in long bones
Following micro-CT and biomechanical testing on femora, the long bones (one femur and tibia from all animals in each group) were “pooled” by group and time point, and analyzed for levels of various trace metals. The C8 vertebrae from all the animals in a group were also tested as a group. Bones were “pooled” in order to have a sufficient amount of material to obtain a detectable signal with several of the minor trace elements thought to be important to bone. Bones were digested in a concentrated nitric acid solution (10 ml) for approximately 30 min, after which they were cooled to room temperature. Concentrated hydrochloric acid (10 ml) was added and the sample was digested for an additional 15 min. After cooling and dilution with distilled water, levels of individual trace elements were determined by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). Bone preparation and assays were done on a fee-for-service by Advanced Laboratories, Inc. Sensitivity by this method is 0.5 μg/g.
2.6. Tartrate-resistant acid phosphatase (TRAP) and N-terminal peptide of type I procollagen (P1NP)
Blood was obtained at the time of necropsy from each animal. TRAP and P1NP were assessed in serum samples using commercially-available enzyme-linked immunosorbent assays (ELISAs) (Immunodiagnostic Systems, Inc.; Fountain Hills, AZ). TRAP is produced by osteoclasts and macrophages and can be detected in serum. The ELISA used here measures TRAP 5b, the form specific to osteoclasts (Hannon et al., 2004). TRAP 5b is thought to be a measure of osteoclast number rather than activity. P1NP is a measure of osteoblast function. Type I collagen is the major collagenous protein in bone (Risteli & Risteli, 2006).
2.7. Data presentation and statistical evaluation
Data from micro-CT analysis and biochemical evaluations were obtained for individual mice. The data were presented as group averages and standard deviations. Differences among groups were compared for statistical significance using ANOVA followed by paired group comparisons (two-tailed). Differences were considered significant at the p < 0.05 level. Trace element assessment was done on bones that were “combined” from all the mice in a group in order to have a sufficient amount for assessment of minor trace elements. While individual “combined” samples could not be analyzed statistically, the data for each mineral from the three different time-points (long bones only) were subjected to two-way factorial ANOVA followed by paired group comparison, to determine if the effect of time was significant. Where the criteria were met (time not a significant variable), data from the three time points were grouped together and analyzed. Differences were considered significant at the p < 0.05 value.
3. Results
3.1. Animal weight and survival data
At the initiation of study, mice had an average weight of 10 ± 1 g. Over the 18-month period, animals gained weight on both diets. Animals in the AIN76A diet groups (with or without AQ) had weights of 42 ± 6 g and 45 ± 4 g at 18-month time-point. As expected, animals on the HFWD gained more weight than animals on AIN76A, but AQ had no effect on weight gain (51 ± 8 g in HFWD without AQ compared to 52 ± 6 g with the AQ). Previous studies have suggested that dietary calcium supplementation can help to control weight-gain under some conditions (Bastie et al., 2012, Pilvi et al., 2007). Neither the present study, nor our previous study with female mice (Aslam et al., 2010a, Aslam et al., 2013), demonstrated this capacity of calcium with either the standard rodent chow diet or the high-fat diet.
Over the entire course of the 18-month maintenance period, 11 animals died or were euthanized for humane reasons. Of these, 9 were on the HFWD without supplementation and 2 were on the HFWD with the mineral supplement. Bones from animals that died prematurely were handled as part of their respective cohort. Serum calcium levels obtained at euthanasia were 9.9 ± 1.3 and 10.6 ± 1.1 mg per dl in mice on AIN76A without and with AQ compared to 9.5 ± 1.2 and 10.1 ± 0.7 mg per dl in mice on the HFWD without and with AQ.
3.2. Femoral bone structure and function: effect of diet and mineral supplementation
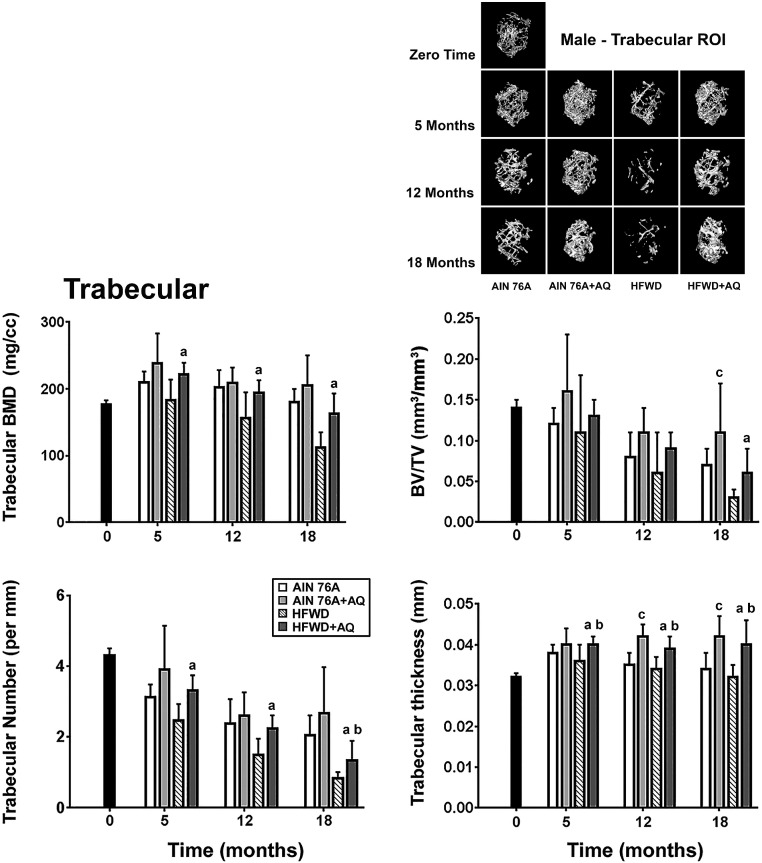
Micro-CT data from the trabecular ROI at 5, 12 and 18-month time-points are shown in Fig. 1. It can be seen from the figure that BMD remained almost constant over the 18-month maintenance period in mice on the rodent chow diet but declined by approximately 40% in mice fed the HFWD as compared to mice fed HFWD plus AQ. BV/TV and trabecular number declined in mice on either diet, but the decline was more severe in mice on the HFWD (BV/TV: 79% reduction in HFWD versus 48% in AIN76A; trabecular number: 77% decline versus 49% in the two diets, respectively). Trabecular thickness remained constant over the 18-month time period on either diet.
Fig. 1.
Femoral bone: Structural features of trabecular bone. Structural features were assessed in the trabecular ROI by micro-CT as described in the Materials and Methods. Data are based on 5 mice at baseline (4 weeks of age), 10 mice at the 5 and 12 month time-points and 15 mice at the 18 month time-point in each group. Values are means and standard deviations. Statistical significance of each parameter was assessed by ANOVA followed by paired group comparisons. Statistical significance at the p < 0.05 level is indicated by the letters “a”, “b”, and “c”. The letter “a” above the HFWD + AQ bar indicates statistically significant improvement relative to HFWD alone. The letter “b” above the HFWD + AQ bar indicates statistically significant improvement relative to AIN76A group. The letter “c” above the AIN76A + AQ bar indicates statistically significant improvement relative to AIN76A alone. All of the trabecular micro-CT parameters measured at the three time points are presented in Supplement Table 3. Insert: Representative 3D micro-CT images of the trabecular ROI from the femora of mice in each diet group, at each time-point.
Femoral bone: Structural features of trabecular bone. Structural features were assessed in the trabecular ROI by micro-CT as described in the Materials and Methods. Data are based on 5 mice at baseline (4 weeks of age), 10 mice at the 5 and 12 month time-points and 15 mice at the 18 month time-point in each group. Values are means and standard deviations. Statistical significance of each parameter was assessed by ANOVA followed by paired group comparisons. Statistical significance at the p < 0.05 level is indicated by the letters “a”, “b”, and “c”. The letter “a” above the HFWD + AQ bar indicates statistically significant improvement relative to HFWD alone. The letter “b” above the HFWD + AQ bar indicates statistically significant improvement relative to AIN76A group. The letter “c” above the AIN76A + AQ bar indicates statistically significant improvement relative to AIN76A alone. All of the trabecular micro-CT parameters measured at the three time points are presented in Supplement Table 3. Insert: Representative 3D micro-CT images of the trabecular ROI from the femora of mice in each diet group, at each time-point.
Fig. 1 also demonstrates the effects of mineral supplementation on trabecular bone parameters. Mineral supplementation improved each of the trabecular bone parameters and this was observed in both diets. Of interest, even though trabecular thickness (unlike the other parameters) did not decline significantly in either diet over the 18-month time-period, AQ led to a statistically significant increase in thickening in both AIN76A and the Western style diet (33–35% increase).
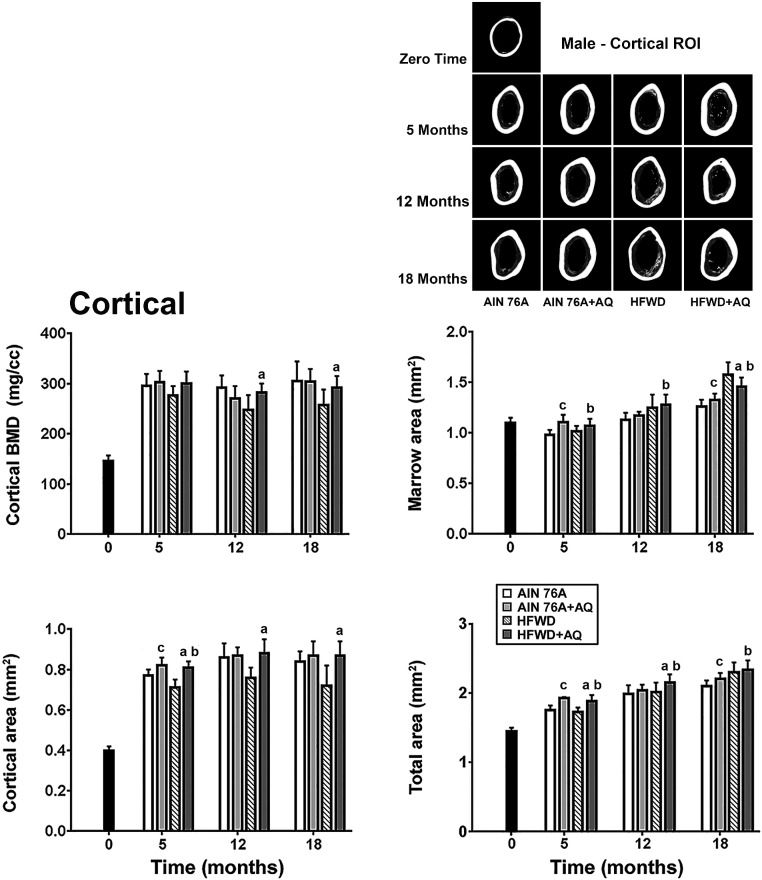
Cortical bone parameters are presented in Fig. 2. In contrast to what was seen with trabecular bone (where there was a significant decline in structural features over time), cortical bone properties – specifically, BMD and cortical area – increased in all groups between the beginning of the study at 3-weeks of age and 5 months, and then remained stable through the end of the study (18 months). The Western style diet had a negative effect on both parameters (both BMD and cortical area were reduced by approximately 16% at the 18-month time-point in the HFWD as compared to HFWD plus AQ). Mineral supplementation with AQ reversed these HFWD-dependent deficits in cortical bone (Fig. 2).
Fig. 2.
Femoral bone: Structural features of cortical bone. Structural features were assessed in the cortical ROI by micro-CT as described in the Materials and Methods. Data are based on 5 mice at baseline (4 weeks of age), 10 mice at the 5 and 12 month time-points and 15 mice at the 18 month time-point in each group. Values are means and standard deviations. Statistical significance of each parameter was assessed by ANOVA followed by paired group comparisons. Statistical significance at the p < 0.05 level is indicated by the letters “a”, “b”, and “c”. The letter “a” above the HFWD + AQ bar indicates statistically significant improvement relative to HFWD alone. The letter “b” above the HFWD + AQ bar indicates statistically significant improvement relative to AIN76A group. The letter “c” above the AIN76A + AQ bar indicates statistically significant improvement relative to AIN76A alone. All of the cortical micro-CT parameters measured at the three time points are presented in Supplement Table 4. Insert: Representative 3D micro-CT images of the cortical ROI from the femora of mice in each diet group, at each time-point.
Femoral bone: Structural features of cortical bone. Structural features were assessed in the cortical ROI by micro-CT as described in the Materials and Methods. Data are based on 5 mice at baseline (4 weeks of age), 10 mice at the 5 and 12 month time-points and 15 mice at the 18 month time-point in each group. Values are means and standard deviations. Statistical significance of each parameter was assessed by ANOVA followed by paired group comparisons. Statistical significance at the p < 0.05 level is indicated by the letters “a”, “b”, and “c”. The letter “a” above the HFWD + AQ bar indicates statistically significant improvement relative to HFWD alone. The letter “b” above the HFWD + AQ bar indicates statistically significant improvement relative to AIN76A group. The letter “c” above the AIN76A + AQ bar indicates statistically significant improvement relative to AIN76A alone. All of the cortical micro-CT parameters measured at the three time points are presented in Supplement Table 4. Insert: Representative 3D micro-CT images of the cortical ROI from the femora of mice in each diet group, at each time-point.
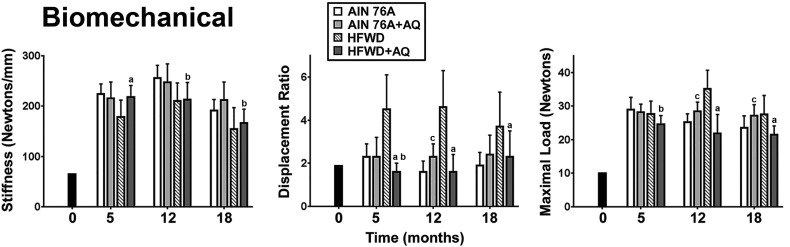
Femoral mechanical properties (bone ductility and strength) were determined by subjecting bones to failure in 4-point bending (Fig. 3). Response in the 4-point bending assay is largely a reflection of cortical bone properties (Bouxsein et al., 2010). Consistent with differences in cortical bone structural properties assessed by micro-CT (i.e., reduced BMD, reduced cortical area and increased marrow area), bones from mice on the unsupplemented HFWD were highly ductile (i.e., with reduced stiffness and high displacement ratio) as compared to bones from mice maintained on the AIN76A diet. Consistent with our understanding of bone mechanics (Meganck et al., 2009, Bouxsein et al., 2010, Turner, 2006, Peterlik et al., 2006), the high ductility of the femora of mice without mineral supplementation was associated with a high load required for catastrophic failure. Mineral supplementation reduced bone ductility while having only a modest effect on bone strength. In contrast to these results in HFWD-fed mice, mineral supplementation had minimal effect on long bone biomechanical properties in mice maintained on the AIN76A diet. All of the micro-CT and biomechanical parameters from femora are presented in Supplement Table 3, Supplement Table 4, Supplement Table 5.
Fig. 3.
Femoral bone: Biomechanical properties. Femoral stiffness and strength were assessed in the 4-point bending assay as described in the Materials and Methods. Data are based on 5 mice at baseline (4 weeks of age), 10 mice at the 5 and 12 month time-points and 15 mice at the 18 month time-point in each group. Values are means and standard deviations. Statistical significance of each parameter was assessed by ANOVA followed by paired group comparisons. Statistical significance at the p < 0.05 level is indicated by the letters “a”, “b”, and “c”. The letter “a” above the HFWD + AQ bar indicates statistically significant improvement relative to HFWD alone. The letter “b” above the HFWD + AQ bar indicates statistically significant improvement relative to AIN76A group. The letter “c” above the AIN76A + AQ bar indicates statistically significant improvement relative to AIN76A alone. All of the biomechanical properties measured in femora at the three time points are presented in Supplement Table 5.
Femoral bone: Biomechanical properties. Femoral stiffness and strength were assessed in the 4-point bending assay as described in the Materials and Methods. Data are based on 5 mice at baseline (4 weeks of age), 10 mice at the 5 and 12 month time-points and 15 mice at the 18 month time-point in each group. Values are means and standard deviations. Statistical significance of each parameter was assessed by ANOVA followed by paired group comparisons. Statistical significance at the p < 0.05 level is indicated by the letters “a”, “b”, and “c”. The letter “a” above the HFWD + AQ bar indicates statistically significant improvement relative to HFWD alone. The letter “b” above the HFWD + AQ bar indicates statistically significant improvement relative to AIN76A group. The letter “c” above the AIN76A + AQ bar indicates statistically significant improvement relative to AIN76A alone. All of the biomechanical properties measured in femora at the three time points are presented in Supplement Table 5.
3.3. Vertebral bone structure and function: Effect of mineral supplementation
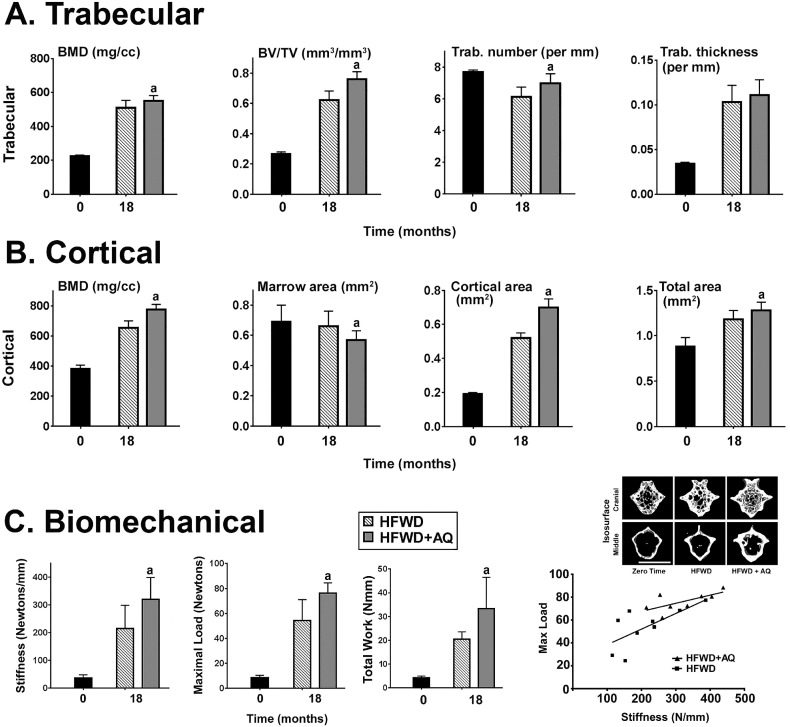
Although the present study was conducted, primarily, with femoral bone, we also carried out a series of experiments with the C8 vertebrae from mice on the HFWD at the 18-month time-point. Micro-CT data are shown in Fig. 4A and 4B. In the trabecular ROI, BMD, BV/TV and trabecular thickness were all increased significantly at the 18-month time-point relative to what was seen at 3 weeks of age. In the cortical ROI, we observed significant increases in BMD, cortical area and total area at the 18-month time-point relative to what was seen at 3 weeks of age. Mineral supplementation with AQ improved bone structural features detected by micro-CT in both trabecular and cortical ROI relative to what was observed in the absence of supplementation (Fig. 4A and 4B). Trabecular BMD was increased by 8% and cortical BMD was increased by 18% in mice on the mineral-supplemented diet as compared to the unsupplemented HFWD (p < 0.05 for both). Cortical area was increased by almost 37% in the mineral-supplemented HFWD relative to the HFWD alone (p < 0.05).
Fig. 4.
Vertebral bone structure and function. A: Structural features of trabecular bone. B: Structural features of cortical bone. C: Biomechanical properties. Data are based on 10 mice at the 18-month time-point in each group. Values are means and standard deviations. Statistical significance of each parameter was assessed using the Student t-test. Statistical significance at the p < 0.05 level is indicated by the letter “a” above the HFWD + AQ bar, which indicates statistically significant improvement relative to HFWD alone. The right lower panel (scatter plot) shows the positive correlation between stiffness and strength in individual mice. All of the C8 vertebral micro-CT trabecular/cortical bone parameters and biomechanical properties measured at the 18 month time-point are presented in Supplement Tables 6 and 7. Insert: A representative 3D micro-CT image of trabecular (surface) and cortical (diaphysis) region from the C8 caudal vertebrae of a mouse in each diet group at zero time and 18 month (magnification bar = 1 mm).
Vertebral bone structure and function. A: Structural features of trabecular bone. B: Structural features of cortical bone. C: Biomechanical properties. Data are based on 10 mice at the 18-month time-point in each group. Values are means and standard deviations. Statistical significance of each parameter was assessed using the Student t-test. Statistical significance at the p < 0.05 level is indicated by the letter “a” above the HFWD + AQ bar, which indicates statistically significant improvement relative to HFWD alone. The right lower panel (scatter plot) shows the positive correlation between stiffness and strength in individual mice. All of the C8 vertebral micro-CT trabecular/cortical bone parameters and biomechanical properties measured at the 18 month time-point are presented in Supplement Tables 6 and 7. Insert: A representative 3D micro-CT image of trabecular (surface) and cortical (diaphysis) region from the C8 caudal vertebrae of a mouse in each diet group at zero time and 18 month (magnification bar = 1 mm).
Vertebral biomechanical properties (i.e., resistance to compression load damage) were assessed following micro-CT analysis. Results in the compression-damage assay are a reflection of both stiffness and strength, and depend on both trabecular and cortical properties (Tommasini et al., 2008). As shown in Fig. 4C, bone stiffness was substantially improved (48% increase with AQ compared to HFWD alone) as was maximum load (41% increase in AQ compared to HFWD alone) (p < 0.05 for both). All of the micro-CT and biomechanical parameters from C8 vertebrae are presented in Supplement Table 6, Supplement Table 7.
3.4. Levels of individual trace elements in bone: effect of diet and mineral supplementation
Following micro-CT analysis and biomechanical testing, bone calcium levels and levels of 11 cationic elements thought to be important for bone health were assessed in femora at each of the three time-points. Minerals included boron, copper, chromium, iron, magnesium, manganese, potassium, selenium, silicon, strontium and zinc. The data are presented in Table 1. As part of the analysis, levels of 30 additional minerals prevalent in AQ were assessed in femoral bone (Supplement Table 8, Supplement Table 9, Supplement Table 10). To summarize the data from Table 1, bone calcium levels from mice on the HFWD (with a calcium concentration of 0.41 g per kg) were decreased by 3–9% as compared to bone calcium levels in mice fed AIN76A (with a calcium concentration of 5.25 g per kg) (not significantly different). When AQ was included in the HFWD, bone calcium levels were increased by 9, 13 and 7% at 5-, 12- and 18-month time-points, respectively, as compared to HFWD alone. These levels are similar to levels seen in AIN76A without supplementation. Levels of four elements – boron, copper, chromium and selenium were below detectable limits (0.5 μg/g) in all diet groups at all three time-points. Among other cationic elements, iron, manganese, silicon and zinc demonstrated a modest but statistically significant decrease in the presence of AQ versus HFWD alone. Finally, and perhaps most interesting, strontium levels were increased dramatically in bones of mice in response to mineral-supplementation with AQ in both diet groups. This was observed with both diets at all three time-points (Table 1). The same minerals were also assessed in vertebral bone (18-month; HFWD and HFWD + AQ). Similar to what was seen with femora, only strontium was significantly increased by AQ (254 μg/g in supplemented HFWD versus 41 μg/g in HFWD). Complete mineral analysis of C8 vertebrae is presented in Supplement Table 11.
Table 1.
Cationic mineral content in femoral bone samples from mice in each diet group.
| Mineral/diet group | 5-months | 12-months | 18-months |
|---|---|---|---|
| Calcium | |||
| AIN76A | 199,500 | 230,300 | 229,100 |
| AIN76A + AQ | 199,400 | 233,800 | 234,100 |
| HFWD | 193,100 | 215,900 | 204,800 |
| HFWD + AQ | 209,300 | 243,800 | 218,600 |
| Iron | |||
| AIN76A | 144 | 266 | 228 |
| AIN76A + AQ | 69 | 218 | 167 |
| HFWD | 380 | 331 | 327 |
| HFWD + AQ | 134 | 201 | 224a |
| Magnesium | |||
| AIN76A | 3036 | 3192 | 3649 |
| AIN76A + AQ | 3250 | 3724 | 3797 |
| HFWD | 2927 | 3175 | 3243 |
| HFWD + AQ | 3208 | 3537 | 3770 |
| Manganese | |||
| AIN76A | 2.01 | 1.22 | 1.16 |
| AIN76A + AQ | 1.97 | 1.27 | 0.97 |
| HFWD | 7.20 | 4.44 | 3.56 |
| HFWD + AQ | 1.63 | 1.36 | 1.07a |
| Potassium | |||
| AIN76 | 759 | 1273 | 1458 |
| AIN76A + AQ | 724 | 1109 | 1227 |
| HFWD | 758 | 1172 | 1306 |
| HFWD + AQ | 903 | 1123 | 1245 |
| Silicon | |||
| AIN76A | 4.75 | 1.20 | 1.76 |
| AIN76A + AQ | < 0.5 | < 0.5 | 0.81 |
| HFWD | 4.34 | 2.40 | 1.78 |
| HFWD + AQ | < 0.5 | < 0.5 | < 0.5a |
| Strontium | |||
| AIN76A | 50 | 25 | 25 |
| AIN76A + AQ | 210 | 260 | 237c |
| HFWD | 112 | 66 | 44 |
| HFWD + AQ | 231 | 244 | 238a,b |
| Zinc | |||
| AIN76A | 111 | 134 | 124 |
| AIN76A + AQ | 124 | 150 | 138 |
| HFWD | 246 | 232 | 198 |
| HFWD + AQ | 140 | 164 | 147a,b |
Femora from all mice in a group were pooled together, digested and analyzed by ICP-OES, as one sample per group (10 mice per group at 5- and 12-months and 15 mice at 18-months). Boron, copper, chromium and selenium were below detectable limit (0.5 μg/g). The data for each mineral from the three time-points grouped together were subjected to two-way factorial ANOVA followed by paired group comparisons. “a” and “b” are placed on the HFWD + AQ group: “a” shows statistically significant difference relative to the HFWD group, and “b” shows statistically significant difference relative to AIN76A. A “c” is placed on the AIN76A + AQ group shows significant difference relative to AIN76A alone (p < 0.05).
3.5. Serum TRAP and P1NP
TRAP-5b and P1NP levels are shown in Table 2. Consistent with what we have seen previously in female C57BL/6 mice (Aslam et al., 2010a, Aslam et al., 2013), levels of TRAP-5b were increased in response to mineral supplementation with AQ in both diets at all three time-points. Eighteen month values were statistically significant. Also consistent with what we have reported previously in females, P1NP levels were not significantly different between the supplemented and unsupplemented diets at any of the three time-points (Table 2).
Table 2.
Serum TRAP and P1NP levels.
| Diet group | 5-months | 12-months | 18 months |
|---|---|---|---|
| TRAP (U/ml) | |||
| AIN76A | 0.6 ± 0.5 | 9.5 ± 2.62 | 5.9 ± 1.4 |
| AIN76A + AQ | 0.9 ± 0.5 | 16.0 ± 6.0 | 11.6 ± 3.9c |
| HFWD | 0.4 ± 0.5 | 9.1 ± 5.6 | 7.8 ± 3.7 |
| HFWD + AQ | 1.1 ± 0.4 | 24.7 ± 11.2 | 10.1 ± 4.7a,b |
| P1NP (ng/ml) | |||
| AIN76A | Not determ | 20.3 ± 4.3 | 17.1 ± 3.4 |
| AIN76A + AQ | Not determ | 17.7 ± 3.2 | 14.8 ± 3.9 |
| HFWD | Not determ | 23.6 ± 5.8 | 21.2 ± 5.1 |
| HFWD + AQ | Not determ | 20.1 ± 4.4 | 17.9 ± 6.2 |
Baseline TRAP = 0.75 ± 0.5 U/ml. Values are means and standard deviations. N = 10, 9 and 10 for AIN76A; 8, 10 and 10 for AIN + AQ; 9, 10 and 10 for HFWD and 10, 10 and 8 for HFWD + AQ at 5, 12 and 18 months, respectively.
P1NP data at baseline and at 5 month - not available. N = 10 and 10 for AIN76A; 10 and 10 AIN + AQ; 9 and 9 for HFWD and 10 and 8 for HFWD + AQ at 12 and 18 months, respectively.
Statistical significance was determined by ANOVA followed by paired group comparisons. “a” and “b” are placed on the HFWD + AQ group: “a” shows statistically significant increase relative to the HFWD group, “b” shows statistically significant increase relative to AIN76A; “c” is placed on the AIN76A + AQ group, and shows significant increase relative to the control (p < 0.05).
4. Discussion
Osteoporosis affects men as well as women, but females make up the majority of people diagnosed with the condition and suffer the majority of osteoporotic bone fractures (Guggenbuhl, 2009, Gielen et al., 2011, Adler, 2014). When findings from the current study in male mice are taken in conjunction with our previous findings with female mice, they allow us to conclude that while female mice lose bone over time on both healthy and unhealthy diets, bone loss in males is, primarily, a consequence of the (unhealthy) HFWD. To the extent that these findings can be extrapolated to humans, they suggest that men can benefit more than women from a life-time healthful diet. Perhaps the higher incidence of osteoporosis in women reflects this.
An important aspect of the present study was determining the extent to which a calcium-rich, multi-mineral supplement could mitigate the effects of the Western-style diet on bone structure and function in male mice. In our previous studies with female mice (Aslam et al., 2013), mineral supplementation reduced bone loss in either diet. Here we demonstrate that the same multi-mineral supplement reduced bone loss in both femora and vertebrae as compared to control. In males, however, the effects were seen primarily in the HFWD, where bone loss was more severe. It is reasonable to suggest based on these data that a lack of dietary minerals contributes significantly to bone deterioration seen by us [present report and (Aslam et al., 2010a, Aslam et al., 2013)] and others (Zernicke et al., 1995, Pelton et al., 2012, Parhaml et al., 2001, Glatt et al., 2007, Ward et al., 2003) in HFWD-fed rodents. The consequences of the mineral deficiency to the negative impact of a HFWD are not unique to bone. Previous studies showed that tumor formation in the liver and colon were increased in mice on the same HFWD as used here, and that both ill-effects were largely overcome with mineral supplementation (Newmark et al., 2009, Aslam et al., 2012a, Aslam et al., 2010b, Aslam et al., 2012b). To the extent that these findings can be extrapolated to humans, the data emphasize the importance of having an adequate supply of calcium and, perhaps, other trace elements in the diet. The Western-style diet may be harmful as much by what it lacks as what it contains.
An intriguing question is whether the beneficial effects of the multi-mineral supplement can be ascribed to its calcium content alone, or whether one or more of the trace elements in the preparation contribute to bone health in a meaningful way. Calcium is, by far, the major cationic mineral in bone, and the necessity for an adequate supply of dietary calcium throughout life is well-recognized. However, several other cationic trace elements including boron, copper, chromium, iron, magnesium, manganese, selenium, silicon, strontium and zinc have all been suggested to play roles in bone health (Odabasi et al., 2008, Lowe et al., 2002, Strause et al., 1994, Jugdaohsingh, 2007, Marie et al., 2001, Cashman and Flynn, 1998, Yamaguchi, 1998). Calcium is the most highly represented mineral in AQ but each of the other elements is present at quantifiable levels (Supplement Table 1). It is not unreasonable to suggest that one or several of these minerals could provide added benefit over and above that seen with calcium alone. In our two previous studies conducted with female C57BL/6 mice (Aslam et al., 2010a, Aslam et al., 2013), one of the comparisons was between mice on the AIN76A diet without supplementation and HFWD-fed mice with the mineral supplement. While the two diets provided comparable levels of calcium, female mice on the HFWD with the mineral supplement fared better than mice on AIN76A without the supplement (in terms of both structural and mechanical properties). Most of the added benefit appeared to be in trabecular bone. Using the same approach with male mice, we found a smaller (but still detectable) added benefit with AQ. Specifically, in male mice, femoral trabecular thickness was greater in AQ-supplemented mice than in control mice on either diet. How valuable the additional minerals will, ultimately, prove to be in human bone health cannot be determined from the present data. We are currently conducting a 90-day intervention biomarker study (ClinicalTrials.gov Identifier: NCT02647671; comparing AQ to both placebo and calcium alone – randomized and double-blinded) in human subjects with serum markers of bone turnover as one of the endpoints.
One thing is clear from this study; the distribution of certain cationic minerals in bones was affected by AQ-supplementation. Levels of iron, manganese and zinc were higher in bones of HFWD-fed mice than in AIN76A-fed mice. When the HFWD was supplemented with AQ, levels of these trace elements declined. Perhaps, more interestingly, strontium content increased several fold in response to AQ supplementation, and this was observed in both diets. The increase in strontium is of particular interest since strontium is known to replace calcium in bone, and is thought to preserve bone microarchitecture and bone strength (Marie et al., 2001, Meunier et al., 2004, Ammann et al., 2004). In addition, strontium has been shown to alter osteoclast/osteoblast function in vitro (Marie et al., 2001, Saidak and Marie, 2012) and so an effect on bone remodeling mechanisms may contribute to its action – independent of incorporation into the crystalline structure, itself. It would be unwise at this time to ascribe all of the added benefit to strontium because certain other minerals present in the multi-mineral supplement are also known to affect osteoclast and osteoblast function (Lowe et al., 2002, Yamaguchi, 1998). Currently, we are comparing calcium along with various combinations of mineral salts (formulated to the same relative amounts as present in the multi-mineral supplement) to calcium alone in rodents on the HFWD.
In summary, we have previously studied bone changes in female C57BL/6 mice maintained over an 18-month period on either a low-fat rodent chow diet or a HFWD, and have assessed the effects of a calcium-rich, multi-mineral supplement on bone structure and function in both diets (Aslam et al., 2013). Our major conclusions from those studies were i) that significant (and comparable) bone loss occurred over time in either diet and ii) that dietary mineral supplementation had a protective effect in either diet. Here we demonstrate with male C57BL/6 mice that diet has a much greater impact on bone structure/function than it appears to have with females – i.e., that bone loss occurs more rapidly and to a greater extent in the “unhealthy” diet than in the rodent chow diet. In spite of this, males benefit from mineral supplementation just as much as females.
The following are the supplementary data related to this article.
Supplemental Methodology.
. Composition of the multi-mineral algae product (Aquamin Soluble®).
. Composition of the four diets.
. Micro-CT analysis of trabecular regions of femora.
. Micro-CT analysis of cortical regions of femora.
. Biomechanical properties of femora.
. Micro-CT analysis of cortical and trabecular regions of Caudal 8 vertebrae at 18 month.
. Biomechanical properties of Caudal 8 vertebrae at 18 month.
. Bone Mineral Analysis at 5 months (μg/g).
. Bone Mineral Analysis at 12 months (μg/g).
. Bone Mineral Analysis at 18 months (μg/g).
. Bone Mineral Analysis at 18 months in C8 vertebrae of male mice (μg/g).
Disclosure of conflict
All authors state that they have no financial or personal conflict of interest (No Competing Interests).
Acknowledgement
This study was supported in part by grant CA140760 from the National Institutes of Health, Bethesda, MD, and by grant 11-0577 from Worldwide Cancer Research (formerly Association for International Cancer Research), St. Andrews, Fife, Scotland. The authors would like to thank Marigot, Ltd. (Cork, Ireland) for providing the multi-mineral supplement (Aquamin®) as a gift.
References
- Adler R.A. Osteoporosis in men: a review. Bone Res. 2014;2:14001. doi: 10.1038/boneres.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann P., Shen V., Robin B., Mauras Y., Bonjour J.P., Rizzoli R. Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J. Bone Miner. Res. 2004;19:2012–2020. doi: 10.1359/JBMR.040906. [DOI] [PubMed] [Google Scholar]
- Aslam M.N., Kreider J.M., Paruchuri T., Bhagavathula N., DaSilva M., Zernicke R.F., Goldstein S.A., Varani J. A mineral-rich extract from the red marine algae lithothamnion calcareum preserves bone structure and function in female mice on a Western-style diet. Calcif. Tissue Int. 2010;86:313–324. doi: 10.1007/s00223-010-9340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M.N., Paruchuri T., Bhagavathula N., Varani J. A mineral-rich red algae extract inhibits polyp formation and inflammation in the gastrointestinal tract of mice on a high-fat diet. Integr Cancer Res. 2010;9:93–99. doi: 10.1177/1534735409360360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M.N., Bergin I., Naik M., Hampton A., Allen R., Kunkel S.L., Rush H., Varani J. A multi-mineral natural product inhibits liver tumor formation in C57BL/6 mice. Biol. Trace Elem. Res. 2012;147:267–274. doi: 10.1007/s12011-011-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M.N., Bergin I., Naik M., Paruchuri T., Hampton A., Rehman M., Dame M.K., Rush H., Varani J. A multimineral natural product from red marine algae reduces colon polyp formation in C57BL/6 mice. Nutr. Cancer. 2012;64:1020–1028. doi: 10.1080/01635581.2012.713160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M.N., Bergin I., Jepsen K., Kreider J.M., Graf K.H., Naik M., Goldstein S.A., Varani J. Preservation of bone structure and function by Lithothamnion sp. derived minerals. Biol. Trace Elem. Res. 2013;156:210–220. doi: 10.1007/s12011-013-9820-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastie C.C., Gaffney-Stomberg E., Lee T.W.A., Dhima E., Pessin J.E., Augenlicht L.H. Dietary cholecalciferol and calcium levels in a Western-style diet alter energy metabolism and inflammatory responses in mice. J. Nutr. 2012;142:859–865. doi: 10.3945/jn.111.149914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey A.L., Coleman R. Aging and bone (Crit. Rev Oral Biol Med) J. Dent. Res. 2010;89:1333–1348. doi: 10.1177/0022034510377791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Müller R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J. Bone Miner. Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Cashman K., Flynn A. Trace elements and bone metabolism. In: Sandstrom B., Walter P., editors. Role of Trace Elements for Health Promotion and Disease Prevention. Bibl Nutr Dieta. Vol. 54. Karger; Basel: 1998. pp. 150–164. [DOI] [PubMed] [Google Scholar]
- Center J.R., Nguyen T.V., Schneider D., Sambrook P.N., Eisman J.A. Mortality after all major types of osteoporotic fractures in men and women: an observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- De Laet C.E., Van Hout B.A., Burger H., Weel A.E., Hofman A., Pols H.A. Hip fracture prediction in elderly men and women: validation in the Rotterdam study. J. Bone Miner. Res. 1998;13:1587–1593. doi: 10.1359/jbmr.1998.13.10.1587. [DOI] [PubMed] [Google Scholar]
- Gielen E., Vanderschueren D., Callewaert F., Boonen S. Osteoporosis in men. Best Pract. Res. Clin. Endocrinol. Metab. 2011;25:321–335. doi: 10.1016/j.beem.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Glatt V., Canalis E., Stadmeyer L., Bouxsein M.L. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Miner. Res. 2007;22:1197–1207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- Guggenbuhl P. Osteoporosis in males and females: is there really a difference. Joint Bone Spine. 2009;76:595–601. doi: 10.1016/j.jbspin.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Hannon R.A., Clowes J.A., Eagleton A.C., Al Hadari A., Eastell R., Blumsohn A. Clinical performance of immunoreactive tartrate resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone. 2004;34:187–194. doi: 10.1016/j.bone.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Heaney R.P., Weaver C.M. Newer perspectives on calcium nutrition and bone quality. J. Am. Coll. Nutr. 2005;24:574S–581S. doi: 10.1080/07315724.2005.10719506. [DOI] [PubMed] [Google Scholar]
- Johnell O., Kanis J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- Jugdaohsingh R. Silicon and bone health. J. Nutr. Health Aging. 2007;11:99–110. [PMC free article] [PubMed] [Google Scholar]
- Lowe N.M., Lowe N.M., Fraser W.D., Jackson M.J. Is there potential therapeutic value of copper and zinc for osteoporosis? Proc. Nutr. Soc. 2002;61:181–185. doi: 10.1079/PNS2002154. [DOI] [PubMed] [Google Scholar]
- Marie P.J., Ammann P., Boivin G., Rey C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 2001;69:121–129. doi: 10.1007/s002230010055. [DOI] [PubMed] [Google Scholar]
- Meganck J.A., Kozloff K.M., Thornton M.M., Broski S.M., Goldstein S.A. Beam hardening artifacts in micro-computed tomography scanning can be reduced by X-ray beam filtration and the resulting images can be used to accurately measure BMD. Bone. 2009;45:1104–1106. doi: 10.1016/j.bone.2009.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier P.J., Roux C., Seeman E., Ortolani S., Badurski J.E., Spector T.D., Cannata J., Balogh A., Lemmel E.M., Pors-Nielsen S., Rizzoli R., Genant H.K., Reginster J.Y. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N. Engl. J. Med. 2004;350:459–468. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- Newmark H.L., Yang K., Kurihara N., Fan K., Augenlicht L.H., Lipkin M. Western style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57BI/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88–92. doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odabasi E., Turan M., Aydin A., Akay C., Kutlu M. Magnesium, zinc, copper, manganese and selenium levels in postmenopausal women with osteoporosis: can magnesium play a key role in osteoporosis? Ann. Acad. Med. Singap. 2008;37:564–567. [PubMed] [Google Scholar]
- Parhaml F., Tintut Y., Beamer W.G., Gharavi N., Goodman W., Demer L.L. Atherogenic high-fat diet reduces bone mineralization in mice. J. Bone Miner. Res. 2001;16:182–188. doi: 10.1359/jbmr.2001.16.1.182. [DOI] [PubMed] [Google Scholar]
- Peacock M., Liu G., Carey M., McClintock R., Ambrosius W., Hui S., Johnston C.C. Effect of a calcium or 25OHD vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. J. Clin. Endocrinol. Metab. 2000;85:3011–3019. doi: 10.1210/jcem.85.9.6836. [DOI] [PubMed] [Google Scholar]
- Pelton K., Krieder J., Joiner D., Freeman M.R., Goldstein S.A., Solomon K.R. Hypercholesterolemia promotes an osteoporotic phenotype. Am. J. Pathol. 2012;181:928–936. doi: 10.1016/j.ajpath.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlik M., Cross H.S. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur. J. Clin. Investig. 2005;35:290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- Peterlik H., Roschger P., Klauhofer K., Fratzl P. From brittle to ductile fracture of bone. Nat. Mater. 2006;5:52–55. doi: 10.1038/nmat1545. [DOI] [PubMed] [Google Scholar]
- Pilvi T.K., Korpela R., Huttunen M., Vapaatalo H., Mervaala E.M. High-calcium diet with whey protein attenuates body-weight gain in high-fat-fed C57BL/6J mice. Br. J. Nutr. 2007;98:900–907. doi: 10.1017/S0007114507764760. [DOI] [PubMed] [Google Scholar]
- Prentice A. Diet, nutrition and the prevention of osteoporosis. Public Health Nutr. 2004;7(1A):227–243. doi: 10.1079/phn2003590. [DOI] [PubMed] [Google Scholar]
- Risteli J., Risteli L. Products of bone collagen metabolism. In: Seibel M.J., Robins S.P., Bilezikian J.P.G., editors. Dynamics of Bone and Cartilage Metabolism: Principles and Clinical Applications. second ed. Academic Press; London: 2006. pp. 391–405. [Google Scholar]
- Rosen C.J., Klibanski A. Bone, fat, and body composition: Evolving concepts in the pathogenesis of osteoporosis. Am. J. Med. 2009;122:409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Saidak Z., Marie P.J. Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 2012;136:216–226. doi: 10.1016/j.pharmthera.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Salem G.J., Zernicke R.F., Martinez D.A., Vailas A.C. Adaptations of immature trabecular bone to moderate exercise: geometrical, biochemical, and biomechanical correlates. Bone. 1993;14:647–654. doi: 10.1016/8756-3282(93)90087-q. [DOI] [PubMed] [Google Scholar]
- Strause L., Saltman P., Smith K.T., Bracker M., Andon M.B. Spinal bone loss in post-menopausal women supplemented with calcium and trace metals. J. Nutr. 1994;124:1060–1064. doi: 10.1093/jn/124.7.1060. [DOI] [PubMed] [Google Scholar]
- Tommasini S.M., Wearne S.L., Hof P.R., Jepsen K.J. Percolation theory relates corticocancellaous architecture to mechanical function in vertebrae of inbred mouse strains. Bone. 2008;42:743–750. doi: 10.1016/j.bone.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C.H. Bone strength: current concepts. Ann. N. Y. Acad. Sci. 2006;1068:429–446. doi: 10.1196/annals.1346.039. [DOI] [PubMed] [Google Scholar]
- US Department of Agriculture . 8th. ed. 2015. 2015–2020 Dietary Guidelines for Americans.http://health.gov/dietaryguidelines/2015/guidelines/ Washington DC. [Google Scholar]
- von Muhlen D., Safii S., Jassal S.K., Svartberg J., Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos. Int. 2007;18:1337–1344. doi: 10.1007/s00198-007-0385-1. [DOI] [PubMed] [Google Scholar]
- Ward W.E., Kim S., Bruce W.R. A western-style diet reduces bone mass and biomechanical bone strength to a greater degree in male compared with female rats during development. Br. J. Nutr. 2003;90:589–595. doi: 10.1079/bjn2003952. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M. Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med. 1998;11:119–135. [Google Scholar]
- Zernicke R.F., Salem G.J., Barnard R.J., Schramm E. Long-term high-fat sucrose diet alters rat femoral neck and vertebral morphology, bone mineral content and mechanical properties. Bone. 1995;16:25–31. doi: 10.1016/s8756-3282(00)80007-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methodology.
. Composition of the multi-mineral algae product (Aquamin Soluble®).
. Composition of the four diets.
. Micro-CT analysis of trabecular regions of femora.
. Micro-CT analysis of cortical regions of femora.
. Biomechanical properties of femora.
. Micro-CT analysis of cortical and trabecular regions of Caudal 8 vertebrae at 18 month.
. Biomechanical properties of Caudal 8 vertebrae at 18 month.
. Bone Mineral Analysis at 5 months (μg/g).
. Bone Mineral Analysis at 12 months (μg/g).
. Bone Mineral Analysis at 18 months (μg/g).
. Bone Mineral Analysis at 18 months in C8 vertebrae of male mice (μg/g).