Abstract
Aluminum salts such as aluminum oxyhydroxide and aluminum hydroxyphosphate are commonly used human vaccine adjuvants. In an effort to improve the adjuvant activity of aluminum salts, we previously showed that the adjuvant activity of aluminum oxyhydroxide nanoparticles is significantly more potent than that of aluminum oxyhydroxide microparticles. The present study was designed to i) understand the mechanism underlying the potent adjuvant activity of aluminum oxyhydroxide nanoparticles, relative to microparticles, and ii) to test whether aluminum hydroxyphosphate nanoparticles have a more potent adjuvant activity than aluminum hydroxyphosphate microparticles as well. In human THP-1 myeloid cells, wild-type and NLRP3-deficient, both aluminum oxyhydroxide nanoparticles and microparticles stimulate the secretion of proinflammatory cytokine IL-1β by activating NLRP3 inflammasome, although aluminum oxyhydroxide nanoparticles are more potent than microparticles, likely related to the higher uptake of the nanoparticles by the THP-1 cells than the microparticles. Aluminum hydroxyphosphate nanoparticles also have a more potent adjuvant activity than microparticles in helping a model antigen lysozyme to stimulate specific antibody response, again likely related to their stronger ability to activate the NLRP3 inflammasome.
Keywords: Aluminum oxyhydroxide, aluminum hydroxyphosphate, nanoparticles, microparticles, IL-1β, NLRP3 inflammasome, antigen binding, antibody response
Graphical abstract
Introduction
Vaccines have been an indispensable tool in the fight against infectious diseases. For most modern vaccines, an adjuvant is often needed to help induce strong pathogen-specific humoral and cellular immune responses. Aluminum salts are the most commonly used human vaccine adjuvants in the U.S., although their adjuvant activity is relatively weak. Two types of aluminum salts, namely aluminum oxyhydroxide (AlO(OH), or AH, also known as aluminum hydroxide) and aluminum hydroxyphosphate (Al(OH)x(PO)y, or AP, also known as aluminum phosphate), are commonly used. Both aluminum oxyhydroxide and aluminum hydroxyphosphate are composed of small nanometer-scale primary particles, which if not stabilized, aggregate in an aqueous solution to form larger microparticles of 1–20 µm [1, 2].
In an effort to improve the adjuvant activity of aluminum salts, previously we reported that the adjuvant activity of aluminum oxyhydroxide nanoparticles of ~110 nm is significantly stronger than that of aluminum oxyhydroxide microparticles of ~9 µm [3]. The present study was designed to understand the mechanism underlying the stronger adjuvant activity of aluminum oxyhydroxide nanoparticles, and to test whether aluminum hydroxyphosphate nanoparticles also have a stronger adjuvant activity than microparticles.
The exact mechanisms underlying the adjuvant activity of aluminum salts have not been fully elucidated [4, 5]. Proposed mechanisms of immunopotentiation by aluminum-containing adjuvants include formation of antigen depot [6, 7], stimulation of dendritic cells [8], complement activation [9], and stimulation of chemokine release [4, 9]. However, recent evidence points out that aluminum salt-based adjuvants activate an intracellular pathogen pattern recognition receptor signaling pathway involving the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome [10, 11]. The activation of NLRP3 inflammasome controls the maturation and secretion of cytokines such as IL-1β and IL-18 whose potent proinflammatory activity directs host responses to infection and injury [12]. Therefore, in the present study, we focused on examining whether the adjuvant activity of aluminum oxyhydroxide nanoparticles (AHNPs) is dependent on NLRP3 inflammasome activation, and whether AH-NPs are more potent than aluminum oxyhydroxide microparticles (AH-MPs) in activating the NLRP3 inflammasome. In addition, we prepared aluminum hydroxyphosphate nanoparticles (AP-NPs) and microparticles (AP-MPs) and compared their abilities in activating NLPR3 inflammasome in cell culture and in helping enhance antigen-specific immune responses in a mouse model.
Materials and Methods
Materials
Aluminum Hydroxide Nanopowder/Nanoparticles (high purity, 99.9%, 10–20 nm, hydrophilic, # US3026) were from US Research Nanomaterials, Inc. (Houston TX). Aluminum chloride hexahydrate, sodium hydroxide, polyvinylpyrrolidone, Laemmli sample buffer, sodium bicarbonate, sodium carbonate, phosphate-buffered saline (PBS), CA-074-Me, beta-mercaptoethanol, and N-acetyl-L-cysteine (NAC) were from Sigma-Aldrich (St. Louis, MO). Aluminum hydroxide Alhydrogel® liquid suspension (Alum), lipopolysaccharide (LPS), phorbol 12-myristate 13-acetate (PMA), monosodium urate (MSU) crystal, HygroGold, and normocin were from InvivoGen (San Diego, CA). The human IL-1β ELISA kit was from R&D Systems (Minneapolis, MN). Goat antimouse immunoglobulins (IgG) were from Southern Biotechnology Associates, Inc. (Birmingham, AL). Bio-safe™ Coomassie blue staining solution and Bio-Rad DC™ protein assay reagents were from Bio-Rad Laboratories (Hercules, CA). Cell culture medium, antibiotics, and fetal bovine serum (FBS) were from Invitrogen (Carlsbad, CA).
Preparation and characterization of aluminum oxyhydroxide particles
Previously, we synthesized aluminum oxyhydroxide nanoparticles by reacting aluminum chloride with sodium hydroxide, whereas the aluminum oxyhydroxide microparticles were prepared by suspending commercially available aluminum hydroxide dried gel in aqueous medium [3]. In the present study, to ensure that the aluminum oxyhydroxide nanoparticles and microparticles are prepared from the same material, the Aluminum Hydroxide Nanopowder/Nanoparticles powder from the US Research Nanomaterials was used. The powder was dispersed in water (5 mg/ml) and separated into AH-NPs and AH-MPs by centrifugation. Briefly, the Aluminum Hydroxide Nanopowder/Nanoparticles powder was slowly added into warm water while stirring. The suspension was probe-sonicated and spun at 900 rpm for 10 min. The supernatant was probe-sonicated repeatedly and spun down again at 900 rpm for 10 min. The resultant supernatant suspension was stabilized by adding polyvinylpyrrolidone (1%, w/v) and used as nanoparticles (AH-NPs). The sediment was re-suspended and used as microparticles (AH-MPs) in subsequent studies.
The size and size distribution of the AH-MPs in suspension were determined using a Sympatec HELOS laser diffraction instrument equipped with a R3 lens (Sympatec GmbH, Germany). The particle size and size distribution of the AH-NPs were determined using a Malvern Zeta Sizer Nano ZS (Westborough, MA). The AH-NPs and AH-MPs were also examined using an FEI Tecnai Transmission Electron Microscope (TEM) in the Institute for Cellular and Molecular Biology (ICMB) Microscopy and Imaging Facility at The University of Texas at Austin. Carbon-coated 400-mesh grids were activated for 1–2 min. One drop of the particle suspension was deposited on the grids and incubated for 2 min at room temperature. The grids were washed with water and dried for 1 min. Extra water was removed using filter paper and allowed to dry for 15 min before observation [3]. The aluminum contents in the AH-NPs and AH-MPs preparations were determined using a Varian 710-ES Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) in the Civil Architectural and Environmental Engineering Department at the University of Texas at Austin.
Synthesis and characterization aluminum hydroxyphosphate particles
To prepare aluminum hydroxyphosphate particles, an aqueous solution of AlCl3 (180 mM) was slowly added under magnetic stirring to an aqueous solution of dibasic hydrogen phosphate (108 mM). The resultant suspension was probe-sonicated and centrifuged at 900 rpm, and the supernatant was collected. The process was repeated twice with the collected supernatant. Particles in the supernatant are considered as nanoparticles (i.e., AP-NPs), while the sediment was re-suspended and considered as microparticles (i.e., AP-MPs). The particle size and size distribution and the aluminum contents in samples were determined as mentioned above.
Cell Culture
Human THP-1 cells from the American Type Culture Collection (ATCC, Manassas, VA) were grown at 37°C with 5% CO2 in RPMI-1640 medium supplemented with 10% FBS (v/v), 100 U/ml-100 µg/ml penicillin-streptomycin, and 50 µM β-mercaptoethanol. The NLRP3-deficient THP-1 cells (THP1-defNLRP3, InvivoGen) were grown at 37°C with 5% CO2 following InvivoGen’s instruction in RPMI-1640 medium supplemented with 10% FBS (v/v), 200 µg/ml HygroGold, and 100 µg/ml normocin. HEK-Blue IL-1β cells that contain an IL-1β-sensitive reporter were from InvivoGen and grown following InvivoGen’s instruction at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% FBS (v/v), 4.5 g/l glucose, 2 mM GlutaMAX medium, 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml zeocin, 200 µg/ml hygromycin, and 100 µg/ml normocin (all from Life Technologies or InvivoGen).The level of secreted embryonic alkaline phosphatase (SEAP) protein, a truncated form of human placental alkaline phosphatase released into the culture medium, was used as a measurement of NF-κB activation through IL-1β stimulation with the QUANTI-Blue™ detection reagent.
IL-1β production after THP-1 cells are stimulated with aluminum salt particles
THP-1 cells, wild type or defNLRP3, in 100 µl culture medium were plated at the density of 3 × 104 per well in 96-well plates in the presence of 1 µg/ml PMA for 16 h to differentiate the monocytes into macrophages. The medium was replaced with fresh medium, and cells were treated with AH-NPs, AH-MPs, AP-NPs, or AP-MPs (50 µg of aluminum/ml) in the presence of LPS (10 ng/ml) for 6 h. Inflammasome activation requires two signals for the production of mature IL-1β, and thus the differentiated THP-1 cells were cultured in the presence of LPS [13, 14]. Our own data also showed that aluminum particles alone (e.g., Alhydrogel®, AH-NPs, AP-NPs) are weak in inducing THP-1 cells to produce IL-1β, but treating the cells with LPS significantly increases the aluminum particles’ ability to induce the production of IL-1β (data not shown). The supernatant of the activated cells was collected to determine IL-1β content using an ELISA kit (R&D Systems) or the InvivoGen’s Quanti-Blue® SEAP reporter assay following the manufacturers’ instructions.
To examine the effect of specific inhibitors on IL-β release, THP-1 cells were pre-treated with NAC (a radical scavenger, antioxidant, and glutathione precursor, 25 mM) or CA-074-Me (a protease cathepsin B inhibitor that also inhibits lysosomal rupture [15], 20-0.31 µM) for 30 min before the addition of aluminum salt particles (i.e., AH-NPs or AH-MPs), and the IL-1β content in the cell culture medium was determined using the Quanti-Blue® SEAP reporter assay. All experiments were repeated at least twice with reproducible results.
Quanti-Blue® SEAP reporter assay
The HEK-Blue™ IL-1β cells are used to measure IL-1β in inflammasome activation studies following the manufacturer’s instruction. HEK-Blue™ IL-1β cells stably express an optimized, secreted alkaline phosphatase (SEAP) reporter gene under the control of a promoter inducible by NF-κB transcription factor. These cells detect the presence of IL-1β in samples, which triggers the production of SEAP. The level of SEAP protein released into the culture medium was used as a measurement of NF-κB activation through IL-1β stimulation with QUANTI-Blue™ detection reagent. HEK-Blue cells (2 × 104/well) were plated in 96-well plates and grown to 70% confluence. Aliquots of THP-1 cell culture medium (20 µl) were added into 96-well plates containing HEK-Blue ™ IL-1β cells in 180 µl of medium. The cells were then incubated overnight at 37°C, and 50 µL of the cell culture medium was added to 150 µl of pre-warmed Quanti-Blue™, a SEAP colorimetric detection medium, as per manufacturer’s instruction. Color was allowed to develop for 1 h, and absorbance was read at 620 nm using a Bio-Tek® microplate reader (Burlington, VT).
MTT assay
MTT assay was used to determine cell viability after THP-1 cells were cultured with various aluminum particles in the presence or absence of different inhibitors as mentioned above. Briefly, 25 µl of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reagent (5 mg/ml) was added to the wells and incubated at 37°C in the dark for 3–4 h. One hundred microliters (100 µL) of dimethyl sulfoxide was added into each well and incubated for an additional 15 min to solubilize the MTT-formazan product. Absorbance was then measured at 540 nm. A cell viability of greater than 90% was considered non-toxic.
Determination of intracellular glutathione (GSH) content
A GSH-Glo assay kit (Promega, Madison, WI) was used to determine the intracellular GSH levels after cells were incubated with aluminum particles. Briefly, THP-1 cells were incubated in the presence of AH-NPs or AH-MPs (aluminum content, 50 µg/ml) in 96-well plates at 37°C and 5% CO2 for 6 h. The cell culture medium was then replaced with 100 µl GSH-Glo reaction buffer containing Luciferin-NT substrate and glutathione S-transferase. After 30 min of incubation at room temperature with constant shaking, 100 µl of Luciferin D detection reagent was added. The plate was incubated again at room temperature with constant shaking for 15 min. The luminescent intensity was determined using a Bio-Tek® microplate reader.
Uptake of aluminum salt particles by THP-1 cells
THP-1 cells, wild type or defNLRP3, in 100 µl culture medium were plated at the density of 3 × 104 per well in 96-well plates in the presence of 1 µg/ml of PMA for 16 h to differentiate the monocytes into macrophages. The medium was replaced with fresh medium, and the primed cells were treated with aluminum salt particles or Alhydrogel® (Alum, aluminum concentration, 50 µg/ml) in the presence of LPS (10 ng/ml) for 6 h. Cells were washed 3 times with PBS and incubated with 6 N HNO3 overnight at 60°C. The cells were then spun down, and the supernatant was passed through a 0.22 µm nylon filter to remove any particulate matter that may interfere with the assay. The protein concentrations in aliquots of each lysate (25 µl per sample) were determined in a 96-well plate using the Bradford test kit from Bio-Rad. The aluminum contents in cells were determined using ICP-OES. The reported aluminum levels were normalized against the protein contents in the respective samples.
Binding between lysozyme and aluminum hydroxyphosphate particles
SDS-PAGE assay was used to determine the extent to which lysozyme as a model protein antigen binds to aluminum hydroxyphosphate particles [3]. Briefly, lysozyme (60 µg) in 0.06 M NaCl was mixed with various amounts of AP-NPs or APMPs in suspension at ratios ranging from 0 to 1 (aluminum to lysozyme, w/w) and incubated at 4oC overnight while stirring. The lysozyme-particle mixtures were then mixed with Laemmli sample buffer (62.5 mM Tris-HCl, pH 6.8, 25% glycerol, 2% SDS, and 0.01% Bromophenol Blue). Electrophoresis was performed with AnyKD® Mini-PROTEAN® TGX™ precast polyacrylamide gels (Bio-Rad). Precision plus protein standards were run along with the samples at 130 V for 1 h. The gels were stained in a Bio-Safe Coomassie blue staining solution and scanned. The intensity of the protein bands in the gel was quantified using the NIH ImageJ software. The percent binding was calculated by subtracting the percent of unbound proteins (i.e., band intensity of the lysozyme in the lysozyme-aluminum hydroxyphosphate particle mixtures) from the total proteins (i.e., band intensity of free lysozyme that was not mixed with aluminum hydroxyphosphate particles).
Mouse immunization study
Animal study was conducted following the U.S. National Research Council guidelines for care and use of laboratory animals. The animal protocol was approved by the Institutional Animal Care and Use Committee at The University of Texas at Austin. Female BALB/c mice (18–20 g, Charles River Laboratories, Wilmington, MA) were subcutaneously injected with the lysozyme-adsorbed aluminum hydroxyphosphate particles twice, 2 weeks apart at a dose of 5 µg of lysozyme per mouse per injection. Lysozyme alone dissolved in 0.06 M NaCl was used as a control. Seven days after the second immunization, mice were bled. Specific antibody levels in serum samples were determined using ELISA [3]. Plates were coated with 2 µg/ml of lysozyme in a carbonate buffer, and lysozyme-specific total IgG in the serum was detected using peroxidase-labeled anti-mouse IgG secondary antibody.
Statistical analysis
Statistical analyses were completed by performing analysis of variance (ANOVA) followed by two-tailed Student’s t-test (GraphPad Prism 5 software, La Jolla, CA). A p value of ≤ 0.05 (two-tail) was considered significant.
Results and Discussion
Aluminum oxyhydroxide nanoparticles are more potent than aluminum oxyhydroxide microparticles in activating NLRP3 inflammasome
Previously we reported that aluminum oxyhydroxide particles of ~110 nm are more potent than the traditional aluminum oxyhydroxide microparticles (~9 µm) in helping antigens adsorbed on them to induce specific antibody responses in a mouse model [3]. The exact role of the NLRP3 inflammasome in vivo remains inconclusive [16– 18], but NLRP3 inflammasome activation is thought to be crucial for the adjuvant activity of aluminum salt adjuvants [18]. Therefore, the ability of the AH-NPs and AH-MPs to activate NLRP3 inflammasome and stimulate IL-1β production were studied using human THP-1 myeloid cells. PMA (1 µg/ml) was used as a monocyte to macrophage differentiator, and LPS (10 ng/ml) as an IL-1β primer. It has been demonstrated that aluminum hydroxide induces IL-1β and IL-18 secretion in a caspase-1-dependent manner in LPS-primed cells in vitro [10, 19–21]. Aluminum hydroxide alone is a known weak activator of immune cells in vitro [22, 23], but it induces the production of mature IL-1β through the activation of NLRP3 inflammasome in the presence of LPS [10, 19, 24]. MSU crystals are known to activate NLRP3 inflammasome in vitro in a similar way as aluminum hydroxide [11, 25] and hence were chosen as a positive control.
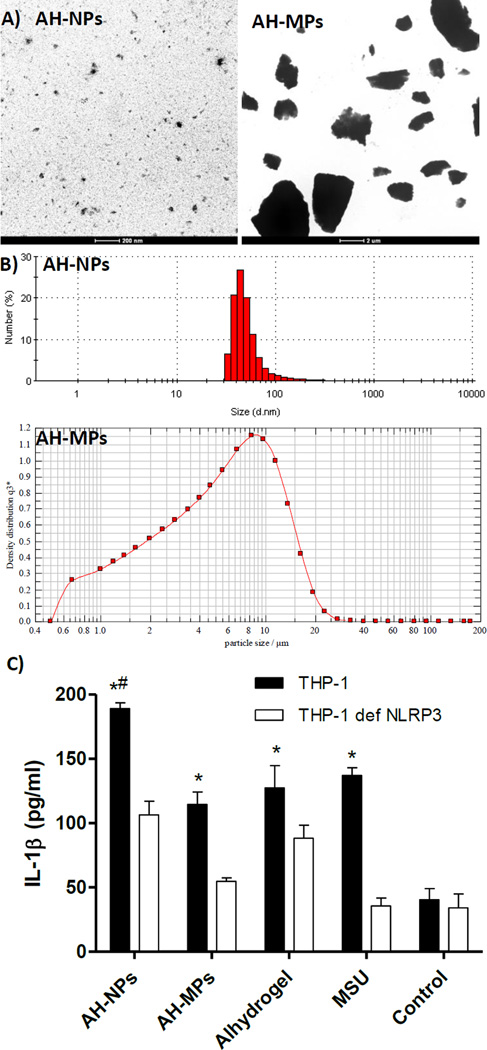
Representative TEM images of the AH-NPs and AH-MPs are shown in Fig. 1A. Shown in Fig. 1B are representative particle size and size distribution of the AH-NPs and AH-MPs. The majority of the AH-NPs are below 100 nm, whereas the media diameter of the AH-MPs (i.e., X50) is 5.36 µm, with X10 and X90 values of 1.16 µm and 12.85 µm, respectively (Fig. 1B). Incubation of THP-1 cells primed with LPS with AH-NPs stimulated a significantly higher level of IL-1β production than with AH-MPs (Fig. 1C); however, in THP1-defNLRP3 cells, the ability for both the AH-NPs and AHMPs in stimulating IL-1β production was relatively lower than in the wild type THP-1 cells (Fig. 1C), demonstrating that both AH-NPs and AH-MPs can activate NLRP3 inflammasome, but the AH-NPs are more potent than the AH-MPs. Therefore, the stronger adjuvant activity of the AH-NPs is likely related to their stronger ability to activate NLRP3 inflammasome. Finally, even in the THP1-defNLRP3 cells, the AH-NPs stimulated significant IL-1β production (Fig. 1C), indicating that the AH-NPs stimulate the production of proinflammatory cytokines through NLRP3-independent pathway(s) as well. Previously, Sun et al. reported that certain aluminum oxyhydroxide nanorods also stimulate the THP1-defNLRP3 cells to produce IL-1β [20].
Fig. 1.
A) Representative TEM images of AH-NPs and AH-MPs. B) Representative particle size and size distribution profiles of AH-NPs and AH-MPs as determined using dynamic light scattering and laser diffraction, respectively. C) IL-1β secretion by THP-1 cells after stimulation with AH-NPs or AH-MPs. Differentiated THP-1 cells, wild type or NLRP3-deficient, were incubated with AH-NPs or AH-MPs for 6 h in the presence of LPS, and the IL-1β levels in cell culture media were measured. Alhydrogel® and monosodium urate (MSU) crystals were positive controls, while cells in the Control group were left untreated. (* p ≤ 0.05, THP-1 vs. THP-1 def NLRP3 received the same treatment; # p ≤ 0.05, AH-NPs vs. AH-MPs in THP-1 cells).
Soluble aluminum ion does not activate NLRP3 inflammasome
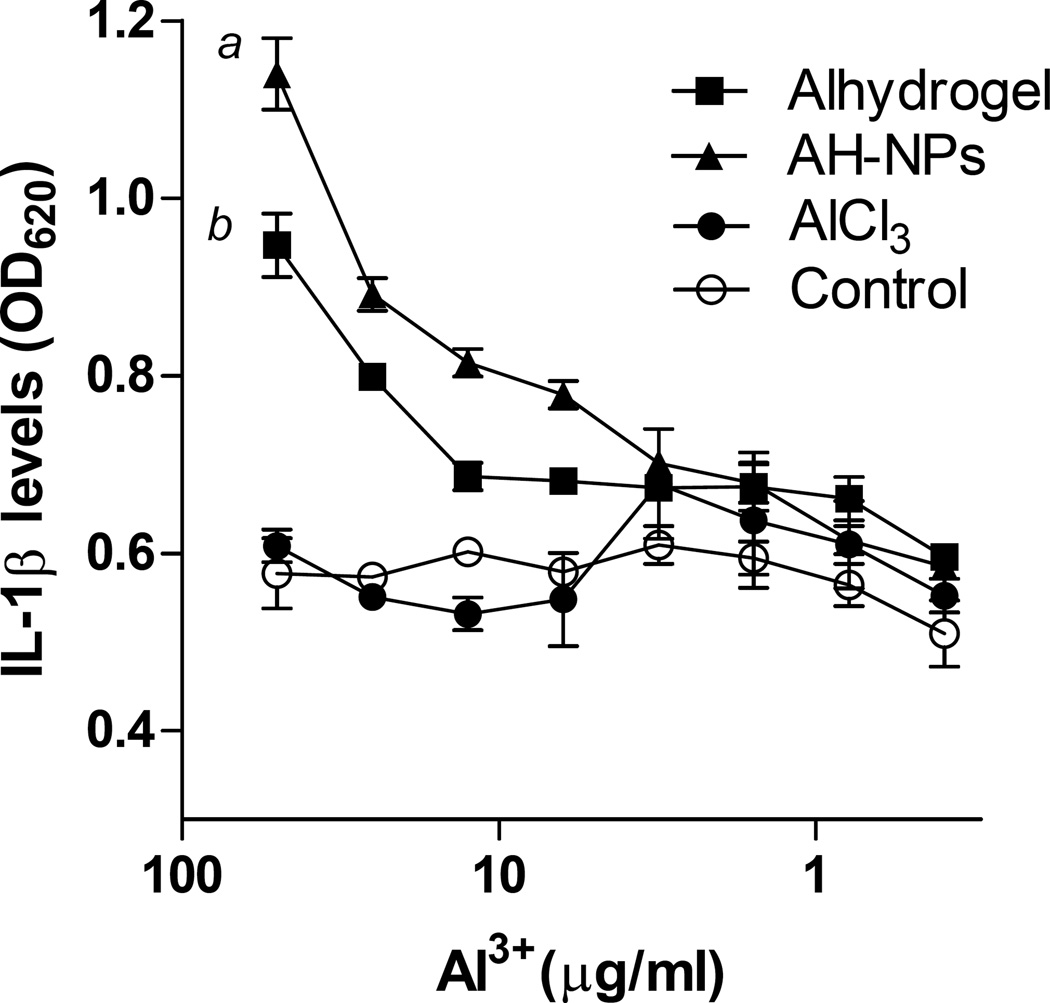
To confirm that it is the particulate aluminum salt (i.e., AH-NPs or AH-MPs), not soluble aluminum ion, that activates NLRP3 inflammasome, differentiated THP-1 cells primed with LPS were cultured in the presence of a AlCl3 solution, and the ability of the AlCl3 (solubility, 45.8 g/100 ml at 20oC) to induce IL-1β production was compared to that of AH-NPs or Alhydrogel®, all containing an equal amount of aluminum. As expected, both AH-NPs and Alhydrogel® stimulated the production of IL-1β in a concentration-dependent manner (Fig. 2). However, AlCl3 at all the concentrations tested (< 50 µg/ml of aluminum) did not stimulate any significant IL-1β production (Fig. 2). Clearly, it is the insoluble aluminum oxyhydroxide particles, not the aluminum ion per se, that activates the NRLP3 inflammasome. As will be explained later, it is thought that insoluble aluminum salt particulates activate NLRP3 inflammasome by inducing lysosomal damage or rupture [15]. Soluble aluminum ions in the AlCl3 solution do not induce lysosomal rupture, and thus are not expected to activate NRLP3 inflammasome.
Fig. 2.
AlCl3 does not stimulate THP-1 cells to secret IL-1β. Differentiated THP-1 cells were incubated with various concentrations of aluminum as AlCl3, AH-NPs, or Alhydrogel® for 6 h in the presence of LPS, and IL-1β levels in cell culture medium were measured using the HEK-Blue™ IL-1β cells-QUANTI-Blue™ assay. a,b p ≤ 0.05, AH-NPs or Alum vs. Control at aluminum concentrations ≥ 25 µg/ml.
More aluminum oxyhydroxide nanoparticles are taken up by THP-1 cells than aluminum oxyhydroxide microparticles
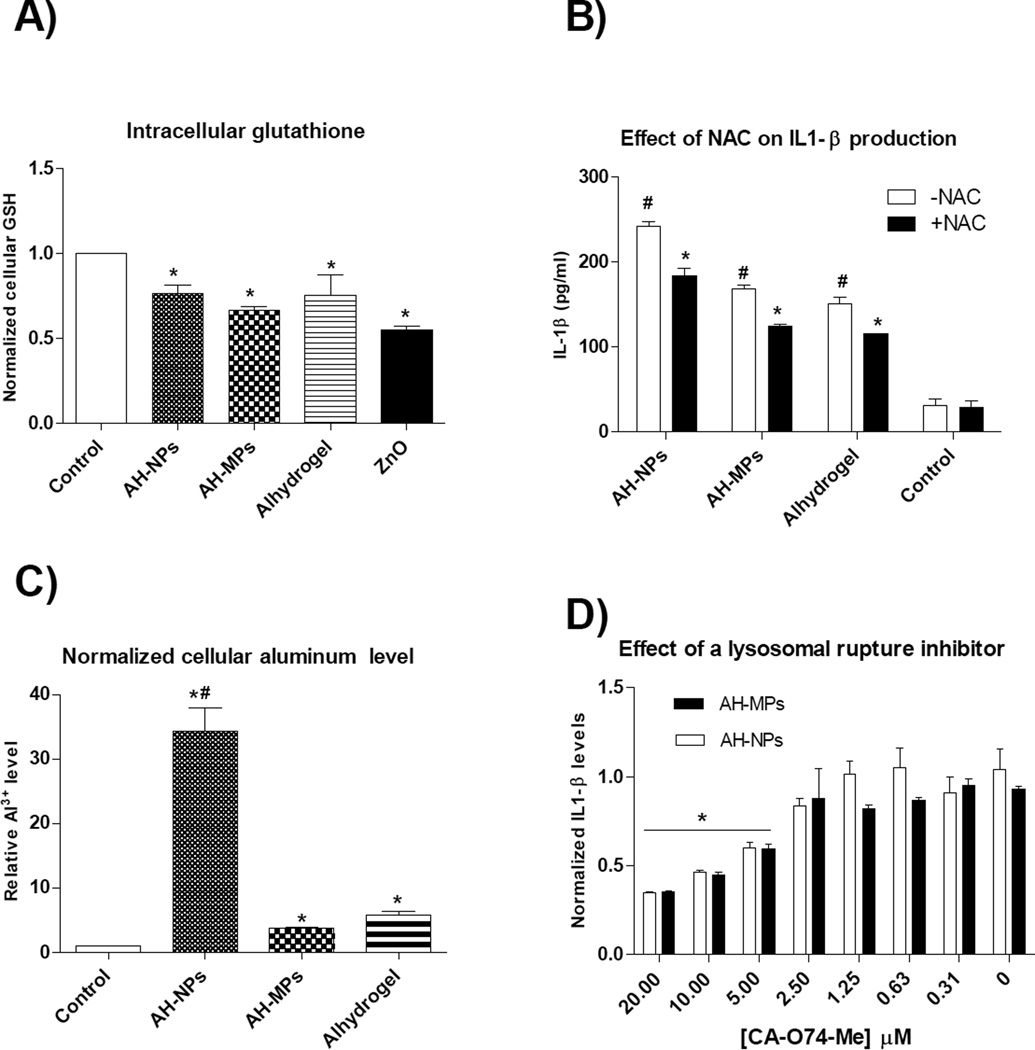
Data from recent studies indicated that NLRP3 inflammasome activation is dependent on the generation of reactive oxygen species (ROS) and the induction of lysosomal rupture [26, 27]. Most NLRP3 agonists induce ROS, and this pathway activates NLRP3 inflammasome [28]. Therefore, the intracellular glutathione level in THP-1 cells was measured after they were treated with AH-NPs or AH-MPs. In addition, NLRP3 activation in THP-1 cells by AH-NPs and AH-MPs, in the presence or absence of NAC, an antioxidant and glutathione precursor [20, 29], was evaluated by measuring IL-1β production. Zinc oxide was used as a control because it is known to induce ROS and reduce intracellular glutathione [30]. It was previously shown that aluminum oxyhydroxide nanorods reduce intracellular glutathione level in vitro [20]. Incubation of THP-1 cells with the AH-NPs or AH-MPs reduced intracellular glutathione levels (Fig. 3A), and NAC reduced the ability of the AH-NPs and AH-MPs to stimulate IL-1β production (Fig. 3B). Therefore, both AH-NPs and AH-MPs induce ROS inside cells, and the ROS induced by them are likely related to their ability to activate NLRP3 inflammasome. However, since the AH-NPs and AH-MPs are not significantly different in their ability to induce ROS (Fig. 3A), the more potent adjuvant activity of the AH-NPs, relative to the AH-MPs, is not likely related to ROS induction.
Fig. 3.
A) Intracellular glutathione level in THP-1 cells after stimulation with AH-NPs or AH-MPs. THP-1 cells were incubated with AH-NPs or AH-MPs (aluminum content, 50 µg/ml) for 24 h, and intracellular GSH level was determined using a GSH-Glo assay. Zinc oxide (ZnO) (50 µg/ml) was used as a positive control (* p < 0.05, vs. Control). B) NAC, an antioxidant, inhibited IL-1β production induced by AH-NPs and AH-MPs. THP-1 cells were pre-treated with 25 mM NAC for 30 min before treatment as in A. IL-1β production was determined using ELISA (* p < 0.05 vs. to Control; # p < 0.05, with NAC vs. without NAC treatment). C) Intracellular aluminum level after THP-1 cells were incubated with AH-NPs or AH-MPs for 6 h (* p < 0.05 vs. Control; # p < 0.05 AH-NPs vs. AH-MPs or Alhydrogel®). D) The effect of CA-074-Me, a cathepsin B inhibitor that inhibits lysosomal rupture, on IL-1β secretion by THP-1 cells. THP-1 cells were pre-treated with up to 20 µM of CA-074-Me for 30 min before incubating with AH-NPs or AH-MPs for 6 h, and the IL-1β levels in cell culture medium were determined using the HEK-Blue™ IL-1β cells-QUANTI-Blue™ assay (* p < 0.05, with CA-074-Me treatment at concentrations ≥ 5 µM vs. without CA-074-Me treatment (i.e., 0 µM of CA-074-Me)).
Particulate or crystalline materials such as MSU, silica, and asbestos, when engulfed by phagocytes, cause lysosomal rupture, resulting in the release of lysosomal contents that are somehow sensed by the NLRP3 inflammasome [15, 31]. When the intracellular levels of aluminum in THP-1 cells were determined after they were incubated with AH-NPs or AH-MPs for 6 h, the level of aluminum in THP-1 cells incubated with the AH-NPs was 9.3-fold higher than in THP-1 cells incubated with AHMPs (Fig. 3C). Inhibition of lysosomal rupture using CA-074-Me, a proteinase cathepsin B inhibitor, reduced the ability of both AH-NPs and AH-MPs to stimulate IL-1β production in THP-1 cells (Fig. 3D), but CA-074-Me appeared to have a similar effect on both AH-NPs’ and MPs’ ability to stimulate IL-1β production (Fig. 3D), indicating that the AH-NPs and AH-MPs are equally effective in inducing lysosomal rupture. Therefore, the more potent NLRP3 inflammasome-activating activity of the AH-NPs, relative to the AHMPs, may be attributed, at least in part, to the increased uptake of the AH-NPs by the THP-1 cells, as compared to the AH-MPs.
The increased uptake of the AH-NPs, relative to the AH-MPs, by the differentiated THP-1 cells is in agreement with previous reports. For example, in a previous study, by measuring the uptake of fluorescein-labeled ovalbumin (OVA) adsorbed on aluminum hydroxide nanoparticles or microparticles, we showed that the uptake of OVA-adsorbed aluminum hydroxide nanoparticles by macrophages (J774A.1 cells) and dendritic cells (DC2.4 cell line and bone marrow derived) are more extensive than the uptake of OVA-adsorbed aluminum hydroxide microparticles [3]. Kanchan and Panda also reported that antigen-loaded polylactide nanoparticles of 200–600 nm are more efficiently taken up by macrophages than microparticles (2–8 µm) prepared with the same materials [32]. Based on micrographs provided by the ATCC, the diameter of the THP-1 cells used in this study is around 15 µm. It is likely that both the AH-NPs and AH-MPs were taken up by the THP-1 cells by endocytosis. However, due to the large particle size of the AH-MPs (Figs. 1A–B), they were likely internalized via phagocytosis, whereas the smaller AH-NPs (Figs. 1A–B) likely entered the THP-1 cells by other types of endocytosis (e.g., macropinocytosis). Regardless of the different endocytosis pathways the AH-NPs and AH-MPs may have taken to enter the THP-1 cells, once endocytosed, however, it is expected that the particles followed the same endo-lysosomal pathway, induce lysosomal rupture, and activate the NLRP-3 inflammasome to stimulate the secretion of proinflammatory cytokines such as IL-1β. The AH-NPs and AH-MPs used in this study may not be different in their ability to induce lysosomal rupture (Fig. 3D). However, based on the intracellular aluminum levels (Fig. 3C), more AH-NPs were taken up by the THP-1 cells and likely traveled to the lysosomes than AH-MPs, explaining why the AH-NPs are more potent than the AH-MPs in activating the NLRP3 inflammasome. Finally, the alternative model proposing that the cytotoxicity of insoluble aluminum salts causes the release of endogenous damage-associated molecular patterns such as uric acid [5], which activate NLRP3 inflammasome, may not apply in the present study, because we only studied NLRP3 inflammasome activation in THP-1 cells in vitro, and based on MTT assay, AH-NPs and AH-MPs at the concentrations used did not cause any significant cytotoxicity against THP-1 cells (data not shown).
Aluminum hydroxyphosphate nanoparticles are more potent than aluminum hydroxyphosphate microparticles in activating NLRP3 inflammasome
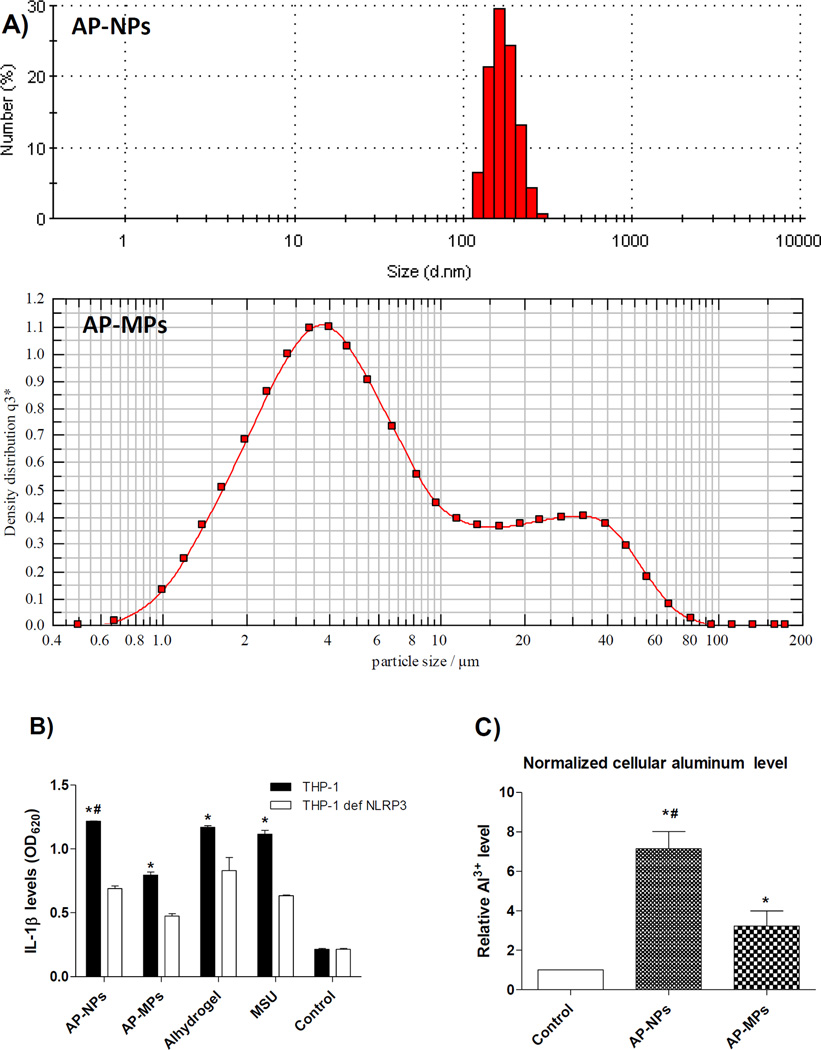
Aluminum hydroxyphosphate has been extensively used as a human vaccine adjuvant as well [33]. In order to understand whether the adjuvant activity of aluminum hydroxyphosphate particles can be increased by reducing their particle size to nanometers, AP-NPs and AP-MPs were prepared. Shown in Fig. 4A are representative particle size and size distribution of the AP-NPs and AP-MPs. The majority of the APNPs are in the range of 122–255 nm, whereas the media diameter of the AH-MPs (i.e., X50) is 4.87 µm, with X10 and X90 values of 1.84 µm and 30.36 µm, respectively (Fig. 4A). The AP-NPs are more potent than the AP-MPs in activating NLRP3 inflammasome, as the THP-1 cells that were stimulated with the AP-NPs produced a higher level of IL-1β than those stimulated with the AP-MPs, and the IL-1β levels were significantly lower in the THP1-defNLRP3 cells (Fig. 4B), again likely due to the increased uptake of the AP-NPs by the THP-1 cells, as compared to the AP-MPs (Fig. 4C). It is noticed however that the exact levels of intracellular aluminum in THP-1 cells incubated with the AP-NPs or AP-MPs were relatively lower than in THP-1 cells incubated with the AH-NPs or AHMPs, which is probably related to the differences in the morphology and consistency of the aluminum oxyhydroxide and aluminum hydroxyphosphate particles (Figs. 1A–B, 4A). The differences in the surface charges and charge densities of the aluminum oxyhydroxide and the aluminum hydroxyphosphate particles could be another contributing factor as well.
Fig. 4.
A) Representative particle size and size distribution profiles of AP-NPs and APMPs as determined using dynamic light scattering and laser diffraction, respectively. B) IL-1β secretion by THP-1 cells after stimulation with AP-NPs or AP-MPs. THP-1, wild type or NLRP3-deficient, were incubated with AP-NPs or AP-MPs for 6 h, and the IL-1β levels in the cell culture media were determined using the HEK-Blue™ IL-1β cells-QUANTI-Blue™ assay. Alhydrogel® and MSU crystals were used as positive controls, while cells in the Control group were not treated (* p < 0.05, THP-1 vs. THP-1 defNLRP3; # p < 0.05, AP-NPs vs. AP-MPs in THP-1 cells). C) Intracellular aluminum levels after THP-1 cells were incubated with AP-NPs or AP-MPs for 6 h (* p < 0.05 vs. Control; # p < 0.05, AP-NPs vs. AP-MPs).
Aluminum hydroxyphosphate nanoparticles show a stronger adjuvant activity than aluminum hydroxyphosphate microparticles in a mouse model
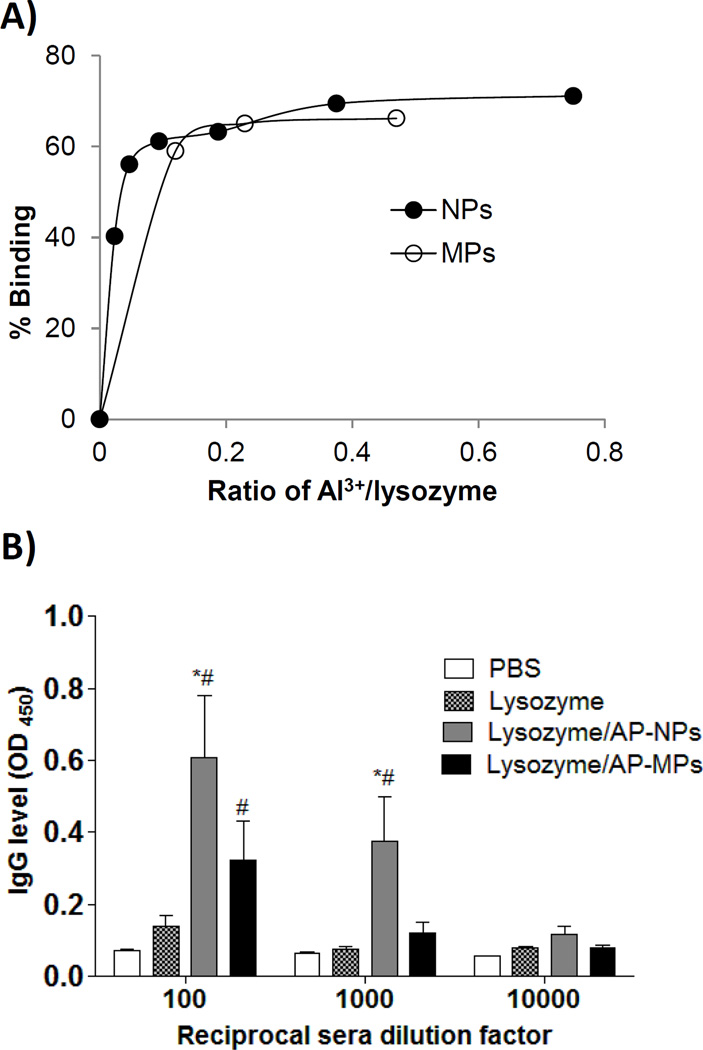
To test whether the AP-NPs have a stronger vaccine adjuvant activity than the AP-MPs in vivo, egg lysozyme, with an isoelectric point of 11.35 [34], was chosen as an antigen to evaluate the specific antibody responses induced in a mouse model. Antigens adsorb on aluminum salt particles via several mechanisms including electrostatic interaction and ligand exchange [2]. The surface charge for aluminum hydroxyphosphate particles can be affected by exposure to phosphate ions [2, 35], and thus 0.06 M NaCl was used when studying the binding of lysozyme by the AP-NPs and AP-MPs. Shown in Fig. 5A are the binding isotherms of lysozyme with the AP-NPs or AP-MPs. The adsorption capacity of the aluminum hydroxyphosphate particles to lysozyme is around 0.65 to 0.7 (Fig. 5A), in agreement with what was reported by Hem et al. [2]. At the aluminum to lysozyme ratio of 1:5 (w/w), the adsorption capacities of the AP-NPs and AP-MPs are approximately equal (Fig. 5A), and thus lysozyme-adsorbed AP-NPs and AP-MPs prepared at an aluminum to lysozyme ratio of 1:5 (w/w) were used to immunize mice (lysozyme, 5 µg/mouse). One week after the second immunization, lysozyme-specific total IgG level in mice immunized with lysozyme-adsorbed AP-NPs was significantly higher than in mice immunized with lysozyme-adsorbed AP-MPs (Fig. 5B). Thus, the vaccine adjuvant activity of aluminum hydroxyphosphate can be enhanced by using aluminum hydroxyphosphate nanoparticles, instead of the conventional microparticles.
Fig 5.
A) The isotherms of the binding between lysozyme and AP-NPs or AP-MPs. Shown are the fractions of lysozyme that bound to the particles at different aluminumto lysozyme ratios (w/w). Data are mean from 3 measurements. Standard deviations are not shown for clarity. B) Serum lysozyme-specific total IgG levels. Mice (n = 5) were dosed on days 7 and 14 with lysozyme-adsorbed AP-NPs or AP-MPs. Lysozyme alone was used as a control. The total anti-lysozyme IgG levels in serum samples were measured 7 days after the second immunization (* p < 0.05, AP-NPs vs. AP-MPs; # p < 0.05, vs. lysozyme alone at the same dilution factor).
Finally, it would be interesting and useful to know whether the aluminum oxyhydroxide nanoparticles or the aluminum hydroxyphosphate nanoparticles have more potent adjuvant activity, but the difference in the zeta potentials of the aluminum oxyhydroxide nanoparticles (~ +50 mV) and the aluminum hydroxyphosphate nanoparticles (~ −20 mV) makes it difficult to design an experiment to test and compare their adjuvant activities in an animal model, because an antigen that strongly adsorbs on one will not likely adsorb on the other, and the degree of adsorption of antigens onto aluminum salt-based adjuvants is thought to significantly affect the immunogenicity of the antigens [36]. Nonetheless, it was found that in stimulating THP-1 cells to produce IL-1β, the aluminum oxyhydroxide nanoparticles were more potent than the aluminum hydroxyphosphate nanoparticles at an equivalent concentration of aluminum (data not shown). However, the IL-1β release data by THP-1 cells in culture do not necessarily indicate that the aluminum oxyhydroxide nanoparticles have a more potent adjuvant activity than the aluminum hydroxyphosphate nanoparticles in vivo.
Conclusions
The vaccine adjuvant activity of aluminum salts such as aluminum oxyhydroxide and aluminum hydroxyphosphate can be significantly enhanced by using small nanometer-scale particles, rather than the traditional microparticles. The potent adjuvant activity of nanometer-scale aluminum salt particles is likely, at least in part, due to their higher uptake by antigen-presenting cells such as macrophages to more efficiently activate NLRP3 inflammasome.
Acknowledgments
This work was supported in part by grants from the U.S. National Institute of Allergy and Infectious Diseases (AI078304 and AI105789 to ZC), the National Natural Science Foundation of China (81460454 to ZC, 81460248 and 81260457 to YS), the National “Twelfth Five-Year” Plan for Science & Technology Support of China (2014BAI13B03 to YS), and the Inner Mongolia Natural Science Funds (2014ZD05 to ZC, 2013MS1138, 2012MS1121, and 2011MS1110 to YS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Romero Mendez IZ, Shi Y, HogenEsch H, Hem SL. Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine. 2007;25:825–833. doi: 10.1016/j.vaccine.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 2.Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert review of vaccines. 2007;6:685–698. doi: 10.1586/14760584.6.5.685. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Aldayel AM, Cui Z. Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. J Control Release. 2014;173:148–157. doi: 10.1016/j.jconrel.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HogenEsch H. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine. 2002;20(Suppl 3):S34–S39. doi: 10.1016/s0264-410x(02)00169-x. [DOI] [PubMed] [Google Scholar]
- 5.Hogenesch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406. doi: 10.3389/fimmu.2012.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He P, Zou Y, Hu Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother. 2015;11:477–488. doi: 10.1080/21645515.2014.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nature reviews Immunology. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mannhalter JW, Neychev HO, Zlabinger GJ, Ahmad R, Eibl MM. Modulation of the human immune response by the nontoxic and non-pyrogenic adjuvant aluminium hydroxide: effect on antigen uptake and antigen presentation. Clin Exp Immunol. 1985;61:143–151. [PMC free article] [PubMed] [Google Scholar]
- 9.Ulanova M, Tarkowski A, Hahn-Zoric M, Hanson LA. The Common vaccine adjuvant aluminum hydroxide up-regulates accessory properties of human monocytes via an interleukin-4-dependent mechanism. Infect Immun. 2001;69:1151–1159. doi: 10.1128/IAI.69.2.1151-1159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenbarth SC, Colegio OR, O'Connor W, Suterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory proper ties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 12.Martinon F, Mayor A, Tschopp J. The Inflammasomes : Guardians of the Body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 13.Sharif O, Bolshakov VN, Raines S, Newham P, Perkins ND. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol. 2007;8:1. doi: 10.1186/1471-2172-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol. 2014;23:37–45. doi: 10.1016/j.intimp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKee AS, Munks MW, MacLeod MK, Fleenor CJ, Van Rooijen N, Kappler JW, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spreafico R, Ricciardi-Castagnoli P, Mortellaro A. The controversial relationship between NLRP3, alum, danger signals and the next-generation adjuvants. Eur J Immunol. 2010;40:638–642. doi: 10.1002/eji.200940039. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 20.Sun B, Ji Z, Liao YP, Wang M, Wang X, Dong J, et al. Engineering an effective immune adjuvant by designed control of shape and crystallinity of aluminum oxyhydroxide nanoparticles. ACS Nano. 2013;7:10834–10849. doi: 10.1021/nn404211j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 22.Seubert A, Monaci E, Pizza M, O'Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180:5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- 23.Rimaniol AC, Gras G, Verdier F, Capel F, Grigoriev VB, Porcheray F, et al. Aluminum hydroxide adjuvant induces macrophage differentiation towards a specialized antigen-presenting cell type. Vaccine. 2004;22:3127–3135. doi: 10.1016/j.vaccine.2004.01.061. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 26.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 27.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Na lp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nature reviews Immunology. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 29.Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, et al. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2:2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aimanianda V, Haensler J, Lacroix-Desmazes S, Kaveri SV, Bayry J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol Sci. 2009;30:287–295. doi: 10.1016/j.tips.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Kanchan V, Panda AK. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials. 2007;28:5344–5357. doi: 10.1016/j.biomaterials.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Hem SL, Johnston CT, HogenEsch H. Imject Alum is not aluminum hydroxide adjuvant or aluminum phosphate adjuvant. Vaccine. 2007;25:4985–4986. doi: 10.1016/j.vaccine.2007.04.078. [DOI] [PubMed] [Google Scholar]
- 34.Weter LR, Deutsch HF. Immunological studies on egg white proteins. IV. Immunochemical and physical studies of lysozyme. J Biol Chem. 1951;192:237–242. [PubMed] [Google Scholar]
- 35.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82:497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 36.Gupta RK. Aluminum compounds as vaccine adjuvants. Advanced drug delivery reviews. 1998;32:155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]