Abstract
Purpose
We investigated if programmed death-ligand 1 (PD-L1) expression levels were prognostic of survival outcomes after intensity-modulated radiation therapy (IMRT) for non-metastatic nasopharyngeal carcinoma (NPC).
Methods and Materials
104 patients with non-metastatic NPC treated with radical IMRT were investigated for their PD-L1 expression by immunohistochemistry (IHC) which were correlated with survival endpoints including locoregional failure-free survival (LRFFS), progression-free survival (PFS), distant metastasis-free survival (DMFS) and overall survival (OS).
Results
After a median follow-up of 7.6 years, 21 (20.2%), 19 (18.3%) and 31 (29.8%) patients suffered from locoregional failure, distant metastases and overall disease progression, respectively, and 31 (29.8%) patients died. Patients whose tumors had PD-L1 IHC 2+ (moderate to strong membrane staining in ≥ 25% of tumor cells) enjoyed longer LRFFS (5-year 100% vs. 74.4%, Hazard ratio [HR], 0.159, 95% confidence interval [CI], 0.021–0.988; P = 0.042) and marginally longer PFS (5-year 95.0% vs. 65.2%, HR, 0.351, 95% CI, 0.08–0.999, P = 0.067) compared to those whose tumors had PD-L1 IHC 0 (minimal membrane staining with PD-L1 in < 5% tumor cells or no staining with PD-L1) or 1+ (minimal to moderate membrane staining with PD-L1 in between 5–24% tumor cells). PD-L1 IHC 2+ was independently prognostic of both LRFFS (P = 0.014) and PFS (P = 0.045) in multivariable analyses. Only induction chemotherapy followed by concurrent chemoradiation was prognostic of DMFS (P = 0.003) and no prognostic factor for OS was identified.
Conclusion
PD-L1 expression levels correlated with LRRFS and PFS in non-metastatic NPC treated with radical IMRT. It may play a role in radiosensitivity for NPC, which should be further confirmed in prospective studies using immunotherapy together with IMRT.
Introduction
Nasopharyngeal carcinoma (NPC) is an endemic malignancy in southern China, Hong Kong, Taiwan and Singapore where there is a strong genetic predisposition [1,2]. The undifferentiated type (WHO Type 3) is highly associated with Epstein-Barr virus (EBV) which carries a better prognosis as compared to the keratinizinig squamous carcinoma (WHO Type 1). Radiation therapy is the mainstay of treatment for non-metastatic NPC especially for early-stage diseases while concurrent cisplatin-based chemoradiation is indicated for stage III to IVB diseases [3]. Intensity-modulated radiation therapy (IMRT) has been considered the standard radiation technique for NPC in virtue of its superior tumor coverage and organ sparing from necessary radiation leading to better locoregional control and reduced toxicities [4–10]. Unlike other head and neck squamous cell carcinoma in which anti-epidermal growth factor receptor (anti-EGFR) antibody cetuximab can be effectively combined with radiation therapy as curative treatment with similar efficacy as chemotherapy for non-metastatic diseases, there has been no conclusive evidence of targeted therapy combined with radiation therapy which showed comparable efficacy as chemotherapy in the same setting [11–14]. Recently, immune checkpoints and immunotherapy have been extensively studied in an attempt to redirect host anti-tumor responses to cancer cells [15]. It is well-recognized that cancer cells can evade host immunosurveillance by inhibiting functions of cytotoxic T-lymphocytes through the expression of certain ligands. Blockage of these ligands or their receptors by specific targeted drugs may result in reactivation of host immune responses thus enhancing anti-tumor effects. Programmed death-1 (PD-1) is an immune checkpoint on the surface of T-lymphocytes [16]. The corresponding ligand called programmed death-ligand 1 (PD-L1) is moderately to strongly expressed in various types of cancer including melanoma, non-small-cell lung cancer and head and neck cancers. Unlike EGFR in which high expression is associated with poor response to radiation therapy in head and neck cancers [17,18], PD-L1 expression has not been found to correlate with treatment outcomes after radiation therapy for head and neck cancers including NPC. Based on the above, we investigated the correlation of expression levels of PD-L1 in tumor cells and tumor infiltrating lymphocytes (TIL) with survival outcomes in patients with non-metastatic NPC treated with IMRT.
Methods and Materials
Patient eligibility and pretreatment investigations
Prior approval from local institutional review board (Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster, approval number UW 15–222) was obtained before study commencement. All clinical investigations were conducted according to the principles of Declaration of Helsinki. All patients provided written informed consent before study recruitment. Patients with previously untreated non-metastatic NPC who were treated with IMRT between 2005 and 2009 were reviewed from a prospectively collected database. Formalin-fixed paraffin-embedded (FFPE) archived nasopharyngeal tumor samples were retrieved for PD-L1 expression analysis as described below. Pretreatment investigations and workup included blood tests for serum hematology, biochemistry, hepatitis B serology, serology for antibodies against EBV viral capsid antigen (VCA) and early antigen (EA), fabrication of customized thermoplastic head and neck cast for the following contrast-enhanced computed tomography (CT) scanning of the head and neck region in IMRT treatment position with 3mm slice thickness co-registered with the T1, T2 and gadolinium-enhanced magnetic resonance images (MRI) of the head and neck region acquired by a 3-tesla scanner for subsequent target and organs-at-risk (OAR) delineation and subsequent IMRT treatment planning and optimization and contrast-enhanced CT scan of the thorax and abdomen to rule out distant metastases. Patients who suffered from stage I to small stage II diseases received IMRT alone while those with bulky stage II disease received concurrent chemoradiation with cisplatin 100mg/m2 on day 1, 22 and 43 of IMRT. Patients who suffered from stage III to IVB diseases received concurrent chemoradiation as mentioned followed by adjuvant chemotherapy with cisplatin 80mg/m2 on day 1 and 5-FU 1000mg/m2 from day 1 to 4 every 4 weeks for 3 more cycles starting 4 weeks after completion of IMRT, according to Intergroup 0099 trial [3, 19]. Some patients received 3 cycles of induction chemotherapy (cisplatin 100mg/m2 on day 1 and 5-FU 1000mg/m2 from day 1 to 5 every 3 weeks) before concurrent chemoradiation due to close proximity of their tumors to the normal critical OARs including brainstem, spinal cord, optic chiasm/nerves, etc, in an attempt for good tumor shrinkage so that a radical radiation dose could be delivered to the tumors with relative sparing of these OARs.
The gross tumor volumes of both the primary tumor (GTV-P) and the radiologically involved cervical nodes (GTV-N) were outlined on the planning CT images with the aid of co-registered MRI images as we described before [20]. Subsequently clinical target volume (CTV-70) and planning target volume containing CTV-70 with a 5mm margin (PTV-70) were generated to take into account the microscopic disease spread, physiological body motions and set-up errors respectively. Another CTV-66 encompassing the high risk areas including the posterior half of the maxillary sinuses, nasal cavities, parapharyngeal spaces, styloid processes, basiocciput, basisphenoid, clivus, foramina rotunda and ovale, pterygopalatine fossae, pterygomaxillary fissures, infra-orbital fissures, cavernous sinuses and level Ib and level V nodal stations was also contoured. A corresponding PTV-66 with a 3mm margin encompassing CTV-66 was created by boolean operations of the treatment planning system (Eclipse version 8.0, USA) which was also used for IMRT planning. OARs included brainstem, spinal cord, globes, optic nerves, optic chiasm, lenses, temporomandibular joints, temporal lobes, auditory nerves, cochleae, mandible, oral cavity, larynx, parotid glands and the vestibules [20]. During IMRT optimization, the maximum dose of brainstem, optic nerves and chiasm must be ≤ 54 Gy (allowing 0.1cc brainstem < 60Gy) and spinal cord ≤ 45Gy (allowing 0.1cc spinal cord < 48Gy). Efforts were also made to limit mean dose of parotid glands to 26 Gy whenever possible and dose to the lenses and temporal lobes as low as reasonably achieved without compromising dose coverage to the PTVs.
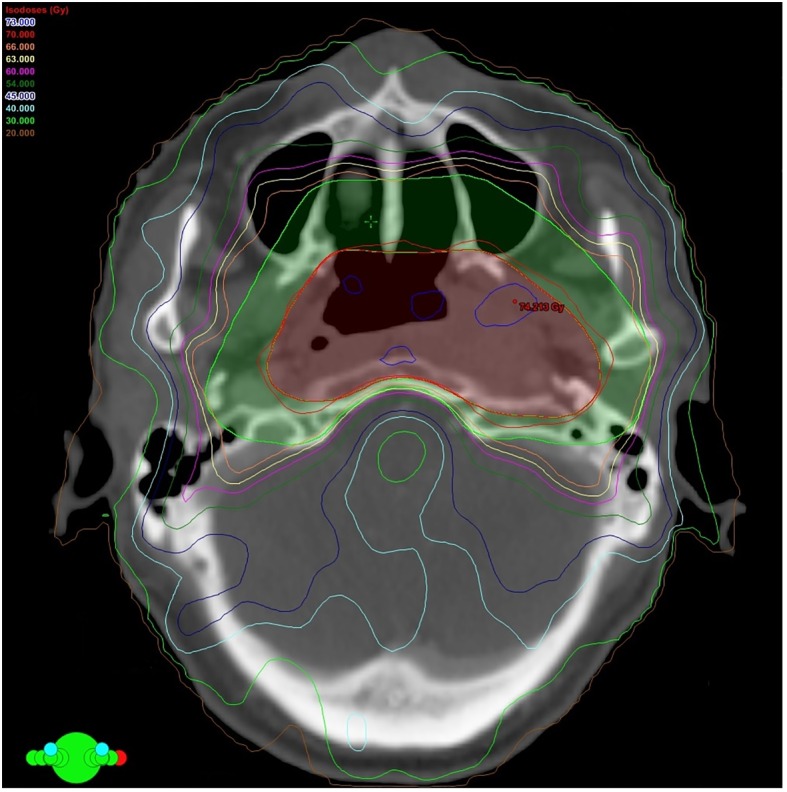
A 9-field IMRT plan delivered by step-and-shoot technique was generated by Eclipse Treatment Planning System version 8.9, Palo Alto, CA, USA) using Anisotropic Analytical Algorithm (Fig 1). A total dose of 70 Gy and 66 Gy was prescribed to PTV-70 and PTV-66 respectively with simultaneous accelerated radiation therapy technique (SMART), as this has been the standard IMRT prescriptions in our institution for the past 10 years [20]. All IMRT plans fulfilled acceptance criteria with at least 95% of PTVs having received the prescribed dose, the maximum dose of PTVs limited to 107% or below and the maximum dose of organs-at-risk within tolerance limits according to International Commission on Radiation Units and Measurements (ICRU) criteria and they were delivered by a 6MV linear accelerator (Varian Medical Systems, Palo Alto, CA, USA). Positional verification with on-board imaging was performed before IMRT commencement. It was repeated again daily immediately before the first 3 fractions of IMRT followed and then weekly afterwards during the whole course of IMRT, to track any anteroposterior and lateral body displacements. All patients received regular clinical follow up and imaging surveillance every 3 to 4 months for the first year after IMRT, then every 6 months for the second year and yearly afterwards to exclude treatment failure and relapse.
Fig 1. Computed tomography image showing the beam orientation and isodose distribution of a 9-field intensity-modulated radiation therapy (IMRT) plan for a patient with non-metastatic nasopharyngeal carcinoma.
PD-L1 expression level by immunohisotchemistry
Immunohistochemical staining of 5μm sections from FFPE archived tissue of nasopharyngeal biopsies or excision specimens of tumor was performed by in the diagnostic laboratory in Anatomical Pathology Unit of our hospital with the antibody 1:200 anti-PD-L1 (E1L3N, Cell Signaling Technology®, Danvers, MA, USA) using the standard protocol for routine diagnostic specimens. Hematoxylin and eosin (H&E) sections were also reviewed for the presence of tumors and TIL. Scoring of the intensity and percentage of tumor cells were done semi-quantitatively. The intensity of immunohistochemical staining with PD-L1 was scored by a 3-point scale. Score 0 meant minimal membrane staining with PD-L1 in less than 5% tumor cells, or no staining with PD-L1 was identified. Score 1 (1+) indicated minimal to moderate membrane staining with PD-L1 in between 5% to 24% tumor cells while score 2 (2+) comprises more positivity defined as at least 25% tumor cells demonstrating moderate to strong complete membrane staining with PD-L1. The same scoring system also applied to PD-L1 expression for TILs but their IHCs were reported separately from the IHCs for tumor cells.
Statistical analysis
Kaplan-Meier methods were employed for survival studies. Log-rank tests were used comparison of survival outcomes among patients with different stages of NPC and different expression levels of PD-L1 as defined above. Cox proportional hazard models with univariable and multivariable analyses were performed to identify prognostic factors of survival endpoints including locoregional failure-free survival (LRFFS), progression-free survival (PFS), distant metastasis-free survival (DMFS) and overall survival (OS) with age as continuous covariate and sex, use of IMRT alone, use of concurrent chemoradiation alone, use of induction chemotherapy followed by concurrent chemoradiation, use of concurrent chemoradiation followed by adjuvant chemotherapy, T-classification (T1/T2 vs. T3/T4), N-classification (N0/N1 vs. N2/N3), overall stage (I/II vs. III/IVA-B), expression levels of PD-L1 as categorical covariates. LRFFS was calculated from the starting date of IMRT to the date of occurrence of locoregional recurrence or death from any cause. PFS started from date of commencement of IMRT to the date of disease progression or death from any cause. DMFS was calculated from the starting date of IMRT to the date of occurrence of distant metastasis or death from any cause. OS was defined from the date of commencement of IMRT to the date of death from any cause. Receiver-operating characteristic (ROC) curves were constructed for the performance of the cut-off value of IHC scores of PD-L1 expression on the survival endpoints. Statistical significance was defined as P < 0.05 (two-sided). All statistical analyses were performed by Statistical Package for Social Sciences (SPSS) version 20.0.
Results
Out of 146 consecutive patients with non-metastatic NPC treated with radical IMRT, only 104 patients were eligible with adequate archived tumor samples for IHC staining and subsequent analysis. The baseline characteristics of these 104 patients were displayed in Table 1. Fourteen patients (13.5%) received IMRT alone because of early-stage (stage I and II) diseases, advanced age or significant medical comorbidities. Seventeen patients (16.3%) received concurrent chemoradiation alone for their bulky stage II disease. Twenty patients (19.2%) received induction chemotherapy followed by concurrent chemoradiation at the discretion of treating radiation oncologists because of the close proximity of their primary tumors to the critical OARs. The remaining 53 patients (51.0%) received concurrent chemoradiation followed by adjuvant chemotherapy. All patients completed IMRT without suspension.
Table 1. Baseline patient characteristics of the 104 patients.
| Characteristic | N (%) |
|---|---|
| Median age in years (range) | 53 (27–80) |
| Male/female | 85 (81.7)/19 (18.3) |
| T-classification | |
| T1 | 24 (23.1) |
| T2 | 30 (28.8) |
| T3 | 39 (37.5) |
| T4 | 11 (10.6) |
| N-classification | |
| N0 | 26 (25.0) |
| N1 | 25 (24.0) |
| N2 | 41 (39.4) |
| N3a | 6 (5.8) |
| N3b | 6 (5.8) |
| Overall stage | |
| I | 12 (11.5) |
| II | 20 (19.2) |
| III | 50 (48.1) |
| IVA | 10 (9.6) |
| IVB | 12 (11.5) |
| IMRT alone | 14 (13.5) |
| Concurrent chemoradiation | |
| Concurrent chemoradiation alone | 17 (16.3) |
| Induction chemotherapy then concurrent chemoradiation | 20 (19.2) |
| Concurrent chemotherapy then adjuvant chemotherapy | 53 (51.0) |
| PD-L1 expression level by immunohistochemistry | |
| 0 | 78 (75.0) |
| 1+ | 4 (3.8) |
| 2+ | 22 (21.2) |
Abbreviations: IMRT, intensity-modulated radiation therapy; PD-L1, programmed death-ligand 1.
After a median follow-up duration of 7.6 years (5.7–10.8 years), 21 (20.2%), 19 (18.3%) and 31 (29.8%) patients suffered from locoregional failure, distant metastases and overall disease progression respectively and 31 (29.8%) patients died during the study period. The median LRFFS, PFS, DMFS and OS were not reached. The mean LRFFS, PFS, DMFS and OS were 78.5, 71.7, 94.0 and 89.4 months respectively. The 5-year corresponding rates were 79.9%, 71.4%, 85.7% and 81.7% respectively.
Patterns of IHC staining with PD-L1
Seventy-eight patients (75.0%) had no membrane staining with PD-L1 for their tumor cells. Four (3.8%) and 22 (21.2%) patients had IHC 1+ and IHC 2+ for their tumor cells respectively (Fig 2). The baseline clinical demographics between these 2 groups of patients were shown, without any statistically significant differences noted (Table 2). This observation suggested that PD-L1 expression might not be common in NPC tumor cells. However, once the gene was induced, expression level can be prominent and in significant proportion of the tumor. Therefore we grouped IHC 0 or 1+ as one group against IHC 2+ in our subsequent univariable and multivariable analyses as we believed that tumour cells with IHC 1+ (minimal to moderate membrane staining) behaved similarly as those with IHC 0. Interestingly, although there were many TILs associated with the tumor cells, PD-L1 was only expressed in these tumor-associated lymphoid cells in a scattered manner. None of the tumors had more than 10% of intimately associated lymphocytes with discernible PD-L1 staining and thus all TILs scored 0 for their PD-L1 expression (Fig 3).
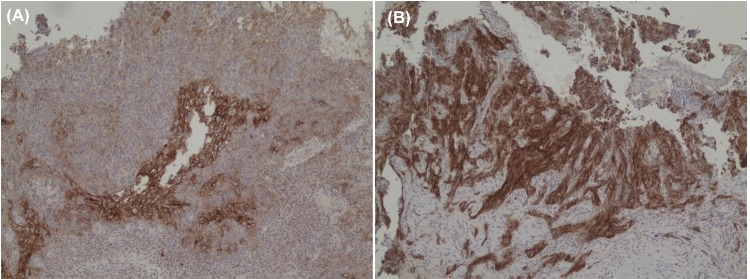
Fig 2. Immunohistochemical staining for PD-L1 in patients with non-metastastic nasopharyngeal carcinoma.
(A) A patient whose tumor was scored 1+ (weak to moderate membrane in about 5% of tumor cells) on immunohistochemical staining with PD-L1. (B) Another patient whose tumor was scored 2+ (moderate to strong membrane staining in more than 25% tumor cells) on immunohistochemical staining with PD-L1.
Table 2. Comparison of baseline clinical parameters of patients stratified by the levels of PD-L1 expression with immunohistochemical staining (0 or 1+ versus 2+).
| IHC 0 or 1+ | IHC 2+ | P | |
|---|---|---|---|
| Median age in years (range) | 52.5 (27–80) | 53.5 (30–75) | 0.855 |
| Male/female | 66/16 | 19/3 | 0.527 |
| T-classification | 0.523 | ||
| T1 | 19 | 5 | |
| T2 | 21 | 9 | |
| T3 | 33 | 6 | |
| T4 | 9 | 2 | |
| N-classification | 0.117 | ||
| N0 | 23 | 3 | |
| N1 | 22 | 3 | |
| N2 | 29 | 12 | |
| N3a | 3 | 3 | |
| N3b | 5 | 1 | |
| Overall stage | 0.865 | ||
| I | 10 | 2 | |
| II | 16 | 4 | |
| III | 40 | 10 | |
| IVA | 8 | 2 | |
| IVB | 8 | 4 | |
| IMRT alone | 12 | 2 | 0.499 |
| Concurrent chemoradiation | 0.793 | ||
| Concurrent chemoradiation alone | 13 | 4 | |
| Induction chemotherapy then concurrent chemoradiation | 16 | 4 | |
| Concurrent chemotherapy then adjuvant chemotherapy | 41 | 12 |
Abbreviations: IHC, immunohistochemical staining; IMRT, intensity-modulated radiation therapy.
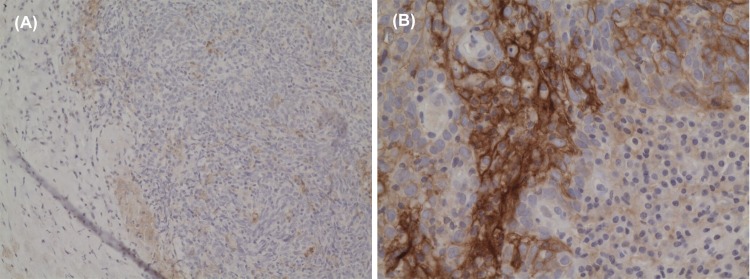
Fig 3. Micrograph showing the absence of immunohistochemical staining for PD-L1 in tumor-infiltrating lymphocytes.
(A) Low-power field. (B) High-power field despite strong immune-positivity to PD-L1 in the adjacent tumor cells.
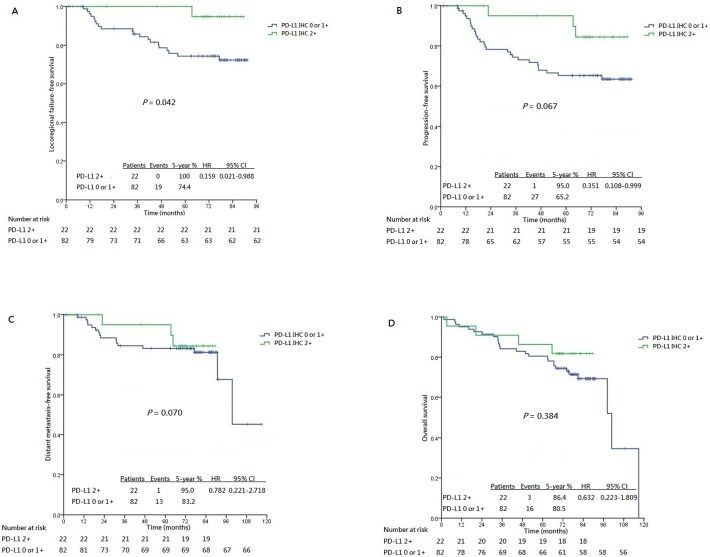
Subgroup analyses revealed that patients whose tumors exhibited PD-L1 IHC 2+ enjoyed a significantly longer LRFFS (5-year 100%, hazard ratio [HR], 0.159, 95% confidence interval [CI], 0.021–0.988; P = 0.042) compared to those whose tumors were PD-L1 IHC 0 or 1+ only (5-year 74.4%) (Fig 4A). Similarly, marginally longer PFS was also seen in those whose tumors exhibited PD-L1 IHC 2+ (5-year 95.0%, HR, 0.351, 95% CI, 0.108–0.999; P = 0.067) compared to those whose tumors was PD-L1 0 or 1+ only (5-year 65.2%) (Fig 4B). No significant differences in DMFS and OS were noticed between patients whose tumors exhibited PD-L1 IHC 2+ and the rest whose tumors exhibited PD-L1 IHC 0 or 1+ only (Fig 4C and 4D), despite a numerical advantage for patients whose tumors demonstrating PD-L1 IHC 2+. ROC analysis showed that the area-under-the-curve (AUC) values for locoregional progression and overall progression was 0.613 (95% CI, 0.479–0.726, P = 0.048) and 0.590 (95% CI, 0.467–0.697, P = 0.059) respectively by using IHC 2+ as the cutoff (Figs 5 and 6).
Fig 4. Kaplan-Meier curves stratified by the patterns of immunohistochemical staining for PD-L1 (2+ vs 0 or 1+).
(A) Loco-regional failure-free survival. (B) Progression-free survival. (C) Distant metastasis failure-free survival. (D) Overall survival.
Fig 5. Receiver operating characteristics curve showing the performance of PD-L1 IHC 2+ as cut-off for locoregional progression of patients with non-metastatic NPC treated with radical intensity-modulated radiation with or without adjunct chemotherapy.
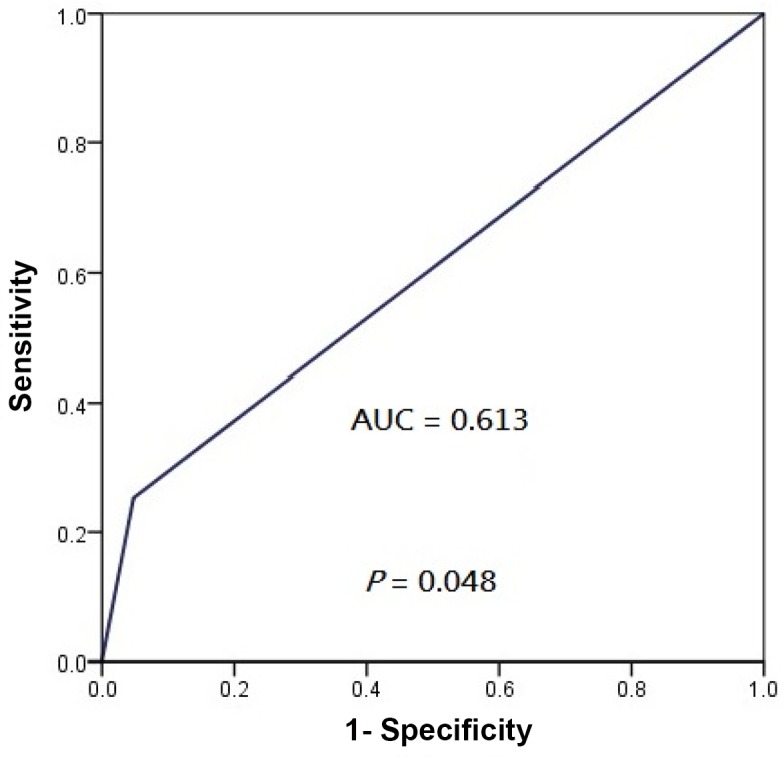
Fig 6. Receiver operating characteristics curve showing the performance of PD-L1 IHC 2+ as cut-off for overall progression of patients with non-metastatic NPC treated with radical intensity-modulated radiation with or without adjunct chemotherapy.
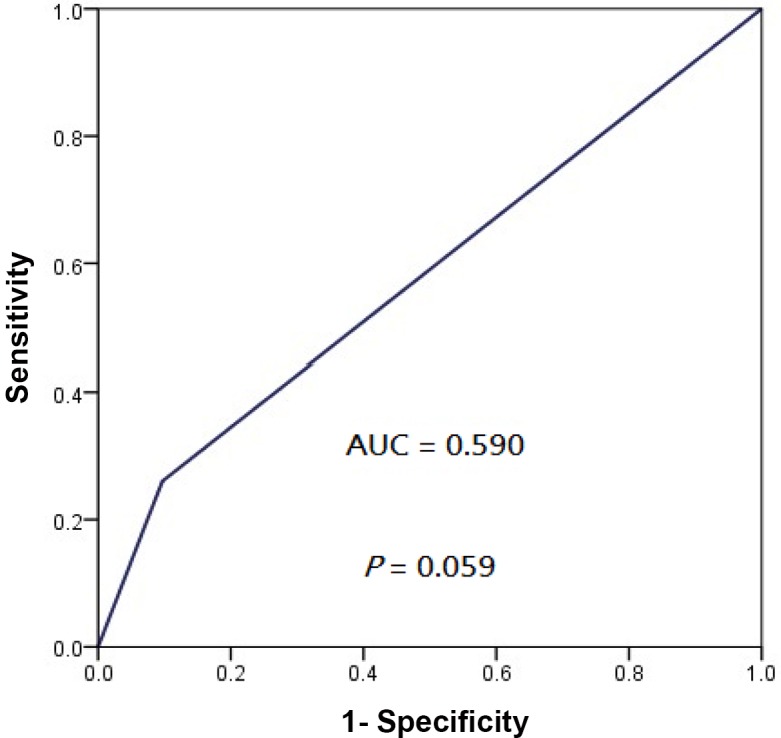
Univariable and multivariable analyses
Univariable and multivariable analyses were performed for LRFFS and PFS (Tables 3 and 4) as well as DMFS and OS (Tables 5 and 6). IMRT alone versus concurrent chemoradiation was not prognostic of any survival endpoint. While T-classification, induction chemotherapy followed by concurrent chemoradiation, PD-L1 IHC 0 or 1+ and PD-L1 IHC 2+ were significant in univariable analysis, only induction chemotherapy followed by concurrent chemoradiation (P = 0.029) and PD-L1 IHC 2+ (P = 0.014) were independently and significantly prognostic of LRFFS in multivariable analysis. For PFS, T-classification, induction chemotherapy followed by concurrent chemoradiation and PD-L1 IHC 0 or 1+ and PD-L1 IHC 2+ were significant in univariable analysis. However only T-classification (P = 0.019) and PD-L1 IHC 2+ (P = 0.045) were significant as independent prognostic factors in the subsequent multivariable analysis. For DMFS, only induction chemotherapy followed by concurrent chemoradiation was prognostic in both univariable (P = 0.003) and multivariable analyses (P = 0.003). Only T-classification was prognostic of OS in both univariable (P = 0.031) and multivariable analyses (P = 0.026). Besides, no statistical analysis was performed for the correlation between PD-L1 expression of TIL and each survival endpoint because no definite PD-L1 staining was observed for all TILs.
Table 3. Univariable and multivariable analysis for locoregional failure-free survival.
| Univariable analysis | Multivariable analysisa | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 0.976 | 0.940–1.014 | 0.216 | ND | ||
| Sex (male as reference) | 1.328 | 0.391–4.512 | 0.650 | ND | ||
| T-classification | ||||||
| T1/T2 vs. T3/T4 | 0.388 | 0.156–0.964 | 0.042 | 0.465 | 0.268–1.254 | 0.162 |
| N-classification | ||||||
| N0N1 vs. N2N3 | 0.737 | 0.310–1.749 | 0.488 | ND | ||
| Overall stage | ||||||
| I/II vs. III/IVA-B | 0.307 | 0.090–1.043 | 0.058 | 0.415 | 0.125–1.297 | 0.161 |
| IMRT alone | 3.546 | 0.476–26.316 | 0.217 | ND | ||
| Concurrent chemoradiation | 0.456 | 0.106–1.961 | 0.291 | ND | ||
| Induction chemotherapy then concurrent chemoradiation | 0.324 | 0.134–0.785 | 0.023 | 0.337 | 0.136–0.802 | 0.029 |
| Concurrent chemoradiation then adjuvant chemotherapy | 1.069 | 0.454–2.518 | 0.879 | ND | ||
| PD-L1 expression level | ||||||
| PD-L1 1+ | 0.268 | 0.062–1.152 | 0.077 | 0.278 | 0.061–1.149 | 0.060 |
| PD-L1 2+ | 0.162 | 0.022–1.000 | 0.042 | 0.159 | 0.021–0.988 | 0.014 |
Abbreviations: CI, confidence interval; HR, hazard ratio; IMRT, intensity-modulated radiation therapy; ND, not done; PD-L1, programmed death-ligand 1.
aOnly covariates found significant (P < 0.1) in the univariable analysis were considered in the multivariable analysis.
Table 4. Univariable and multivariable analyses for progression-free survival.
| Univariable analysis | Multivariable analysisa | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 0.992 | 0.963–1.022 | 0.606 | ND | ||
| Sex (male as reference) | 0.899 | 0.368–2.194 | 0.815 | ND | ||
| T-classification | ||||||
| T1/T2 vs. T3/T4 | 0.357 | 0.168–0.759 | 0.007 | 0.363 | 0.170–0.761 | 0.009 |
| N-classification | ||||||
| N0/N1 vs. N2/N3 | 0.708 | 0.347–1.446 | 0.343 | ND | ||
| Overall stage | ||||||
| I/II vs. III/IVA-B | 0.437 | 0.179–1.066 | 0.069 | 0.456 | 0.176–1.131 | 0.110 |
| IMRT alone | 2.632 | 0.628–10.989 | 0.186 | ND | ||
| Concurrent chemoradiation | 0.643 | 0.225–1.838 | 0.410 | ND | ||
| Induction chemotherapy then concurrent chemoradiation | 0.335 | 0.160–0.702 | 0.014 | 0.401 | 0.205–1.563 | 0.254 |
| Concurrent chemoradiation then adjuvant chemotherapy | 0.820 | 0.404–1.664 | 0.582 | ND | ||
| PD-L1 expression level | ||||||
| PD-L1 1+ | 0.386 | 0.135–1.104 | 0.076 | 0.398 | 0.140–1.108 | 0.056 |
| PD-L1 2+ | 0.346 | 0.105–1.010 | 0.067 | 0.351 | 0.108–0.999 | 0.045 |
Abbreviations: CI, confidence interval; HR, hazard ratio; IMRT, intensity-modulated radiation therapy; ND, not done; PD-L1, programmed death-ligand 1.
aOnly covariates found significant (P < 0.1) in the univariable analysis were considered in the multivariable analysis.
Table 5. Univariable and multivariable analysis for distant metastasis-free survival.
| Univariable analysis | Multivariable analysisa | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.006 | 0.968–1.045 | 0.775 | ND | ||
| Sex (male as reference) | 0.676 | 0.241–1.896 | 0.457 | ND | ||
| T-classification | ||||||
| T1/T2 vs. T3/T4 | 0.392 | 0.148–1.034 | 0.058 | 0.495 | 0.183–1.335 | 0.165 |
| N-classification | ||||||
| N0N1 vs. N2/N3 | 0.486 | 0.188–1.255 | 0.136 | ND | ||
| Overall stage | ||||||
| I/II vs. III/IVA-B | 0.550 | 0.181–1.670 | 0.291 | ND | ||
| IMRT alone | 2.899 | 0.386–21.739 | 0.301 | ND | ||
| Concurrent chemoradiation | 1.060 | 0.304–3.689 | 0.927 | ND | ||
| Induction chemotherapy then concurrent chemoradiation | 0.217 | 0.083–0.568 | 0.003 | 0.219 | 0.080–0.565 | 0.003 |
| Concurrent chemoradiation then adjuvant chemotherapy | 0.472 | 0.177–1.263 | 0.135 | ND | ||
| PD-L1 expression level | ||||||
| PD-L1 1+ | 0.533 | 0.155–1.835 | 0.319 | ND | ||
| PD-L1 2+ | 0.782 | 0.225–2.725 | 0.700 | ND | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; IMRT, intensity-modulated radiation therapy; ND, not done; PD-L1, programmed death-ligand 1.
aOnly covariates found significant (P < 0.1) in the univariable analysis were considered in the multivariable analysis.
Table 6. Univariable and multivariable analyses for overall survival.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.068 | 0.968–1.100 | 0.975 | ND | ||
| Sex (male as reference) | 0.985 | 0.398–2.439 | 0.974 | ND | ||
| T-classification | ||||||
| T1/T2 vs. T3/T4 | 0.432 | 0.201–0.928 | 0.031 | 0.429 | 0.211–0.919 | 0.026 |
| N-classification | ||||||
| N0/N1 vs. N2/N3 | 0.810 | 0.393–1.667 | 0.567 | ND | ||
| Overall stage | ||||||
| I/II vs. III/IVA-B | 0.428 | 0.162–1.125 | 0.085 | 0.452 | 0.189–1.273 | 0.145 |
| IMRT alone | 2.101 | 0.499–8.850 | 0.311 | ND | ||
| Concurrent chemoradiation | 0.534 | 0.161–1.767 | 0.304 | ND | ||
| Induction chemotherapy then concurrent chemoradiation | 0.965 | 0.365–2.551 | 0.943 | ND | ||
| Concurrent chemoradiation then adjuvant chemotherapy | 0.532 | 0.245–1.152 | 0.109 | ND | ||
| PD-L1 expression level | ||||||
| PD-L1 1+ | 0.576 | 0.220–1.508 | 0.261 | ND | ||
| PD-L1 2+ | 0.627 | 0.217–1.812 | 0.388 | ND | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; IMRT, intensity-modulated radiation therapy; ND, not done; PD-L1, programmed death-ligand 1.
aOnly covariates found significant (P < 0.1) in the univariable analysis were considered in the multivariable analysis.
Discussion
In our study, PD-L1 was overexpressed in 25% (26 of 104 patients) of NPC patients and 21.2% of our patients whose tumors were IHC 2+ with PD-L1. Our result revealed that PD-L1 IHC 2+ was associated with better LRRFS and PFS. These advantages of PD-L1 expressed tumors have been observed in stage I resected pulmonary adenocarcinoma which was associated with a better relapse-free survival [21]. In a previous study by Zhang et al, PD-1 alone, an immune checkpoint expressed on the surface of T-lymphocytes upon activation, or in combination with PD-L1, was associated with a worse disease-free survival in NPC patients [22]. However this study also included stage IVC metastatic diseases and their patients were all treated with conventional radiation technique. As the latter radiation modality was considered outdated in the modern era of IMRT or other precision radiation technique, their conclusion on the prognostic significance of PD-L1 expression was not directly comparable to our current observations. Our study, on the contrary, recruited a more representative population of non-metastatic NPC patients who were all treated with standard dosing schedules of IMRT. On the other hand, other clinical parameters for instance IMRT alone versus concurrent chemoradiation and the sequence of chemotherapy (except induction chemotherapy followed by concurrent chemoradiation which was prognostic of LRRFS and DMFS and advanced T-classification which was prognostic of PFS and OS) were not prognostic factors of the survival endpoints.
The role of TILs on survival outcome should be further explored. Hsu et al demonstrated that the presence of PD-1 expressing TILs was prognostic of locoregional relapse-free survival, disease-free survival and OS [23]. However only 46 patients were analyzed in their study, which were fewer than our study population. In addition, the definition of PD-1 positivity in both the tumor cells and TILs was not clearly stated. Besides, the chosen cut-off value of median PD-1 expression rate set at 27.8% was rather arbitrary and haphazard without detailed explanation. Instead, all TILs virtually did not express PD-L1 in our study and their prognostic role needs further investigation in future studies.
The prognostic role of PD-L1 in various types of malignancies remains to be deciphered. Though most studies delineated negative correlation of PD-L1 expression with survival in general [24–28], other studies in specific tumors such as colorectal cancer, non-small cell lung cancer and melanoma demonstrated more favorable correlations between its expression and survival [29–32]. This reflected that the interaction between PD-L1 expression and host immune responses differs among various types of cancer. In fact, PD-L1 expression is not directly proportional to tumor immune evasion and it may just signify an ongoing anti-tumor immune response that leads to production of interferon-γ and other inflammatory markers [32]. The tumor microenvironment may be even more complication in virus-associated tumors like NPC, as neoantigens associated with infection by these tumor-promoting viruses may give rise to inflammatory responses leading to PD-L1 expression [29,33]. In addition, whether it has another role on radiosensitivity or radioresistance for head and neck cancer and NPC warrants further studies for confirmation.
The scoring system of IHC for PD-L1 has yet to be defined and it may vary for different types of malignancies. Traditionally, the median value of the percentage of tumor cells positive for PD-L1 in order to balance the patient groups is most often used. Alternatively, the semiquantitative H-score, obtained by multiplying the percentage of positively-stained tumor cells and the intensity of staining was also commonly used. In addition, definition of staining positivity (for example cell surface versus cytoplasmic expression, by tumor cells only or by other cells in the tumor milieu, percentage of stained cells regarded as positive) has not reached a unanimous consensus. For instance, positivity defined as ≥ 5% tumor cells positively stained with PD-L1 has recently gained popularity in lung cancer studies [21,34]. In fact even for phase III randomized-controlled trials using the FDA-approved pembrolizumab and nivolumab for advanced non-small cell lung cancer, the cutoffs (1% and 50% for pembrolizumab in their KEYNOTE-001) and (1%, 5% and 10% for nivolumab in their CheckMate 017 trial) chosen were arbitrary at the drug sponsor’s discretion without detailed explanation and their subsequently statistical analyses were also exploratory without detailed elaboration [35–37]. The percentage of PD-L1 positivity was also heavily dependent on the performance of the test kits and antibodies, the number of slides reviewed and the amount of tumor cells and TIL retrieved during micro-dissection of the specimens which can be highly operator dependent. As a result, we thought that choosing 5% and 25% (instead of 1% and 5% in the drug-sponsored trials) as cutoffs were less susceptible to the effect of sampling size and sampling errors of the tumor cells retrieved during micro-dissection. The general impression of PD-L1 expression studies in various types of tumors suggested that different tumors would have different scoring systems. This might include not just differences in the percentage and intensity of PD-L1 expression in tumor cells to be considered positive or negative IHC results, but might extend to include PD-L1 expression in the TIL or even the stromal cells. In the context of immune checkpoints and immunotherapy, these observations suggested that consideration of the overall tumor and microenvironment should be important in predicting the success of immunotherapy. Ultimately, it would be most desirable if the definition and scoring system for PD-L1 IHC correlate with treatment response and survival.
Conclusion
We conclude that PD-L1 expression level can be used as a prognostic factor of LRRFS and PFS in non-metastatic NPC patients treated with radical IMRT. This has provided insight in designing immunotherapy targeting the PD-L1 pathway in combination with IMRT in the future, which should be validated in prospective randomized studies.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Jia WH, Huang QH, Liao J, Ye W, Shugart YY, Liu Q, et al. Trends in incidence and mortality of nasopharyngeal carcinoma over a 20–25 year period (1978/1983–2002) in Sihui and Cangwu counties in southern China. BMC Cancer. 2006; 6:178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55(2):74–108. [DOI] [PubMed] [Google Scholar]
- 3.Baujat B, Audry H, Bourhis J, Chan AT, Onat H, Chua DT, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006; 64(1):47–56. [DOI] [PubMed] [Google Scholar]
- 4.Lee N, Xia P, Quivey JM, Poon I, Akazawa C, Akazawa P, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002; 53(1):12–22. [DOI] [PubMed] [Google Scholar]
- 5.Kam MK, Teo PM, Chau RM, Cheung KY, Choi PH, Kwan WH, et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: The Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004; 60(5):1440–50. [DOI] [PubMed] [Google Scholar]
- 6.Wolden SL, Chen WC, Pfister DG, Kraus DH, Berry SL, Zelefsky MJ, et al. Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer: update of the Memorial Sloan-Kettering experience. Int J Radiat Oncol Biol Phys. 2006; 64(1):57–62. [DOI] [PubMed] [Google Scholar]
- 7.Lee N, Harris J, Garden AS, Glisson B, Xia P, Bosch W, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol 2009; 27(22):3684–90. 10.1200/JCO.2008.19.9109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong FC, Ng AW, Lee VH, Lui CM, Yuen KK, Sze WK, et al. Whole-field simultaneous integrated boost intensity modulated radiotherapy for patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2010; 76(1):138–45. 10.1016/j.ijrobp.2009.01.084 [DOI] [PubMed] [Google Scholar]
- 9.Ng WT, Lee MC, Hung WM, Choi CW, Lee KC, Chan OS, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011; 79(2):420–8. 10.1016/j.ijrobp.2009.11.024 [DOI] [PubMed] [Google Scholar]
- 10.Lee NY, Zhang Q, Pfister DG, Kim J, Garden AS, Mechalakos J, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012; 13(2):172–80. 10.1016/S1470-2045(11)70303-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006; 354(6):567–78. [DOI] [PubMed] [Google Scholar]
- 12.Ma BB, Kam MK, Leung SF, Hui EP, King AD, Chan SL, et al. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2012; 23(5):1287–92. 10.1093/annonc/mdr401 [DOI] [PubMed] [Google Scholar]
- 13.Niu X, Hu C, Kong L. Experience with combination of cetuximab plus intensity-modulated radiotherapy with or without chemotherapy for locoregionally advanced nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2013; 139(6):1063–71. 10.1007/s00432-013-1419-z [DOI] [PubMed] [Google Scholar]
- 14.He X, Xu J, Guo W, Jiang X, Wang X, Zong D. Cetuximab in combination with chemoradiation after induction chemotherapy of locoregionally advanced nasopharyngeal carcinoma: preliminary results. Future Oncol. 2013; 9(10):1459–67. 10.2217/fon.13.151 [DOI] [PubMed] [Google Scholar]
- 15.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12(4): 252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PDL1) in the treatment of advanced human cancer. Clin Cancer Res. 2013; 19(19):1021–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys. 2003; 57(1):246–254. [DOI] [PubMed] [Google Scholar]
- 18.Bonner JA, Maihle NJ, Folven BR, Christianson TJ, Spain K. The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivities. Int J Radiat Oncol Biol Phys. 1994; 29(2):243–7. [DOI] [PubMed] [Google Scholar]
- 19.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized intergroup study 0099. J Clin Oncol. 1998; 16(4):1310–7. [DOI] [PubMed] [Google Scholar]
- 20.Lee VHF, Ng SCY, Leung TW, Au GKH, Kwong DLW. Dosimetric predictors of radiation-induced acute nausea and vomiting in IMRT for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2012; 84(1):176–82. 10.1016/j.ijrobp.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 21.Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014; 50(7):1361–9. 10.1016/j.ejca.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Fang W, Qin T, Yang Y, Hong S, Liang W, et al. Co-expression of PD-1 and PD-L1 predicts poor outcome in nasopharyngeal carcinoma. Med Oncol. 2015; 32(3):86 10.1007/s12032-015-0501-6 [DOI] [PubMed] [Google Scholar]
- 23.Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ, Jin YT, et al. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasophyarngeal carcinoma. Mod Pathol. 2010; 23(10):1393–403. 10.1038/modpathol.2010.130 [DOI] [PubMed] [Google Scholar]
- 24.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005; 11(8):2947–53. [DOI] [PubMed] [Google Scholar]
- 25.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006; 8(3):190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006; 108(1):19–24. [DOI] [PubMed] [Google Scholar]
- 27.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007; 104(9):3360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007; 13(7):2151–7. [DOI] [PubMed] [Google Scholar]
- 29.Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, et al. PD-L1 expression in the merkel cell carcinoma microenvironment: association with inflammation, merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013; 1(1):54–63. 10.1158/2326-6066.CIR-13-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013; 49(9):2233–42. 10.1016/j.ejca.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 31.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014; 94(1):107–16. 10.1038/labinvest.2013.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012; 4(127):127ra37 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013; 19(2):462–8. 10.1158/1078-0432.CCR-12-2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boland JM, Kwon ED, Harrington SM, Wampfler JA, Tang H, Yang P, et al. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer 2013; 14(2):157–63. 10.1016/j.cllc.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 35.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010); a randomised controlled trial. Lancet 2016; 387(10027):1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 36.Brahmer J, Reckamp KL, Bass P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373(2):123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, e tal. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373(17):1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.