Overview
Despite the declining incidence of cervical cancer as a result of the introduction of screening programs, globally it remains a leading cause of cancer-related death in women. Outcomes for patients who are diagnosed with anything but early-stage disease remain poor. Here we examine emerging strategies to improve the treatment of locally advanced disease. We discuss emerging biologic data, which are informing our investigation of new therapeutic interventions in persistent, recurrent, and metastatic cervical cancer. We recognize the importance of interventions to improve quality of life and to prevent long-term sequelae in women undergoing treatment. Finally, and perhaps most importantly, we recognize the need for global collaboration and advocacy to improve the outcome for all women at risk of and diagnosed with this disease.
Cervical cancer remains one of the leading causes of cancer-related morbidity and mortality worldwide and is the fourth leading cause of cancer death in women.1 Human papillomavirus (HPV), particularly types 16 and 18, is associated with subsequent development of cervical cancer, with an increased risk also seen among smokers.2 The incidence and mortality rates of cervical cancer are substantially higher in resource-poor regions of the world; the age standardized incidence of cervical cancer being 1.6 times higher in less developed countries. These regional discrepancies are attributable to reductions made in the incidence of cervical cancer in resource-rich countries with the introduction of widely accessed screening programs.3 This decline is expected to continue as a result of the implementation and increased availability of vaccination against HPV.4 These gains, however, remain challenging to replicate in resource-poor regions, which lack the infrastructure and funding to implement screening and vaccination programs, and where access to treatment remains an important problem. Within the United States, over 12,000 women will be diagnosed with cervical cancer in 2015 with approximately 4,000 women expected to die from their disease. Cervical cancer is disproportionately more common in women of African American or Hispanic ethnicity and in patients with limited access to health care.3 Despite the advances in cervical cancer prevention and diagnosis, the outcome for patients diagnosed with later-stage and recurrent disease remains poor.
NONSURGICAL MANAGEMENT OF LOCALLY ADVANCED CERVICAL CANCER
The use of low-dose chemotherapy concurrent with pelvic radiation has been proven to improve survival, and became the established standard of care for locally advanced cervical cancer after the National Cancer Institute issued a clinical alert in 1999 about the benefit of chemoradiation compared with radiation alone as observed in five randomized clinical trials.5 The Medical Research Council (MRC) individual patient data meta-analysis found that the addition of concurrent chemotherapy to radiation increased the 5-year overall survival (OS) rate by 6% (hazard ratio [HR], 0.81; 60% vs. 66%).6 On the basis of the studies in this analysis, weekly cisplatin at a dose of 40 mg/m2 during pelvic radiation has been adopted as the standard of care for the treatment of locally-advanced disease. However, the 5-year disease-free survival rate was only 58% in the chemoradiation group, which although superior to 50% with radiation alone, still leaves substantial room for improvement.
Identifying Patients Most at Risk of Recurrent Disease
The main prognostic factor for outcome in cervical cancer has traditionally been the International Federation of Gyne-cology and Obstetrics (FIGO) staging system, which is based on clinical examination alone. In those with FIGO stage 1B2 or higher, treatment with primary chemoradiation in the recommended approach.7 In the meta-analysis, the additional benefit of chemotherapy was seen regardless of age, tumor histology, or grade. However, there appeared to be a lesser degree of benefit in higher staged tumors, with an absolute 5-year survival benefit of 10% for women with stages Ib to IIa cervical cancer, 7% for those with stage IIb, and 3% for women with stage III to IVa disease.6
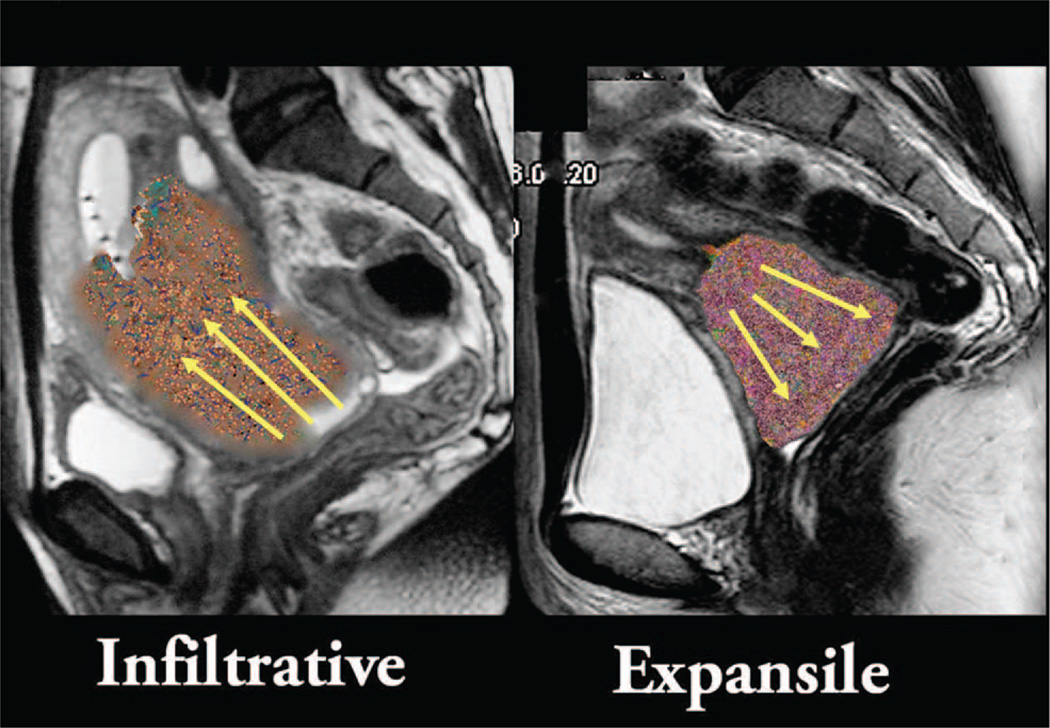
In the meta-analysis, a benefit of adding chemotherapy was also seen regardless of nodal involvement, although it was only possible to look at this in five of the 18 included trials. However, Narayan et al and others have highlighted the important prognostic role of both nodal and uterine corpus involvement as detected by imaging, with MRI and PET now accepted modalities for noninvasively determining these features.8–10 Nodal involvement has been documented to predict disease relapse in multiple studies, but is not part of the current FIGO staging system.8,11–14 Uterine corpus invasion tends to be associated with tumors that grow endophytically rather than exophytically, and has also been shown to predict worse outcomes, in part because it predicts nodal metastasis (Fig. 1).15–20 The relapse rate in those with PET-positive node disease has been reported to be approximately 50% after standard chemoradiation.17,21,22 Some suggest that a follow-up fludeoxyglucose (FDG)-PET scan done 3 to 4 months postchemoradiation can predict patient outcome and may help to determine the intensity of follow-up needed.23
FIGURE 1. Infiltrative versus Expansile Cervical Cancer.
Infiltrative corpus invasive cervix cancer has higher local failure rates, increased frequency of nodal metastases at presentation, and poor survival compared with exophytic tumor of similar volume.
Optimizing Local Therapy
Although the development of distant disease after chemoradiation is the predominant cause of mortality, local relapse may also be a problem that causes substantial morbidity. Although the patterns of treatment failure have not been well described in all studies, in the meta-analysis, locoregional treatment failure was responsible for 35%of the failure events across trials.6
Standard radiation treatment involves 40 to 50.4 Gy of external beam radiation therapy (EBRT) delivered in fractions of 1.8 to 2 Gy to the pelvis. The upper border for treatment is usually L4-S1, unless an extended field is required to cover involved node disease. Parametrial or nodal boost may also be given if these areas are involved. In addition to EBRT, brachytherapy direct to the primary tumor is considered to be an essential component of therapy. It is recommended that the overall treatment time for chemoradiation should not exceed 8 weeks. The role for intensity modulated radiation therapy (IMRT) is controversial and an area of active research because of the recognition that the cervix moves as a result of changes in bladder and bowel filling, and uterine movement.
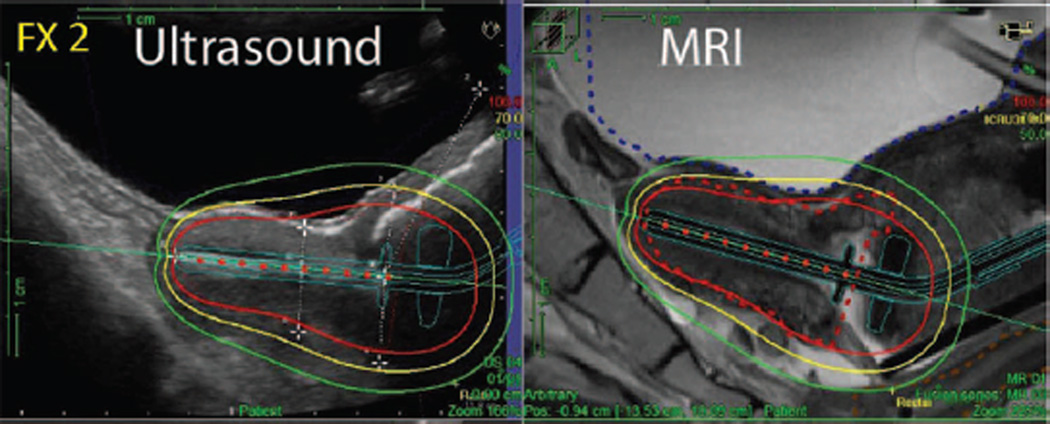
There is also ongoing controversy about the best approach to delivery of brachytherapy. Traditionally, brachytherapy has been planned to give a recommended cumulative dose of 80 to 90 Gy EBRT and brachytherapy to “point A,” which is an anatomic landmark 2 cm lateral to the central canal of the uterus and 2 cm up from the mucous membrane of the lateral fornix in the axis of the uterus.24 There has also been increased interest in the value of using conformal brachytherapy, in which the dose is prescribed to the residual tumor volume at the end of EBRT rather than to point A. Proponents report that this approach may increase the effectiveness of local treatment and also reduce toxicity.25 It is also recommended that image-guidance using either MRI or ultrasound is used to ensure that the tandem and ovoids used for the delivery of brachytherapy are correctly positioned within the uterus (Fig. 2).
FIGURE 2. Ultrasound and MRI Images for Delivery of Brachytherapy.
The left ultrasound image shows the tandem position and superimposed isodoses in the treatment position. The ultrasound images correlates very well with the corresponding MRI image.
A variety of approaches to intensifying the concurrent chemotherapy component of chemoradiation have been tested including the addition of cytotoxic agents. To date, no chemotherapy regimen has been found to be superior to 40 mg/m2 (for most of the GOG studies, the does was capped at a maximum of 70 mg) of cisplatin weekly. However, the meta-analysis does suggest that substituting other agents that have demonstrated efficacy such as carboplatin or 5-flurouracil (5-FU) should be considered for women with a contraindication to cisplatin.6 Addition of biologic agents to chemoradiotherapy is an active area of research. Despite success in head and neck cancers, the combination of the epidermal growth factor receptor (EGFR) inhibitor cetuximab, cisplatin, and radiotherapy proved toxic in patients with cervical cancer.26 RTOG 0417 investigated 10 mg/kg of intravenous bevacizumab every 2 weeks (for three doses only) in combination with chemoradiation in 47 patients.27 Results were promising and the toxicity profile was acceptable with no perforations or fistulas observed; further investigation is warranted.28
Multidisciplinary care is essential during chemoradiation for cervical cancer. In addition to monitoring side effects such as diarrhea and nausea, given the nature of the disease and the patient demographic, financial and psychosocial concerns are common. Early involvement of social work and psychology may assist women to cope with these issues. Younger women may require referral to discuss options for fertility preservation and hormone-replacement therapy. Smoking cessation is highly encouraged as ongoing smoking during chemoradiation may reduce the effectiveness of treatment, as well as increase the risk for the development of a second malignancy.29 Treatment of anemia may also be required and it is generally recommended that the hemoglobin is maintained at 10 g/dL or higher during treatment.30 Eryth-ropoietin during chemoradiation, however, is not recommended because of an increased risk of thromboembolic events.31
Adjuvant Chemotherapy
Although chemoradiation is effective treatment for the primary disease, relapsed disease most commonly develops as distant metastatic disease.32 It is therefore reasonable to predict that the addition of further cycles of adjuvant chemotherapy following completion of chemoradiation may decrease the development of distant metastases and thus improve survival. GOG109 was a U.S. study that randomly assigned patients who had been initially treated with radical hysterectomy and pelvic lymphadenectomy, and subsequently found to have positive pelvic nodes and/or positive margins and/or microscopic parametrial involvement, to receive adjuvant radiation alone or adjuvant chemoradiation. The chemotherapy consisted of four cycles of cisplatin and 5-FU, given as two cycles concurrent with radiation and two cycles post radiation. Progression-free survival (PFS) and OS rates were substantially improved for patients who received the additional chemotherapy. Although only 60% completed all planned chemotherapy, a higher number of chemotherapy courses was positively associated with improved survival rates.33 This trial was one of two trials considered in a subset analysis of the MRC meta-analysis that considered the potential value of giving additional adjuvant chemotherapy. In this subset, there was an impressive absolute improvement of 19% in 5-year survival (from 60% to 79%) compared with radiation alone.33,34
More recently, Duenes-Gonzalez et al demonstrated a benefit of adding concurrent gemcitabine to the standard regimen of weekly cisplatin during radiation, followed by two further cycles of adjuvant cisplatin/gemcitabine. This multicenter, randomized, phase III trial showed a significant 9% improvement in the primary outcome of PFS at 3 years (65% to 74%; p = 0.029).35 Toxicity, however, was a concern with two deaths in the experimental arm and a doubling of grade 3 to 4 adverse events (86.5% vs. 46.3%). In addition, prior investigators had been unable to safely deliver similar doses of drugs combined with radiotherapy in a North American population. Furthermore, it remains unclear how much of the benefit observed in this trial was a result of the additional chemotherapy given following the chemoradiation. Finally, follow-up data were truncated at 1 year, and as a result, an accurate measure of the effect on OS or rate of serious late complications cannot be properly assessed.36
Although not practice changing, these studies do raise important and unresolved questions regarding the potential value of adjuvant chemotherapy for women with locally advanced cervical cancer.
Current Clinical Trials
The Gynecologic Cancer InterGroup (GCIG) has an ongoing series of international, randomized, phase III trials aiming to test the effect of different chemotherapy strategies during chemoradiation on OS rates. The OUTBACK trial, led by the Australia New Zealand Gynaecological Oncology Group (ANZGOG) in collaboration with NRG Oncology, is testing the value of administering additional adjuvant chemotherapy after standard cisplatin-based chemoradiation compared with chemoradiation alone (ACTRN12610000732088). The primary aim is to determine if the addition of four cycles of adjuvant carboplatin and paclitaxel to standard cisplatin-based chemoradiation can improve OS.
The TACO trial, led by the Korean Gynecologic Oncology Group (KGOG) and Thai Cooperative Group, is comparing standard treatment with weekly cisplatin during chemoradiation to 3-weekly cisplatin (NCT01561586). This is based on a prior phase II trial from the KGOG, which suggested that the triweekly cisplatin maybe more effective and feasible to deliver.37 It may also be an attractive regimen to use in low-resource countries because of the reduced number of chemotherapy treatments required.
Finally, INTERLACE, a trial led by the National Cancer Research Institute in the United Kingdom, is testing the value of administering additional neoadjuvant chemotherapy before chemoradiation compared with chemoradiation alone (NCT01566240). Although a previous meta-analysis suggested no improvement in OS with neoadjuvant chemotherapy in locally advanced cervical cancer, there was a suggestion of improved outcomes in those trials with a shorter cycle length of 14 days or less or higher dose intensity of cisplatin.38 The regimen being tested of six doses of weekly carboplatin and paclitaxel before standard chemoradiation has been shown to be feasible to deliver in a prior phase II study39
QUALITY OF LIFE FOR PATIENTS WITH CERVICAL CANCER AFTER TREATMENT
Cervical cancer survivors often experience substantial quality of life (QOL) disruptions associated with the disease and treatment, many of which persist long into survivorship.40–45 A recent analysis of health-related QOL data among U.S. cancer survivors indicates that cancer survivors are more likely to have poor physical and mental health–related QOL (25% and 10%, respectively > 1 standard deviation above the U.S. population mean) compared with adults with no cancer history (10% and 5%, respectively). Furthermore, cervical cancer survivors and short-survival cancer survivors report the worst mental health–related QOL.46
Persistent sequelae include pain, bladder and bowel dysfunction,47–51 sexual dysfunction,52–56 lymphedema, and menopausal symptoms,57 as well as reproductive concerns among women of childbearing age.45,58–62 Adverse psychologic consequences are shared with women diagnosed with other gynecologic tumors, and include depression and anxiety,63 sleep disturbance, and concentration difficulties to a greater magnitude than many other populations of patients with cancer.41,42,64–68 Despite challenges inherent in this cancer survivor population, supportive interventions may assist in substantially improving QOL, with potential to also improve stress-related biomarkers.69 This could, in turn, improve disease outcomes.70–72
A recent study indicated that of patients with cervical cancer diagnosed 9 to 30 months earlier, patients who reported the worst QOL also reported more gynecologic problems and less social support.73 Gynecologic problems were substantially worse in patients treated with radiation with or without chemotherapy compared with those treated with surgery only, with a moderate-to-large effect size which is both statistically significant and clinically relevant (FACT-Cx, p = 0.014; FACT-TOI,p = 0.006). Treatment with radiation with or without chemotherapy also contributed to substantially poorer QOL, higher perceived stress, and greater depression, with modest-to-moderate effect sizes. Further, patients with three or more comorbidities before cancer diagnosis have also been reported to have substantially worse QOL, higher perceived stress, more depression and anxiety, and lower social support. In identifying subpopulations who are likely to benefit from supportive care interventions, it appears that a brief screening of type and number of premorbid medical problems, including mood disorders, could target the patients who have the greatest need for more immediate care and attention, as well as future cancer control studies. Therefore, further study of supportive care interventions to improve distress and decrease gynecologic problems in this vulnerable population appear warranted, particularly for women whose cancer treatment extends beyond surgery.
A recent supportive care study examined the effect of a psychosocial telephone counseling (PTC) intervention on QOL domains and associations with biomarkers. In this randomized clinical trial, after adjusting for age and baseline scores, participants receiving PTC had significantly improved depression and improved gynecologic and cancer-specific concerns at 4 months compared with usual care participants (all p < 0.05); significant differences in gynecologic and cancer-specific concerns (p < 0.05) were sustained at 9 months. Participants with decreasing interleukins-4, −5,−10, and −13 had substantially greater improvement in QOL than patients with increasing cytokine levels. This trial confirms that PTC benefits mood, QOL regarding cancer-specific and gynecologic concerns, for a multiethnic underserved cancer survivor population. The improvement in patient-reported outcomes with decreases in T-helper type 2, and counter-regulatory cytokines support a potential bio-behavioral pathway relevant to cancer survivorship.74 Providing supportive care during treatment, and evaluating the effects of supportive care, may reduce the prevalence and magnitude of long-term sequelae of cervical cancer, which will in turn improve QOL and quality of care.
TREATMENT OF METASTATIC OR RECURRENT CERVICAL CANCER
Patients with distant metastases and/or with recurrent disease not suitable for local control have a very poor prognosis, with 5-year survival rates between 5% and 15%.75 In this setting, any treatment is palliative, aiming to prolong survival but also to maintain or improve QOL. Platinum-based combinations have shown the most promising response rates with the combination of cisplatin (or carboplatin) with paclitaxel considered the standard of care. Responses to platinum-based chemotherapy are short-lived; median OS is around 12 months76 and can be less than 6 months for those women who have poor prognostic features.77 Response to chemotherapy is influenced by site of recurrence in relation to previous treatment, with progressive disease within a previously irradiated field being particularly resistant to cytotoxic agents.78 Receipt of prior platinum-based chemoradiation and a short time to relapse after primary treatment are also important negative prognostic factors. There are no effective second-line chemotherapy options for women whose disease progresses.
Targeting Angiogenesis
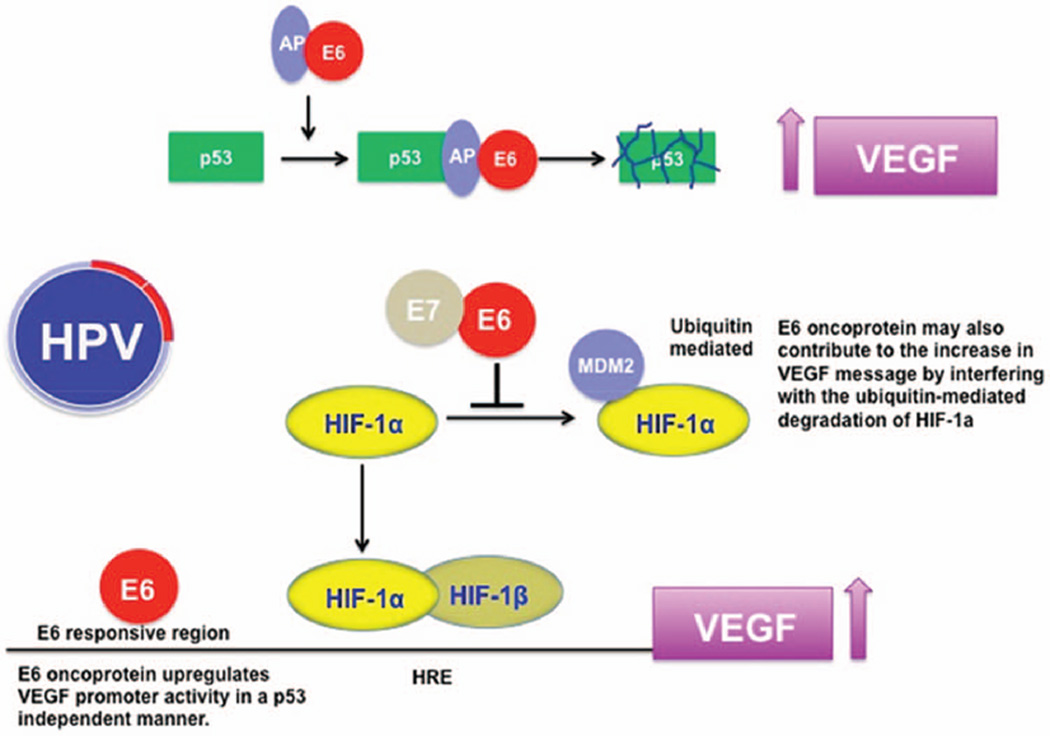
Persistent HPV infection leads to neovascularization and tumor growth promotion, with many studies having demon-strated a prognostic role for vascular endothelial growth factor (VEGF) and other markers of increased angiogenesis in cervical cancer (Fig. 3).79–89 Targeting angiogenesis has therefore emerged as a rational therapeutic strategy in the treatment of cervical cancer. Early phase clinical studies with the anti-VEGF antibody bevacizumab, either alone or in combination with chemotherapy, suggested promising activity. Toxicity was acceptable and responses seen even in previously irradiated sites of disease.89–91 As a result, a four-arm prospective, randomized clinical trial, GOG 240, was conducted. Over 400 patients were randomly assigned to receive treatment with one of two chemotherapy regimens: cisplatin plus paclitaxel versus paclitaxel plus topotecan with or without bevacizumab. Although there was no difference in outcome noted between the two chemotherapy regimens, the addition of bevacizumab led to a significant improvement in median OS, 17 months compared with 13.3 months in the chemotherapy alone arms (HR 0.71; 98% CI, 0.54 to 0.95; p = 0.004). Response rates were also higher for bevacizumab-containing arms (48% vs. 36%; p = 0.008). The benefit from bevacizumab was maintained in women with prior platinum exposure, recurrent/persistent disease, and responses were seen in previously irradiated fields. Toxicity, however, was increased by the addition of bevacizumab with an increased risk of fistula formation in gastrointestinal and genitourinary tracts (10.9% vs. 1%), grade 2 hypertension (25% vs. 2%), neutropenia (35% vs. 26%), and thromboembolism (8% vs. 1%). As a result of this study bevacizumab received a U.S. Food and Drug Administration label for the treatment of cervical cancer in combination with chemotherapy. This has become a new standard of care for women with cervical cancer in resource-rich populations, but expense precludes its use in most parts of the world.70 A better understanding of the risk factors for fistulae development and identification of predictive biomarkers for response would help us to further refine the use of this drug by identifying the subgroups of women who may derive benefit while minimizing toxicity risk.
FIGURE 3. Rationale for Targeting Angiogenesis in Cervical Cancer.
Abbreviations: TSP-1, thrombospondin-1; VEGF, vascular endothelial growth factor; HIF1α, hypoxia inducible factor 1α.
Antiangiogenic agents targeting other parts of the pathway have also been investigated in cervical cancer. Single agent, orally administered, multitargeted receptor tyrosine kinases inhibitors pazopanib (VEGFR 1, 2, and 3; PDGFR-α and β; and c-KIT) and sunitinib (VEGFR 1,2 and 3; PDGFR, c-KIT, and FLT3) were studied in phase II trials. Sunitinib did not display sufficient activity to warrant further investigation and was associated with an unacceptably high (26%) rate of fistula formation.92 In the second, larger study, 230 patients were randomly assigned to one of three arms: pazopanib alone, lapatinib (a tyrosine kinase targeting EGFR and HER2/neu) alone, or a combination of the two agents. Pazopanib improved PFS (HR 0.66; 90% CI, 0.48 to 0.91; p = 0.013) and OS (HR 0.67; 90% CI, 0.46 to 0.99; p = 0.045) compared with lapatinib alone. Median OS was 50.7 weeks compared with 39.1 week for pazopanib and lapatinib, respectively. Pazopanib alone was well tolerated, but the combination of the two drugs lacked efficacy and was associated with more serious adverse events.93
Clearly, targeting angiogenesis in cervical cancer has benefits in terms of efficacy, but patient selection is key and consideration of maintenance of QOL essential when considering future investigation of this therapeutic approach.
Targeting the EGFR
EGFR is expressed at moderate to high levels in cervical carcinoma. However, activating mutations are rare and studies evaluating the association of EGFR protein expression and prognosis in cervical cancer have yielded conflicting results.95–96 Trials investigating the monoclonal antibody cetuximab, either alone or in combination with chemotherapy, failed to demonstrate sufficient clinical activity to warrant further investigation and reports of increased toxicity in combination with chemotherapy are concerning.97–99 Clinical studies with the EGFR tyrosine kinase inhibitors gefitinib, erlotinib, and lapatinib were also disappointing.100,101
Molecular Profiling and Potential Therapeutic Targets
Our understanding of cervical cancer biology has focused around the role of HPV infection in the development of this disease.102 The HPV oncoproteins E5, E6, and E7 are the primary viral factors responsible for initiation and progression of cervical cancer, and act largely by overcoming negative growth regulation by host cell proteins, including downstream effects that increase angiogenesis (Fig. 3).
Recent emerging data, however, are helping us to understand more about the genomic profile of cervical cancer. These data are helping to identify potentially “drugable targets” and thus new therapeutic approaches for investigation. Activating mutations and amplification of PIK3CA (the gene encoding phosphoinositol-3-kinase) have been reported for some time, occurring in 23% to 36% of cervical cancer cases. Reports of somatic mutations in other genes including PTEN, TP53, STK11, and KRAS were also reported.103–105 A more comprehensive analysis was published in 2014, which included whole-exome sequencing. Previously unknown somatic mutations were identified in 79 primary squamous cell cervical carcinomas (SCC), including recurrent substitutions in MAPK and inactivating mutations in HLA-A, -B and B2M, suggesting a role for immune evasion in cervical cancer (Table 1). HPV integration appeared to be a common mechanism for gene overexpression, including ERBB2, which also appears to occur as a result of somatic mutation and amplification.106 A further paper from Wright et al focused on the differences between adenocarcinoma, which account for 10% to 20% of cervical cancers but have a worse prognosis, and SCC.107 Although PIK3CA mutations and PTEN loss were observed in both histologic subtypes, KRAS mutations were detected only in adeno-carcinomas (17.5% vs. 0%), and EGFR mutations only in SCC (0% vs. 7.5%).108
TABLE 1.
Agents Currently under Investigation for the Treatment of Recurrent, Persistent, and Metastatic Cervical Cancer
| Target | Phase of Study | Agent(s) | Clinical Trials No. |
|---|---|---|---|
| Immunotherapy | |||
| CTLA | II | Ipilumimab | NCT01693783 |
| PD-1 | II | Nivolomab | NCT02257528 |
| TiLs | TiLs | NCT01585428 | |
| T-cell immunotherapy | HPV16 only | T cells | NCT02280811 |
| Pathway-Targeted Therapy | |||
| RAS/ERK/PI3K/AKT/MTOR | II | Trametanib (MEK inhibitor)/GSK2141795 (AKT inhibitor) | NCT01958112 |
| PI3K | II | BKM120 | NCT01613677 |
| RTK/Angiogenesis | II | Pazopanib/topotecan | NCT02348398 |
| RTK/Angiogenesis | II | Carboplatin/paclitaxel ± nintedanib or placebo followed by maintenance |
NCT02009579 |
| HPV-Related Therapy | |||
| HPV 16 and 18-positive cancer | II | VGX-3100 (plasmids encoding E6 and E7 protein)/INO-9012 (plasmid encoding interleukin 2) delivered via electroporation |
NCT01693783 |
| Therapeutic vaccine | I-II | ADXS11-001 high dose (therapeutic vaccine) | NCT02164461 |
| I-II | |||
| HPV 16 only | ISA101 (HPV 16 E6/E7 long peptides vaccine) with or without interferon alpha with carboplatin paclitaxel |
NCT02128126 | |
| Cytotoxic Agents | II | Albumin-bound paclitaxel/nedaplatin | NCT01667211 |
| II | Eribulin mesylate | NCT0167818 | |
| Other | |||
| Chromosome Region 1 Maintenance Protein | II | Selinexor | NCT02025985 |
Abbreviations: TiLs, tumor-infiltrating lymphocytes; HPV, human papillomavirus.
Studies are single arm unless otherwise indicated.
The prevalence of mutations within the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway, regardless of histologic subtype, make this an attractive therapeutic target in cervical cancer. An initial phase II study of the mTOR inhibitor temsirolimus for the treatment of women with metastatic or recurrent cervical cancer demonstrated limited activity.109 However, further investigation of newer agents (such as PI3K inhibitors) alone or in combination are warranted, potentially in patient populations enriched for PIK3CA mutations. Other potential therapeutic directions include exploring MAPK1 inhibition or ERBB2 inhibition in patients with activating mutations and mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK) inhibitors in patients with KRAS mutations (extrapolating from the experience in low-grade serous ovarian cancer).
Immunotherapy
There is a strong rationale for investigating immunotherapy in cervical cancer given the host/HPV-induced immune evasion, which leads to persistent infection and carcinogenesis. Regulatory T cells are known to modulate the maintenance of an immunologically tolerant environment to HPV-associated preinvasive and malignant lesions.110,111 Furthermore, the presence of tumor-infiltrating lymphocytes (TiLs) in tumor specimens has been associated with improved outcomes.112,113 Currently, investigation of immu-nomodulating agents and strategies which either enhance the innate immune response to cervical cancer or repress immune-protective pathways are a very active area of cervical cancer research (Table 1). Upregulation of cytotoxic T lymphocyte-associated molecule-4 (CTLA-4) receptor on T lymphocytes is a negative regulator of T-cell activation. Ipilimumab is a fully human immunoglobulin (IgG1 kappa) that blocks CTLA-4. CTLA-4 blockade results in the expansion of activated T-cell clones directed at tumor epitopes, theoretically increasing immunovigilance and eradication of tumor cells.114–116 Ipilimumab has demonstrated substantial clinical activity in patients with metastatic melanoma and is currently being investigated in two clinical trials enrolling women with advanced cervical cancer with results expected soon. (GOG 9929/NCT01711515; NCT01693783). A second attractive immunomodulatory strategy under investigation utilizes antibodies directed against another coinhibitory pathway on activated T-cells, the inhibitory receptor programmed cell death 1 (PD-1) and its ligand PD-L1. It remains to be seen if this approach will yield results in cervical cancer.117 The use of bacterial vectors directed against E7 has been shown to induce tumor regression in preclinical models, and a phase II trial conducted in India with a live-attenuated Listeria monocytogenes vaccine suggests that this approach may be successful with further studies ongoing (GOG 265/NCT01266460).118 Finally, patients with cervical cancer are being included in adoptive immunotherapy programs exploring the potential of TiLs harvested from patient tumor samples and then reinfused after immunodepletion (NCT01266460).
Targeting DNA Repair
Repair of DNA damage occurring in cells is essential for their survival. Therefore, inhibition of DNA repair following radio-therapy is a potentially interesting strategy in cervical cancer. Furthermore, there are reports that a subgroup of cervical cancers may have defective homologous recombination as a result of epigenetic modification of the Fanconi anemia (FA) complementation group F (FANCF).119,120 As a result, investigation of the poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors, which block base excision repair of single stranded DNA breaks, in cervical cancer a potentially interesting idea.121 Other proposed strategies inhibiting the repair of DNA damage include inhibition of ribonucleotide reductase (RNR)122,123 and agents which abrogate the G2/M arrest induced by radiation or chemotherapy, such as the Wee1 inhibitor MK1774 (NCT01076400).124
CHALLENGES IN CERVICAL CANCER RESEARCH
The majority of women affected globally by cervical cancer are unlikely to have access to trials or be able to afford new biologic therapies. Conducting clinical trials in patients with cervical cancer in the developed world is becoming increasingly challenging. Ironically, the falling incidence of cervical cancer in the developed world not only results in fewer women who are eligible for clinical trials, but may also result in a lack of interest by pharmaceutical companies to explore new agents in this patient population, despite the major mortality the disease causes worldwide. International collaboration is increasingly required to complete studies in a timely fashion, which, despite efforts in harmonization by organizations such as the GCIG, continue to pose substantial logistical barriers between countries. Within the United States, the patient demographic makes trial enrollment challenging.125 The participation of ethnic minorities and medically underserved populations in clinical trials is critical to making progress. However, multiple well-documented factors account for disproportionally low enrollment rates among minority patients in clinical trials.126–128 These include patients and their families being unaware of clinical trials, a fear of being “treated like a guinea pig,”129–131 and the presence of mistrust of medical research and researchers among certain ethnic groups including American Indian, Asian American, and African American communities.126,132,133 Furthermore, patients with cancer who are immigrants, live in rural areas, have a poor socioeconomic status, and work frequently cite practical concerns, including issues with transportation, family responsibilities, and out-of-pocket expenses as factors that inhibit their ability to participate in research.126,134,135 It is imperative that clinical researchers of cervical cancer acknowledge these issues and reach out to our most vulnerable patients to provide assistance in helping them to become aware of all of their treatment options.
Given the relatively small numbers of patients available to enroll in clinical trials, it is essential that studies are rationally designed and based on biologically sound hypotheses. To limit the administrative burden and maximize participation, creative trial design is essential. Trial designs such as multiarm (or umbrella) or rolling phase II studies are essential if we are to investigate multiple agents in a time- and resource-efficient manner. Incorporation of translational substudies and functional imaging studies will allow us to gain the maximum information from each trial. Commonality with other HPV-induced malignancies, such as cancers of the oropharynx and anal canal, suggest there might be underdeveloped routes of collaboration. Patients with cervical cancer may be eligible for studies requiring the presence of specific mutational profiles which are not limited to a particular cancer type.125
FINAL REMARKS
Much progress is still needed in the treatment of cervical cancer. It is important that we remember that the majority of women affected globally by cervical cancer are unlikely to be able to access new biologic therapies or have access to clinical trials. If we are to achieve maximum benefit for women with this disease, we need to reach out and form partnerships that allow us to raise the standards for all women. Costs must be considered in the development of new agents so that our results may be globally relevant, and advocacy is essential. Data from randomized trials exploring the role of adjuvant chemotherapy are expected soon that may change our approach to the front-line management of women with locally-advanced cervical cancer. However, the key to ensuring truly improved quality of care for patients is to recognize and identify patients who require supportive interventions both during and following therapy.
KEY POINTS.
Cervical cancer remains one of the leading causes of cancer-related morbidity and mortality in women worldwide.
A number of ongoing clinical trials are examining the role of adjuvant chemotherapy in addition to the standard-of-care treatment, low-dose chemotherapy (cisplatin) concurrent with pelvic radiotherapy for locally advanced cervical cancer.
Women undergoing treatment for locally advanced cervical cancer experience significant psychosocial distress. Multidisciplinary supportive care may reduce the magnitude of long-term sequelae and improve quality of life.
Outcome for women diagnosed with metastatic or recurrent cervical cancer remains poor; there are a number of potential therapeutic targets actively under investigation. The first biologic agent, in combination with chemotherapy, to show a survival benefit was bevacizumab.
International collaboration and engagement of medically underserved communities are essential to making progress in the treatment of cervical cancer.
Footnotes
Disclosures of Potential Conflicts of Interest
Relationships are considered self-held and compensated unless otherwise noted. Relationships marked “L” indicate leadership positions. Relationships marked “I” are those held by an immediate family member; those marked “B” are held by the author and an immediate family member. Institutional relationships are marked “Inst.” Relationships marked “U” are uncompensated.
Employment: None. Leadership Position: None. Stock or Other Ownership Interests: None. Honoraria: None. Consulting or Advisory Role: None. Speakers’ Bureau: None. Research Funding: None. Patents, Royalties, or Other Intellectual Property: None. Expert Testimony: None. Travel, Accommodations, Expenses: Helen Mackay, AstraZeneca. Other Relationships: None.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Australian Institute of Health and Welfare, Australasian Association of Cancer Registries 2010. Cancer in Australia: an overview 2010. Canberra, Australia: Australian Institute of Health and Welfare; 2010. [Accessed February 26, 2015]. http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=6442472684 Published 2010. [Google Scholar]
- 3.World Cancer Research Fund International. [Accessed February 26, 2015];Cervical cancer statistics. www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/cervical-cancer-statistics.
- 4.Choi YH, Jit M, Gay N, et al. Transmission dynamic modelling of the impact of human papillomavirus vaccination in the United Kingdom. Vaccine. 2010;28:4091–4102. doi: 10.1016/j.vaccine.2009.09.125. [DOI] [PubMed] [Google Scholar]
- 5.Greer BE, Koh WJ, Abu-Rustum NR, et al. Cervical cancer. J Natl Compr Canc Netw. 2010;8:1388–1416. doi: 10.6004/jnccn.2010.0104. [DOI] [PubMed] [Google Scholar]
- 6.Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eifel PJ, Burke TW, Delclos L, et al. Early stage I adenocarcinoma of the uterine cervix: treatment results in patients with tumors less than or equal to 4 cm in diameter. Gynecol Oncol. 1991;41:199–205. doi: 10.1016/0090-8258(91)90308-r. [DOI] [PubMed] [Google Scholar]
- 8.Gien LT, Covens A. Lymph node assessment in cervical cancer: prognostic and therapeutic implications. J Surg Oncol. 2009;99:242–247. doi: 10.1002/jso.21199. [DOI] [PubMed] [Google Scholar]
- 9.Grigsby PW. The prognostic value of PET and PET/CT in cervical cancer. Cancer Imaging. 2008;8:146–155. doi: 10.1102/1470-7330.2008.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trimble EL. Cervical cancer state-of-the-clinical-science meeting on pretreatment evaluation and prognostic factors, September 27–28, 2007: proceedings and recommendations. Gynecol Oncol. 2009;114:145–150. doi: 10.1016/j.ygyno.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Goudy G, Stoeckle E, Thomas L, et al. [Prognostic impact of tumour volume and lymph node involvement in intermediate stage T1b1 to T2b cancer of the uterine cervix] Bull Cancer. 2009;96:685–694. doi: 10.1684/bdc.2009.0873. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita H, Nakagawa K, Tago M, et al. Treatment results and prognostic analysis of radical radiotherapy for locally advanced cancer of the uterine cervix. Br J Radiol. 2005;78:821–826. doi: 10.1259/bjr/13147816. [DOI] [PubMed] [Google Scholar]
- 13.Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009;105:107–108. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Unger JB, Lilien DL, Caldito G, et al. The prognostic value of pretreatment 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography scan in women with cervical cancer. Int J Gynecol Cancer. 2007;17:1062–1067. doi: 10.1111/j.1525-1438.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 15.Hope AJ, Saha P, Grigsby PW. FDG-PET in carcinoma of the uterine cervix with endometrial extension. Cancer. 2006;106:196–200. doi: 10.1002/cncr.21573. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Kim W, Lee M, et al. Tumor volume and uterine body invasion assessed by MRI for prediction of outcome in cervical carcinoma treated with concurrent chemotherapy and radiotherapy. Jpn J Clin Oncol. 2007;37:858–866. doi: 10.1093/jjco/hym109. [DOI] [PubMed] [Google Scholar]
- 17.Narayan K, Fisher RJ, Bernshaw D, et al. Patterns of failure and prognostic factor analyses in locally advanced cervical cancer patients staged by positron emission tomography and treated with curative intent. Int J Gynecol Cancer. 2009;19:912–918. doi: 10.1111/IGC.0b013e3181a58d3f. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi H, Shiozawa I, Kitahara T, et al. Uterine body invasion of carcinoma of the uterine cervix as seen from surgical specimens. Gynecol Oncol. 1988;30:173–182. doi: 10.1016/0090-8258(88)90021-2. [DOI] [PubMed] [Google Scholar]
- 19.Perez CA, Camel HM, Askin F, et al. Endometrial extension of carcinoma of the uterine cervix: a prognostic factor that may modify staging. Cancer. 1981;48:170–180. doi: 10.1002/1097-0142(19810701)48:1<170::aid-cncr2820480128>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Prempree T, Patanaphan V, Viravathana T, et al. Radiation treatment of carcinoma of the cervix with extension into the endometrium: a reappraisal of its significance. Cancer. 1982;49:2015–2020. doi: 10.1002/1097-0142(19820515)49:10<2015::aid-cncr2820491012>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Narayan K, Fisher R, Bernshaw D. Significance of tumor volume and corpus uteri invasion in cervical cancer patients treated by radiotherapy. Int J Gynecol Cancer. 2006;16:623–630. doi: 10.1111/j.1525-1438.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 22.Narayan K, McKenzie AF, Hicks RJ, et al. Relation between FIGO stage, primary tumor volume, and presence of lymph node metastases in cervical cancer patients referred for radiotherapy. Int J Gynecol Cancer. 2003;13:657–663. doi: 10.1046/j.1525-1438.2003.13026.x. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz JK, Grigsby PW, Dehdashti F, et al. The role of 18F–FDG PET in assessing therapy response in cancer of the cervix and ovaries. J Nucl Med. 2009;50(Supl 1):64S–73S. doi: 10.2967/jnumed.108.057257. [DOI] [PubMed] [Google Scholar]
- 24.Tod M, Meredith W. A dosage system for use in the treatment of cancer of the uterine cervix. Br J Radiol. 1938;11:809–824. [Google Scholar]
- 25.Narayan K, van Dyk S, Bernshaw D, et al. Ultrasound guided conformal brachytherapy of cervix cancer: survival, patterns of failure, and late complications. J Gynecol Oncol. 2014;25:206–213. doi: 10.3802/jgo.2014.25.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore KN, Sill MW, Miller DS, et al. A phase I trial of tailored radiation therapy with concomitant cetuximab and cisplatin in the treatment of patients with cervical cancer: a gynecologic oncology group study. Gynecol Oncol. 2012;127:456–461. doi: 10.1016/j.ygyno.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Schefter TE, Winter K, Kwon JS, et al. A phase IIstudy of bevacizumab in combination with definitive radiotherapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma: preliminary results of RTOG 0417. Int J Radiat Oncol Biol Phys. 2012;83:1179–1184. doi: 10.1016/j.ijrobp.2011.10.060. [DOI] [PubMed] [Google Scholar]
- 28.Schefter T, Winter K, Kwon JS, et al. RTOG 0417: efficacy of bevaci-zumab in combination with definitive radiation therapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:101–105. doi: 10.1016/j.ijrobp.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Mileshkin L, Paramanathan A, Kondalsamy-Chennakesavan S, et al. Smokers with cervix cancer have more uterine corpus invasive disease and an increased risk of recurrence after treatment with chemoradiation. Int J Gynecol Cancer. 2014;24:1286–1291. doi: 10.1097/IGC.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 30.Bishop AJ, Allen PK, Klopp AH, et al. Relationship between low hemoglobin levels and outcomes after treatment with radiation or chemoradiation in patients with cervical cancer: has the impact of anemia been Ooerstated? Int J Radiat Oncol Biol Phys. 2014:pii:S0360–pii:S3016. doi: 10.1016/j.ijrobp.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Thomas G, Ali S, Hoebers FJ, et al. Phase III trail to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108:317–325. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedlander M, Grogan M. U.S. Preventative Services Task Force. Guidelines for the treatment of recurrent and metastatic cervical cancer. Oncologist. 2002;7:342–347. [PubMed] [Google Scholar]
- 33.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 34.Kantardzic´ N, Beslija S, Begic´ D. [Comparative parameters of myelo-toxicity in patients treated with simultaneous chemotherapy and radiotherapy or only radiotherapy] Med Arh. 2004;58:19–22. [PubMed] [Google Scholar]
- 35.Duen˜as-Gonza´lez A, Zarba´ JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29:1678–1685. doi: 10.1200/JCO.2009.25.9663. [DOI] [PubMed] [Google Scholar]
- 36.Thomas G. Are we making progress in curing advanced cervical cancer? J Clin Oncol. 2011;29:1654–1656. doi: 10.1200/JCO.2010.34.1966. [DOI] [PubMed] [Google Scholar]
- 37.Ryu SY, Lee WM, Kim K, et al. Randomized clinical trial of weekly vs. triweekly cisplatin-based chemotherapy concurrent with radiotherapy in the treatment of locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2011;81:e577–e581. doi: 10.1016/j.ijrobp.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta-Analysis Collaboration. Neoadjuvant chemotherapy for locally advanced cervical cancer: a systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur J Cancer. 2003;39:2470–2486. doi: 10.1016/s0959-8049(03)00425-8. [DOI] [PubMed] [Google Scholar]
- 39.McCormack M, Kadalayil L, Hackshaw A. A phase II study of weekly neoadjuvant chemotherapy followed by radical chemoradiation for locally advanced cervical cancer. Br J Cancer. 2013;108:2464–2469. doi: 10.1038/bjc.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wenzel L, DeAlba I, Habbal R, et al. Quality of life in long-term cervical cancer survivors. Gynecol Oncol. 2005;97:310–317. doi: 10.1016/j.ygyno.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Ashing-Giwa KT, Tejero JS, Kim J, et al. Cervical cancer survivorship in a population based sample. Gynecol Oncol. 2009;112:358–364. doi: 10.1016/j.ygyno.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Ashing-Giwa KT, Lim JW, Tang J. Surviving cervical cancer: does health-related quality of life influence survival? Gynecol Oncol. 2010;118:35–42. doi: 10.1016/j.ygyno.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 43.Korfage IJ, Essink-Bot ML, Mols F, et al. Health-related quality of life in cervical cancer survivors: a population-based survey. Int J Radia-tOncol Biol Phys. 2009;73:1501–1509. doi: 10.1016/j.ijrobp.2008.06.1905. [DOI] [PubMed] [Google Scholar]
- 44.Distefano M, Riccardi S, Capelli G, et al. Quality of life and psychological distress in locally advanced cervical cancer patients administered pre-operative chemoradiotherapy. Gynecol Oncol. 2008;111:144–150. doi: 10.1016/j.ygyno.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 45.Bodurka DC, von Gruenigen VE. Women’s cancer survivorship: time to gear up! Gynecol Oncol. 2012;124:377–378. doi: 10.1016/j.ygyno.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Weaver KE, Forsythe LP, Reeve BB, et al. Mental and physical health-related quality of life among U.S. cancer survivors: population estimates from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21:2108–2117. doi: 10.1158/1055-9965.EPI-12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lutgendorf SK, Anderson B, Rothrock N, et al. Quality of life and mood in women receiving extensive chemotherapy for gynecologic cancer. Cancer. 2000;89:1402–1411. [PubMed] [Google Scholar]
- 48.Bergmark K, Avall-Lundqvist E, Dickman PW, et al. Lymphedema and bladder-emptying difficulties after radical hysterectomy for early cervical cancer and among population controls. Int J Gynecol Cancer. 2006;16:1130–1139. doi: 10.1111/j.1525-1438.2006.00601.x. [DOI] [PubMed] [Google Scholar]
- 49.Skjeldestad FE, Rannestad T. Urinary incontinence and quality of life in long-term gynecological cancer survivors: a population-based cross-sectional study. Acta Obstet Gynecol Scand. 2009;88:192–199. doi: 10.1080/00016340802582041. [DOI] [PubMed] [Google Scholar]
- 50.Grover S, Hill-Kayser CE, Vachani C, et al. Patient reported late effects of gynecological cancer treatment. Gynecol Oncol. 2012;124:399–403. doi: 10.1016/j.ygyno.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 51.Abbott-Anderson K, Kwekkeboom KL. A systematic review of sexual concerns reported by gynecological cancer survivors. Gynecol Oncol. 2012;124:477–489. doi: 10.1016/j.ygyno.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 52.Ferrandina G, Mantegna G, Petrillo M, et al. Quality of life and emotional distress in early stage and locally advanced cervical cancer patients: a prospective, longitudinal study. Gynecol Oncol. 2012;124:389–394. doi: 10.1016/j.ygyno.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 53.Bodurka DC, Sun CC. Sexual function after gynecologic cancer. Obstet Gynecol Clin North Am. 2006;33:621–630. doi: 10.1016/j.ogc.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Lindau ST, Gavrilova N, Anderson D. Sexual morbidity in very long term survivors of vaginal and cervical cancer: a comparison to national norms. Gynecol Oncol. 2007;106:413–418. doi: 10.1016/j.ygyno.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baze C, Monk BJ, Herzog TJ. The impact of cervical cancer on quality of life: a personal account. Gynecol Oncol. 2008;109:S12–S14. doi: 10.1016/j.ygyno.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 56.Sun C, Jhingran A, Gallegos J, et al. Longitudinal quality of life in medically underserved women with locally advanced cervical cancer. Gy-necol Oncol. 2012;125(suppl 1):S50. abstr 117. [Google Scholar]
- 57.Bodurka D, Sun C, Jhingran A, et al. A longitudinal evaluation of sexual functioning and quality of life in cervical cancer survivors. Gynecol Oncol. 2010;120(suppl 1):S81–S82. abstr 187. [Google Scholar]
- 58.Wenzel L, Dogan-Ates A, Habbal R, et al. Defining and measuring reproductive concerns of female cancer survivors. J Natl Cancer Inst Monogr. 2005:94–98. doi: 10.1093/jncimonographs/lgi017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frumovitz M, Sun CC, Schover LR, et al. Quality of life and sexual functioning in cervical cancer survivors. J Clin Oncol. 2005;23:7428–7436. doi: 10.1200/JCO.2004.00.3996. [DOI] [PubMed] [Google Scholar]
- 60.Mantegna G, Petrillo M, Fuoco G, et al. Long-term prospective longitudinal evaluation of emotional distress and quality of life in cervical cancer patients who remained disease-free 2-years from diagnosis. BMC Cancer. 2013;13:127. doi: 10.1186/1471-2407-13-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bradley S, Rose S, Lutgendorf S, et al. Quality of life and mental health in cervical and endometrial cancer survivors. Gynecol Oncol. 2006;100:479–486. doi: 10.1016/j.ygyno.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 62.Herzog TJ, Wright JD. The impact of cervical cancer on quality of life—the components and means for management. Gynecol Oncol. 2007;107:572–577. doi: 10.1016/j.ygyno.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 63.Ashing-Giwa KT, Lim JW, Gonzalez P. Exploring the relationship between physical well-being and healthy lifestyle changes among European- and Latina-American breast and cervical cancer survivors. Psychooncology. 2010;19:1161–1170. doi: 10.1002/pon.1687. [DOI] [PubMed] [Google Scholar]
- 64.Nelson EL, Wenzel LB, Osann K, et al. Stress, immunity, and cervical cancer: biobehavioral outcomes of a randomized clinical trail. Clin Cancer Res. 2008;14:2111–2118. doi: 10.1158/1078-0432.CCR-07-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antoni MH. Psychosocial intervention effects on adaptation, disease course and biobehavioral processes in cancer. Brain Behav Immun. 2013;30(suppl):S88–S98. doi: 10.1016/j.bbi.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Powell ND, Tarr AJ, Sheridan JF. Psychosocial stress and inflammation in cancer. Brain Behav Immun. 2013;30(suppl):S41–S47. doi: 10.1016/j.bbi.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 67.Chida Y, Hamer M, Wardle J, et al. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 68.Chase DM, Huang HQ, Wenzel L, et al. Quality of life and survival in advanced cervical cancer: a Gynecologic Oncology Group study. Gy-necol Oncol. 2012;125:315–319. doi: 10.1016/j.ygyno.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 69.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 70.Tewari KS, Sill MW, Long HJ, 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wenzel L, Osann K, Hsieh S, et al. Quality of life outcomes of a randomized counseling trial for cervical cancer survivors. Qual Life Res. 2012;21(suppl):15. abstr 108.3. [Google Scholar]
- 72.Wenzel L. Late effects and quality of life following aggressive management. Paper presented at: 1st American Brachytherapy Society GYN School; July, 2014; Chicago, IL. [Google Scholar]
- 73.Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266–272. doi: 10.1016/j.ygyno.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wenzel L, Osann K, Hsieh S, et al. Psychosocial telephone counseling for survivors of cervical cancer: results of a randomized biobehavioral trial. J Clin Oncol. Epub. 2015 Feb 23; doi: 10.1200/JCO.2014.57.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waggoner SE. Cervical cancer. Lancet. 2003;361:2217–2225. doi: 10.1016/S0140-6736(03)13778-6. [DOI] [PubMed] [Google Scholar]
- 76.Monk BJ, Sill MW, McMeekin DS, et al. Phase III trialoffour cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moore DH, Tian C, Monk BJ, et al. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2010;116:44–49. doi: 10.1016/j.ygyno.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rose PG, Blessing JA, Gershenson DM, et al. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 1999;17:2676–2680. doi: 10.1200/JCO.1999.17.9.2676. [DOI] [PubMed] [Google Scholar]
- 79.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith-McCune KK, Weidner N. Demonstration and characterization of the angiogenic properties of cervical dysplasia. Cancer Res. 1994;54:800–804. [PubMed] [Google Scholar]
- 81.Kodama J, Seki N, Tokumo K, et al. Vascular endothelial growth factor is implicated in early invasion in cervical cancer. Eur J Cancer. 1999;35:485–489. doi: 10.1016/s0959-8049(98)00410-9. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura M, Bodily JM, Beglin M, et al. Hypoxia-specific stabilization of HIF-1alpha by human papillomaviruses. Virology. 2009;387:442–448. doi: 10.1016/j.virol.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tokumo K, Kodama J, Seki N, et al. Different angiogenic pathways in human cervical cancers. Gynecol Oncol. 1998;68:38–44. doi: 10.1006/gyno.1997.4876. [DOI] [PubMed] [Google Scholar]
- 84.Loncaster JA, Cooper RA, Logue JP, et al. Vascular endothelial growth factor (VEGF) expression is a prognostic factor for radiotherapy outcome in advanced carcinoma of the cervix. Br J Cancer. 2000;83:620–625. doi: 10.1054/bjoc.2000.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee IJ, Park KR, Lee KK, et al. Prognostic value of vascular endothelial growth factor in Stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 2002;54:768–779. doi: 10.1016/s0360-3016(02)02970-x. [DOI] [PubMed] [Google Scholar]
- 86.Bachtiary B, Selzer E, Knocke TH, et al. Serum VEGF levels in patients undergoing primary radiotherapy for cervical cancer: impact on progression-free survival. Cancer Lett. 2002;179:197–203. doi: 10.1016/s0304-3835(01)00872-2. [DOI] [PubMed] [Google Scholar]
- 87.Mitsuhashi A, Suzuka K, Yamazawa K, et al. Serum vascular endothelial growth factor (VEGF) and VEGF-C levels as tumor markers in patients with cervical carcinoma. Cancer. 2005;103:724–730. doi: 10.1002/cncr.20819. [DOI] [PubMed] [Google Scholar]
- 88.Kim YH, Kim MA, Park IA, et al. VEGF polymorphisms in early cervical cancer susceptibility, angiogenesis, and survival. Gynecol Oncol. 2010;119:232–236. doi: 10.1016/j.ygyno.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 89.Eskander RN, Tewari KS. Targeting angiogenesis in advanced cervical cancer. Ther Adv Med Oncol. 2014;6:280–292. doi: 10.1177/1758834014543794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Monk BJ, Sill MW, Burger RA, et al. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2009;27:1069–1074. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wright JD, Viviano D, Powell MA, et al. Bevacizumab combination th erapy in heavily pretreated, recurrent cervical cancer. Gynecol Oncol. 2006;103:489–493. doi: 10.1016/j.ygyno.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 92.Mackay HJ, Tinker A, Winquist E, et al. A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG Trial IND.184. Gynecol Oncol. 2010;116:163–167. doi: 10.1016/j.ygyno.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 93.Monk BJ, Mas Lopez L, Zarba JJ, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2010;28:3562–3569. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 94.Scambia G, Ferrandina G, Distefano M, et al. Epidermal growth factor receptor (EGFR) is not related to the prognosis of cervical cancer. Cancer Lett. 1998;123:135–139. doi: 10.1016/s0304-3835(97)00421-7. [DOI] [PubMed] [Google Scholar]
- 95.Kersemaekers AM, Fleuren GJ, Kenter GG, et al. Oncogene alterations in carcinomas of the uterine cervix: overexpression of the epidermal growth factor receptor is associated with poor prognosis. Clin Cancer Res. 1999;5:577–586. [PubMed] [Google Scholar]
- 96.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 97.Farley J, Sill MW, Birrer M, et al. Phase II study of cisplatin plus cetux-imab in advanced, recurrent, and previously treated cancers of the cervix and evaluation of epidermal growth factor receptor immuno-histochemical expression: a Gynecologic Oncology Group study. Gy-necol Oncol. 2011;121:303–308. doi: 10.1016/j.ygyno.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Santin AD, Sill MW, McMeekin DS, et al. Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;122:495–500. doi: 10.1016/j.ygyno.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kurtz JE, Hardy-Bessard AC, Deslandres M, et al. Cetuximab, topotecan and cisplatin for the treatment of advanced cervical cancer: a phase II GINECO trial. Gynecol Oncol. 2009;113:16–20. doi: 10.1016/j.ygyno.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 100.Schilder RJ, Sill MW, Lee YC, et al. A phase II trial of erlotinib in recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Int J Gynecol Cancer. 2009;19:929–933. doi: 10.1111/IGC.0b013e3181a83467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goncalves A, Fabbro M, Lhomme´ C, et al. A phase II trial to evaluate gefitinib as second- or third-line treatment in patients with recurring locoregionally advanced or metastatic cervical cancer. Gynecol Oncol. 2008;108:42–46. doi: 10.1016/j.ygyno.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 102.Bosch FX, Lorincz A, Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Janku F, Lee JJ, Tsimberidou AM, et al. PIK3CA mutations frequently coexist with RAS and BRAF mutations in patients with advanced cancers. PLoS One. 2011;6:e22769. doi: 10.1371/journal.pone.0022769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bertelsen BI, Steine SJ, Sandvei R, et al. Molecular analysis of the PI3K–AKT pathway in uterine cervical neoplasia: Frequent PIK3CA amplification and AKT phosphorylation. Int J Cancer. 2006;118:1877–1883. doi: 10.1002/ijc.21461. [DOI] [PubMed] [Google Scholar]
- 105.McIntyre JB, Wu JS, Craighead PS, et al. PIK3CA mutational status and overall survival in patients with cervical cancer treated with radical chemoradiotherapy. Gynecol Oncol. 2013;128:409–414. doi: 10.1016/j.ygyno.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 106.Ojesina AI, Lichtenstein L, Freeman SS, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506:371–375. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Galic V, Herzog TJ, Lewin SN, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol. 2012;125:287–291. doi: 10.1016/j.ygyno.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 108.Wright AA, Howitt BE, Myers AP, et al. Oncogenic mutations in cervical cancer: genomic differences between adenocarcinomas and squamous cell carcinomas of the cervix. Cancer. 2013;119:3776–3783. doi: 10.1002/cncr.28288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tinker AV, Ellard S, Welch S, et al. Phase II study of temsirolimus (CCI-779) in women with recurrent, unresectable, locally advanced or metastatic carcinoma of the cervix. A trial of the NCIC Clinical Trials Group (NCIC CTG IND 199) Gynecol Oncol. 2013;130:269–274. doi: 10.1016/j.ygyno.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 110.Sasagawa T, Takagi H, Makinoda S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J Infect Chemother. 2012;18:807–815. doi: 10.1007/s10156-012-0485-5. [DOI] [PubMed] [Google Scholar]
- 111.Patel S, Chiplunkar S. Host immune responses to cervical cancer. Curr Opin Obstet Gynecol. 2009;21:54–59. doi: 10.1097/GCO.0b013e32831a9890. [DOI] [PubMed] [Google Scholar]
- 112.Piersma SJ, Jordanova ES, van Poelgeest MI, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 113.Shah W, Yan X, Jing L, et al. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4(+)FOXP3(+) regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol. 2011;8:59–66. doi: 10.1038/cmi.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tuve S, Chen BM, Liu Y, et al. Combination of tumor site-located CTL-associated antigen-4 blockade and systemic regulatory T-cell depletion induces tumor-destructive immune responses. Cancer Res. 2007;67:5929–5939. doi: 10.1158/0008-5472.CAN-06-4296. [DOI] [PubMed] [Google Scholar]
- 115.Calabro L, Danielli R, Sigalotti L, et al. Clinical studies with anti-CTLA-4 antibodies in non-melanoma indications. Semin Oncol. 37:460–467. doi: 10.1053/j.seminoncol.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 116.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Basu P, Petit RG. ADXS11-001 immunotherapy targeting HPV-E7: preliminary survival data from a phase II study in Indian women with recurrent/refractory cervical cancer. J Clin Oncol. 2012;30(suppl) abstr 5106. [Google Scholar]
- 119.Pulido HA, Fakruddin MJ, Chatterjee A, et al. Identification of a 6-cM minimal deletion at 11q23.1-23.2 and exclusion of PPP2R1B gene as a deletion target in cervical cancer. Cancer Res. 2000;60:6677–6682. [PubMed] [Google Scholar]
- 120.Narayan G, Arias-Pulido H, Nandula SV, et al. Promoter hypermethylation of FANCF: disruption of Fanconi Anemia-BRCA pathway in cervical cancer. Cancer Res. 2004;64:2994–2997. doi: 10.1158/0008-5472.can-04-0245. [DOI] [PubMed] [Google Scholar]
- 121.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 122.Kuo ML, Kinsella TJ. Expression of ribonucleotide reductase after ionizing radiation in human cervical carcinoma cells. Cancer Res. 1998;58:2245–2252. [PubMed] [Google Scholar]
- 123.Kunos CA, Radivoyevitch T, Kresak A, et al. Elevated ribonucleotide reductase levels associate with suppressed radiochemotherapy response in human cervical cancers. Int J Gynecol Cancer. 2012;22:1463–1469. doi: 10.1097/IGC.0b013e318270577f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brana I, Mackay HJ. WEE1 inhibition as an anticancer strategy: first advances. Drugs Fut. 2014;39:207. [Google Scholar]
- 125.Dizon DS, Mackay HJ, Thomas GM, et al. State of the science in cervical cancer: where we are today and where we need to go. Cancer. 2014;120:2282–2288. doi: 10.1002/cncr.28722. [DOI] [PubMed] [Google Scholar]
- 126.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting under-represented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 127.Lara PN, Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23:9282–9289. doi: 10.1200/JCO.2005.02.6245. [DOI] [PubMed] [Google Scholar]
- 128.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 129.Comis RL, Miller JD, Aldige´ CR, et al. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21:830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 130.Roberson NL. Clinical trial participation. Viewpoints from racial/ethnic groups. Cancer. 1994;74(9 Suppl):2687–2691. doi: 10.1002/1097-0142(19941101)74:9+<2687::aid-cncr2820741817>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 131.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 132.Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine. 2008;87:1–9. doi: 10.1097/MD.0b013e3181625d78. [DOI] [PubMed] [Google Scholar]
- 133.Giarelli E, Bruner DW, Nguyen E, et al. Research participation among Asian American women at risk for cervical cancer: exploratory pilot of barriers and enhancers. J Immigr Minor Health. 2011;13:1055–1068. doi: 10.1007/s10903-011-9461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Corbie-Smith G, Thomas SB, Williams MV, et al. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14:537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guadagnolo BA, Petereit DG, Helbig P, et al. Involving American Indians and medically underserved rural populations in cancer clinical trials. Clin Trials. 2009;6:610–617. doi: 10.1177/1740774509348526. [DOI] [PMC free article] [PubMed] [Google Scholar]