Abstract
The reaction products from limonene ozonolysis were investigated using the new carbonyl derivatization agent, O-tert-butylhydroxylamine hydrochloride (TBOX). With ozone (O3) as the limiting reagent, five carbonyl compounds were detected. The yields of the carbonyl compounds are discussed with and without the presence of a hydroxyl radical (OH•) scavenger, giving insight into the influence secondary OH radicals have on limonene ozonolysis products. The observed reaction product yields for limonaketone (LimaKet), 7-hydroxyl-6-oxo-3-(prop-1-en-2-yl)heptanal (7H6O), and 2-acetyl-5-oxohexanal (2A5O) were unchanged suggesting OH• generated by the limonene + O3 reaction does not contribute to their formation. The molar yields of 3-isopropenyl-6-oxo-heptanal (IPOH) and 3-acetyl-6-oxoheptanal (3A6O) decreased by 68% and >95%; respectively, when OH• was removed. This suggests that OH• radicals significantly impact the formation of these products. Nitric oxide (NO) did not significantly affect the molar yields of limonaketone or IPOH. However, NO (20 ppb) considerably decreased the molar reaction product yields of 7H6O (62%), 2A5O (63%), and 3A6O (47%), suggesting NO reacted with peroxyl intermediates, generated during limonene ozonolysis, to form other carbonyls (not detected) or organic nitrates. These studies give insight into the transformation of limonene and its reaction products that can lead to indoor exposures.
Keywords: Ozone, Reaction products, Oxygenated organic compounds, Derivatization
1. Introduction
Volatile organic compounds (VOCs) are introduced indoors by outdoor ventilation, emissions from building materials, and the use of various cleaning products (Nazaroff and Weschler, 2004; Singer et al., 2006). In indoor environments, these VOCs can react with oxidants such as ozone (O3) and/or hydroxyl radicals (OH•) in the gas phase or on indoor surfaces and can transform into a variety of intermediate and stable oxygenated organics (e.g. peroxyl radicals, aldehydes, ketones, di- and tricarbonyls, and carboxylic acids). Peroxyl radicals may further react with NO or NO2 to generate organic nitrates (e.g alkyl nitrates, peroxyacyl nitrates (PANs), hydroxynitrates, and dinitrates) (Finlayson-Pitts and Pitts, 2000). Indoor concentrations of O3, NO, and NO2 in the US have been measured with average values of 50, 50, and 25 ppb, respectively (Nazaroff and Cass, 1986; Weschler and Shields, 1997; Weschler et al., 1994). Although, hydroxyl radical concentrations have not been measured indoors, they have been estimated to be in the range of 0.12–2 × 106 mol cm−3 (0.48–8 × 10−5 ppb) (Alvarez et al., 2013; Sarwar et al., 2002; Waring and Wells, 2015).
Given these measured oxidant concentrations indoors and the reactivity of specific VOCs (e.g. terpenes such as α-pinene, limonene, terpinolene), it is expected that oxidation products are formed and lead to potential indoor exposures. As an example, the bimolecular rate constant for terpinolene + O3 is 19.0 × 10−16 cm3 molecule−1 s−1 (0.169 ppb−1 h−1) (Atkinson and Arey, 2003; Nazaroff and Weschler, 2004). Assuming an indoor O3 concentration of 50 ppb, the pseudo-first order rate for terpinolene ozonolysis would be 8.45 hr−1 indicating terpinolene would likely be removed by reaction with O3 before removal by a typical air-exchange of 0.6 hr−1 (Wilson et al., 1996). Therefore, identifying reaction products from terpene ozonolysis that occurs indoors is critical to characterizing occupant exposures.
Limonene (1-methyl-4-(prop-1-en-2-yl)cyclohexene), is a prevalent terpene with a strong orange-like fragrance found in a number of household consumer products used indoors. The National Library of Medicine’s (NLM) Household Products Database (HHS/NIH, 2015) lists 166 consumer products that contain D-limonene as an ingredient. A significant fraction (59 of 166) of these products are used inside the home (e.g., in cleaning agents) which frequently use D-limonene as an odorant and for its antimicrobial properties. Recent work by Singer et al. determined the one hour concentration of limonene after the application of a full strength cleaning product to be 300–6000 μg/m3 (~80–1600 ppb) (Singer et al., 2006).
The ozonolysis of limonene has been extensively studied using a variety of analytical techniques. However, most of this research has focused on the characterization of secondary organic aerosols (SOAs) from the formation of gas-phase species (Donahue et al., 2014; Ebben et al., 2012; Jiang et al., 2012; Pan et al., 2009; Pathak et al., 2012b; Youssefi and Waring, 2014). This research has provided information about the particle size distribution, aerosol yields and chemical composition, but only limited information of the gas-phase yields from limonene ozonolysis has been determined. Questions still remain on the carbon mass balance of limonene oxidation. The answers may be related to undetected highly oxygenated products (e.g. tricarbonyls). Reaction models (e.g. Master Chemical Mechanism) propose the formation of tri-carbonyl species from limonene ozonolysis (Carslaw, 2013; Jenkin et al., 2015; Norgaard et al., 2013; Pathak et al., 2012a). Recently, the tricarbonyl (3-acetyl-6-oxoheptanal (3A6O)) from limonene ozonolysis was detected using the new derivatization agent, TBOX (Wells and Ham, 2014).
In this study, limonene ozonolysis with and without addition of nitric oxide (NO) and cyclohexane (OH• scavenger) was investigated using a Teflon® impinger to capture and characterize gas-phase reaction products. Identification and quantification of the reaction products (i.e., aldehydes, ketones, and di- and tri-carbonyls) was made using O-tert-butylhydroxylamine hydrochloride (TBOX) to derivatize the carbonyl products (Wells and Ham, 2014). This method provides the sensitivity, ease of use, and applicability needed for detection of carbonyl compounds at expected indoor air concentrations.
2. Experimental methods
2.1. Chemicals and solvents
All compounds were used as received and had the following purities: from Sigma-Aldrich/Fluka (St. Louis, MO): O-tert-butyl-hydroxylamine hydrochloride (TBOX, 99%), limonene (97%), toluene (HPLC grade, 99+%), cyclohexane (HPLC grade, 99+%), cyclohexanone (98%), methylglyoxal (40 wt% in water), and glutaraldehyde (50 wt% in water). Methanol (HPLC grade, 99+%) was purchased from Fisher Scientific (Pittsburgh, PA). Water (DI H2O) was distilled, deionized to a resistivity of 18 MΩ cm, and filtered using a Milli-Q® filter system (Billerica, MA). Helium (UHP grade), the carrier gas, was supplied by Butler Gas (McKees Rocks, PA) and used as received. Experiments were carried out at (297 ± 3) K and 1 atm pressure. Compressed air from the National Institute for Occupational Safety and Health (NIOSH) facility was passed through anhydrous CaSO4 (Drierite, Xenia, OH) and molecular sieves (Drierite) to remove both moisture and organic contaminants. This treated dry air from the NIOSH facility flowed through a mass flow controller and into a humidifying chamber and was subsequently mixed with dry air to the pre-determined relative humidity (RH) of 50%. An 80 L Teflon® reaction chamber contained in a light-tight wooden box was filled through a heated syringe injection port facilitating the introduction of liquid reactants into the chamber. Background measurements of the NIOSH facility air showed concentrations of O3, NO, and NO2 at less than 1.0, 1.2, and 0.5 ppb, respectively. All reactant mixtures were generated by this system.
Ozone was produced by photolyzing air with a mercury pen lamp (Jelight, Irvine, CA) in a separate Teflon® chamber. Aliquots of this O3/air mixture were added to the Teflon® reaction chamber using a gas-tight syringe. O3 concentrations were measured using a Thermo Electron (Waltham, MA) UV photometric ozone analyzer Model 49C. Aliquots of NO were added to the reaction chamber from a 100 ppm tank (Butler Gas, McKees Rocks, PA) using a gas-tight syringe. NO and NO2 concentrations were measured using a Thermo Electron (Waltham, MA) NOx analyzer Model 49i.
2.2. Methods
2.2.1. Calibration
Experiments to measure the gas-phase carbonyls (cyclohexanone, and glutaraldehyde, Table 1) used for calibration of the gas-phase reaction products formed from the reaction of limonene with O3 were conducted with a previously described apparatus (Ham et al., 2015; Wells and Ham, 2014). A brief description is provided here. Reactants were introduced and samples were withdrawn through a 6.4-mm Swagelok (Solon, OH) fitting attached to an 80 L Teflon®-film chamber. The chamber was filled with 50% relative humidity (RH) air (described above).
Table 1.
Compounds used for system calibration. Chromatographic retention time, structure and molecular weight and observed ions are listed.
| RT (min.) | Structure (name) | Derivatized M.W. | El ions (Rel.Intensity) |
|---|---|---|---|
| 12.4 |
 M.W. = 98 cyclohexanone |
169 | 41(58), 57(85), 67(30), 81(40), 96(45), 114(100), 170(65) |
| 20.5 20.7 21.0 |
M.W. = 100 glutaraldehyde |
242 | 41(55), 57(95), 68(35), 99(35), 113(100), 130(50), 186(18), 242(5), 243(5) |
Calibration plots were made by analyzing triplicate measurements of standard solutions that were injected into an 80 L Teflon® chamber at 50% RH, ranging in concentration from 5 to 30 ppb (1.2–7.4 × 1011 mol cm−3). Samples were obtained by pulling 60 L of air from the chamber using a pump (URG 3000-02Q, Chapel Hill, NC) into 25 mL of DI H2O in a 60 mL Teflon® impinger (Savillex, Eden Prairie, MN). After collection, samples were decanted into 40 mL vials, then derivatized with 100 μL aqueous 250 mM TBOX, and placed in a heated water bath at 70 °C for 2 h. After removing the vial from the water bath and allowing to cool to room temperature, 0.5 mL of toluene was added to the vial. The vial was then shaken for 30 s and allowed to separate into a toluene layer and aqueous layer. Then 100 μL of the toluene layer was then removed with a pipette and placed in a 2 mL autosampler vial with a 100 μL glass insert (Resetk, Bellefonte, PA). Then 1 μL of the extract was analyzed by gas chromatography-mass spectrometry (GC/MS) (conditions described below).
2.2.2. Limonene + O3 reactions
In an 80 L volume of air at 50% RH, O3 (20–100 ppb; 0.5–2.5 × 1012 molecule cm−3) was added to 1.7 ppm limonene (4.25 × 1013 molecule cm−3), and allowed to react for 30 min. After the reaction, 60 L of sample was collected into 25 mL of deionized water using an impinger, TBOX derivatized, extracted, and analyzed (as described above). Additional experiments included the addition of cyclohexane (CH) to the reaction mixture to scavenge OH• formed from Criegee intermediates of limonene ozonolysis (Aschmann et al., 2002; Carslaw, 2013; Criegee, 1975; Forester and Wells, 2009). NO’s effect on limonene ozonolysis with and without OH• was also investigated. The results from each of these experiments are described below. Each experiment was done in duplicate.
All samples were analyzed using a Varian (Palo Alto, CA) 3800/Saturn 2000 GC/MS system operated in the electron impact (EI) mode. Compound separation was achieved by an Agilent (Santa Clara, CA) HP-5MS (0.25 mm I.D., 30 m long, 0.25 μm film thickness) column and the following GC oven parameters: 40 °C for 2 min, then 5 °C min−1 to 200 °C, then 25 °C min−1 to 280 °C and held for 5 min. One μL of each sample was injected in the splitless mode, and the GC injector was returned to split mode 1 min after sample injection, with the following injector temperature parameters: 130 °C for 2 min then 200 °C min−1 to 300 °C and held for 10 min. The Saturn 2000 ion trap mass spectrometer was tuned using perfluorotributylamine (FC-43). Full-scan EI ionization spectra were collected from m/z 40–650.
3. Results
3.1. Cyclohexanone, glutaraldehyde calibration
The two carbonyls cyclohexanone (surrogate for singly derivatized LimaKet) and glutaraldehyde (surrogate for doubly derivatized 7H6O, IPOH, 2A5O, and 3A6O), see Table 1) were used for the calibration of all limonene + O3 reaction products, since standards of observed oxidation products were not readily available (Ham et al., 2015). The following retention times were observed: 12.4 min for singly derivatized cyclohexanone (MW = 169) and 20.5, 20.7, 21.0 min for doubly or triply derivatized glutaraldehyde (MW = 242). The limit of detection (determined from three times the standard deviation of the slope of the calibration curve for cyclohexanone and glutaraldehyde (all peaks summed) divided by the slope) for single and multi-carbonyls was 0.06, and 0.11 ppb, respectively.
Derivatization of nonsymmetric carbonyls using TBOX typically resulted in multiple chromatographic peaks due to stereoisomers of the oximes. Typically an M+1 ion was observed for the derivatized oxime compounds. Identification of multiple peaks of the same oxime compound is relatively simple since the mass spectra for each chromatographic peak of a particular oxime are almost identical. TBOX adds a mass of 71 to the molecular weight of each derivatized carbonyl. In most cases, the m/z = 57 ion relative intensity for the chromatographic peaks of the oximes was greater than 50% in the mass spectrum. This ion was attributable to the t-butyl group ( fragment) and could be effectively used to generate selected ion chromatograms to identify derivatized carbonyl compounds in a mixture. All molar yields were determined from the total ion chromatograms.
3.2. Limonene ozonolysis: observed reaction products
The five main products: limonaketone (LimaKet), 7-hydroxy-6-oxo-3-(prop-1-en-2yl)heptanal (7H6O), 3-isopropenyl-6-oxo-heptanal (IPOH), 2-acetyl-5-oxohexanal (2A5O), and 3-acetyl-6-oxoheptanal (3A6O) from limonene ozonolysis are listed below and shown in Table 2. All limonene ozonolysis products are predicted using the Master Chemical Mechanism v. 3.3.1 (Jenkin et al., 2015). Specific product yields determined from plots (See Figures S1–S3) for the reactions: limonene + O3, limonene + O3 + cyclohexane, and limonene + O3 + NO (20 ppb) are described below and results shown in Table 3. The errors reported in Table 3 were calculated by doubling the standard regression slope errors from the yield plots.
Table 2.
Reaction products observed from limonene ozonolysis. Chromatographic retention time, structure and molecular weight and observed ions are listed.
| RT (min) | Structure (name) | Derivatized (MW) | El ions (Rel.Intensity) |
|---|---|---|---|
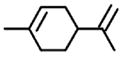 M.W. = 136 Limonene |
|||
| 18.6 |
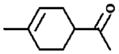 M.W. = 138 Limonaketone (LimaKet) |
208 | 41(10), 57 (12), 65(17), 108(18), 121(19), 136(14), 153(12), 209(17) |
| 23.7 24.1 |
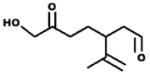 M.W. = 184 7-hydroxyl-6-oxo-3-(prop-1-en-2yl)heptanal (7H6O) |
255 | 41(40), 43(50), 57(80), 79(20), 107(55), 126(68), 139 (28), 182(18), 199(10), 256(1) |
| 27.0 27.3 27.6 |
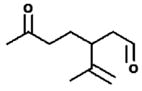 M.W. = 168 3-isopropenyl-6-oxo-heptanal (IPOH) |
310 | 41(50), 57(100), 73(28), 91(28), 107(34), 140(42), 166(40), 181(90), 198(60), 237(20), 254(30), 311(2) |
| 29.3 29.6 29.9 |
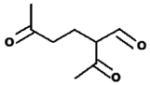 M.W. = 156 2-acetyl-5-oxohexanal (2A5O) |
369 | 41(28), 57(48), 116(35), 125(25), 169(35), 172(15), 184(22), 240(22), 370(8) |
| 31.5 31.7 31.9 32.0 |
 M.W. = 170 3-acetyl-6-oxoheptanal (3A6O) |
383 | 41(15), 57(25), 65(17), 94(10), 165(25), 183(15), 198(17), 254(16), 310(15), 384(2) |
Table 3.
Molar yields of reaction products from limonene ozonolysis under different experimental conditions. CH = cyclohexane (OH• radical scavenger).
| Experiment | Molar yields
|
||||
|---|---|---|---|---|---|
| LimaKet | 7H6O | IPOH | 2A5O | 3A6O | |
| Limonene + O3 | 0.0076 ± 0.0008 | 0.021 ± 0.001 | 0.160 ± 0.005 | 0.019 ± 0.003 | 0.015 ± 0.002 |
| Limonene + O3 + CH | 0.0081 ± 0.0009 | 0.019 ± 0.003 | 0.050 ± 0.008 | 0.016 ± 0.003 | X |
| Limonene + O3 (20 ppb NO) | 0.0052 ± 0.0005 | 0.008a | 0.11 ± 0.01 | 0.007a | 0.008a |
X –Indicates 3A6O was only observed in the Limonene + O3 (100 ppb) + CH experiment, no yield was measured.
Indicates yields based on data from 100 to 50 ppb O3 only just trace amounts of products were observed at 30 ppb O3.
3.2.1. Retention time 18.5 min: 4-acetyl-1-methylcyclohexene (limonaketone)(LimaKet)
The chromatographic peak for the oxime observed at 18.5 min was observed as a reaction product of limonene + O3 as seen in Fig. 1. The main ions (% relative peak height) are 41(10), 57(12), 65(17), 108(18), 121(19), 136(14), 153(12) and 209(17). If m/z = 210 is the M+1 ion, then a molecular weight of 138 (209−71 = 138) is expected for the carbonyl compound. Based on the ions observed, the proposed identity of this product is limonaketone (Hakola et al., 1994). The product yield determined using the cyclohexanone calibration curve as a surrogate was 0.0076 ± 0.0008 for limonene + O3, 0.0081 ± 0.0009 for limonene + O3 + CH, and 0.0052 ± 0.0005 for limonene + O3 + NO (see Table 3). Gas-phase yields of limonaketone in this investigation are significantly lower than those reported by Hakola et al., 1994, who reported yields of 0.20 ± 0.03 for the OH reaction and ≤0.04 for the O3 reaction using a GC-FID.
Fig. 1.
Chromatograms from limonene ozonolysis with and without the addition of NO. (A) With and without addition of cyclohexane (OH• scavenger). (B) With and without addition of nitric oxide (NO).
3.2.2. Retention time 23.7 and 24.1 min: 7-hydroxyl-6-oxo-3-(prop-1-en-2-yl)heptanal – (7H6O)
The chromatographic peaks for the oxime observed at 23.7 and 24.1 min were observed as a reaction product of limonene + O3 as seen in Fig. 1. The main ions (% relative peak height) are 41(40), 43(50) 57(80), 79(20), 107(55), 126(68), 139 (28), 182(18), 199(10), 256(1). If m/z = 256 is the M+1 ion, then a molecular weight of 184 (255−71 = 184) is expected for the carbonyl compound. Based on the ions observed, the proposed identity of this product is 7-hydroxyl-6-oxo-3-(prop-1-en-2-yl)heptanal. The product yield determined using the glutaraldehyde calibration curve as a surrogate was 0.021 ± 0.001 for limonene + O3, 0.019 ± 0.003 for limonene + O3 + CH, and 0.008 for limonene + O3 + NO (see Table 3).
3.2.3. Retention time 27.0, 27.3, 27.4 and 27.6 min: 3-isopropenyl-6-oxo-heptanal – (IPOH)
The chromatographic peaks for the oxime observed at 27.0, 27.3, 27.4 and 27.6 min was observed as a reaction product of limonene + O3 as seen in Fig. 1. The main ions (% relative peak height) are 41(50), 57(100), 73(28), 91(28), 107(34), 140(42), 166(40), 181(90), 198(60), 237(20), 254(30), 311(2). If m/z = 311 is the M+1 ion, then a molecular weight of 168 (310−71−71 = 168) is expected for a dicarbonyl compound. Based on the ions observed, the proposed identity of this product is 3-isopropenyl-6-heptanal (IPOH). Further confirmation of this product was made from previous gas-phase limonene + O3 studies (Hakola et al., 1994; Wells and Ham, 2014). The product yield determined using the glutaraldehyde calibration curve as a surrogate was 0.160 ± 0.005 for limonene + O3, 0.050 ± 0.008 for limonene + O3 + CH, and 0.11 ± 0.01 for limonene + O3 + NO (see Table 3). Gas-phase yields of IPOH in this investigation are higher than in previous studies. Investigations of limonene + O3 by Clausen et al. reported IPOH yields of 2–4% using GC-FID detection and Forester and Wells reported IPOH yields of 0.4% using GC/MS with PFBHA derivatization (Clausen et al., 2001; Forester and Wells, 2011).
3.2.4. Retention time 29.3, 29.6, 29.9 min: 2-acetyl-5-oxohexanal -(2A5O)
The chromatographic peaks for the oxime observed at 29.3, 29.6, and 29.9 min was observed as a reaction product of limonene + O3 as seen in Fig. 1. The main ions (% relative peak height) are 41(28), 57(48), 116(35), 125(25), 169(35), 172(15), 184(22), 240(22), 370(8), see Fig. 2. If m/z = 370 is the M+1 ion, then a molecular weight of 156 (369−71−71−71 = 156) is expected for a tricarbonyl compound. Based on the ions observed, the proposed identity of this product is 2-acetyl-5-oxohexanal. The product yield determined using the glutaraldehyde calibration curve as a surrogate was 0.019 ± 0.003 for limonene + O3, 0.016 ± 0.003 for limonene + O3 + CH, and 0.007 for limonene + O3 + NO (see Table 3). This oxidation product has not been observed previously.
Fig. 2.
Mass spectrum of 2-acetyl-5-oxohexanal (2A5O).
3.2.5. Retention time 31.5, 31.7, 31.9 and 32.0 min: 3-acetyl-6-oxoheptanal – (3A6O)
The chromatographic peaks for the oxime observed at 31.5, 31.7, 31.9 and 32.0 min was observed as a reaction product of limonene + O3 as seen in Fig. 1. The main ions (% relative peak height) are 41(15), 57(25), 65(17), 94(10), 165(25), 183(15), 198(17), 254(16), 310(15), 384(2). If m/z = 384 is the M+1 ion, then a molecular weight of 170 (383−71−71−71 = 170) is expected for a tri-carbonyl compound. Based on the ions observed, the proposed identity of this product is 3-acetyl-6-oxoheptanal (Wells and Ham, 2014). The product yield determined using the glutaraldehyde calibration curve as a surrogate was 0.015 ± 0.002 for limonene + O3 and 0.008 for limonene + O3 + NO. (see Table 3). This product was not observed in the limonene + O3 + CH experiments.
4. Discussion
As stated earlier, the ozonolysis of limonene has been extensively studied using a variety of analytical techniques (Hakola et al., 1994; Larsen et al., 2001; Leungsakul et al., 2005; Wells and Ham, 2014). Ozone can react with limonene via addition to either the endocyclic or exocyclic carbon-carbon double bonds with calculated rate constants (AOPWIN v.1.92a) of 43 and 1.2 × 10−17 cm3 molecule −1 s−1, respectively (EPA, 2000). These numbers suggest that the endocyclic O3 addition is favored by about 35 to 1 over the exocyclic O3 addition.
When O3 adds to either of these double bonds, a primary ozonide is formed which subsequently decomposes to a Criegee intermediate (Aschmann et al., 2002; Atkinson et al., 1992; Criegee, 1975; Forester and Wells, 2011). Further unimolecular decay of the Criegee intermediate may then lead to formation of OH• which can further react with excess limonene or newly formed reaction products. The OH• yield from ozonolysis of limonene has been reported to be 64–80% (Aschmann et al., 2002; Forester and Wells, 2009; Herrmann et al., 2010). The OH• can add to double bonds (similarly to O3) and/or abstract available hydrogens leading to product formation. The calculated OH• rate constants for reaction with limonene from the endocyclic double bond and the exocyclic double bond obtained from AOPWIN are (in units of 10−12 cm3 molecule −1 s−1) 86.9 and 51.4, respectively. To determine which oxidation products were formed via ozonolysis alone and/or a combination of OH• and O3, the hydroxyl radical scavenger (cyclohexane) was added to the system. The effect of OH• on the formation of each product is described below.
4.1. Limonene + O3 and OH• reaction products and yields
Previous investigations of limonene ozonolysis have reported two of the reaction products (LimaKet, IPOH) shown in the current investigation (Clausen et al., 2001; Forester and Wells, 2011; Hakola et al., 1994). Moreover, use of TBOX as the derivatization agent has afforded the observance of three additional oxidation products that have been predicted through models such as the MCM. A proposed reaction mechanism based on the data is described below.
LimaKet, 7H6O, and 2A5O are primary oxidation products formed only from the limonene + O3 reaction, and not from secondary OH• formation as observed in Fig. 3 and Table 3. When OH• are scavenged, no statistically significant effects (7, 10, and 15% change, respectively) based on molar yields were observed, indicating that OH• is not influential in their formation. However, the formations of IPOH and 3A6O were strongly dependent on the presence of OH•. When OH• was scavenged, the molar yields decreased by 68% and >95%, respectively. This data further emphasizes the importance of OH• chemistry and how it strongly influences indoor reactions.
Fig. 3.
Concentrations of observed reaction products after addition of NO and cyclohexane (CH) to scavenge OH radicals. IPOH has been omitted from figure for clarity.
The mechanisms for the formation of LimaKet, IPOH, and 3A6O from limonene ozonolysis have been previously discussed (Carslaw, 2013; Lee et al., 2006; Wells and Ham, 2014). A mechanism for their formation can be found in the Supplementary Information, Figure S4. The ozonolysis product, 7H6O, is likely formed via ozone addition to the endocyclic carbon-carbon double bond, formation of a primary ozonide, and then subsequent cleavage to form the radical LIMOOA, as seen in the MCM. Subsequent decomposition of LIMOOA leads to the dicarbonyl peroxyl radical (LIMALBO2) which reacts with an alkoxyl radical to form the stable oxidation product, 7H6O.
The ozonolysis product, 2A5O, can be formed through multiple mechanisms as detailed in the MCM v.3.3.1. One mechanism (Figure S4) may involve initial ozone addition to the exocyclic double bond to form LimaKet, as previously described (Hakola et al., 1994; Wells and Ham, 2014). Subsequent, ozone addition to the endocyclic double bond of LimaKet, followed by cleavage of the primary ozonide, leads to the radical CH3C(= O)CH2CH2CH(C(= O) CH3)CH2C·(OO•)H. The reaction then likely proceeds via hydrogen abstraction of the adjacent CH2 and decomposition to form CH3C(= O)CH2CH2CH(C(= O)CH3)CH2• and CH2·OOH. The larger radical adds O2 and stabilizes to form the tricarbonyl, 2A5O. Although this proposed mechanism does explain the formation of 2A5O through plausible steps, secondary addition of ozone to LimaKet seems unlikely due to the low concentration of ozone used in these experiments. Alternatively, it is possible that the reaction proceeds through initial O3 addition to the endocyclic bond of limonene to form an intermediate similar to IPOH. This intermediate then reacts with another O3 molecule to form the tricarbonyl. While the mechanism for 2A5O is not clear, the observation of this product highlights the formation of tricarbonyl species.
4.2. Nitric oxide (NO) effect
When NO is added to the limonene + O3 reaction system, there are several pathways for NO to affect the reaction system. NO can react with O3 leading to NO2 with a rate constant of kO3+NO = 2 × 10−14 cm3 molecule−1 s−1 (Wallace et al., 1980). NO can also react with peroxyl radicals (RO2•) generated from OH• and/or O3 reactions to form stabilized carbonyls and/or organic nitrates (Finlayson-Pitts and Pitts, 2000). These reactions are typically fast with a rate constant of kRO2• +NO = 7 × 10−12 cm3 molecule−1 s−1 (Finlayson-Pitts and Pitts, 2000).
Addition of NO (20, 50, and 100 ppb) to the limonene + O3 (50 ppb) system had no statistical effect in the formation of LimaKet or IPOH, Fig. 3. However, when NO is held constant (20 ppb) and O3 was increased (30, 50, and 100 ppb), the molar yields of both LimaKet and IPOH decreased by 31.6% and 31.2%, respectively (Table 3). As O3 was increased, the ratio of RO2•/NO also increased which affected the rate of the RO2• + NO reaction leading to the formation of organic nitrates. The addition of NO had a more significant effect on 7H6O, 2A5O, and 3A6O. A decrease in their formation was observed with the addition of NO (20, 50, and 100 ppb) to the limonene + O3 (50 ppb) system, Fig. 3. Furthermore, when NO is held constant (20 ppb) and O3 was increased (30, 50, and 100 ppb), the molar yields of 7H6O, 2A5O, and 3A6O decreased by 61.9%, 63.3%, and 46.7%, respectively, Table 3. This suggests that peroxyl radical intermediates likely react with NO to form organic nitrates.
Interestingly, 7H6O and 2A5O (Fig. 3) increase when cyclohexane is added in the presence of NO. This may be explained by the side reaction of NO with cyclohexylperoxyl (CHRO2•) radicals. When OH• is scavenged by cyclohexane, CH• can be formed, which then reacts in a diffusion-controlled manner with O2 to form CHO2• (Finlayson-Pitts and Pitts, 2000; Neuenschwander et al., 2010). These CHO2• effectively out-compete RO2• that form 7H6O and 2A5O for reaction with NO, resulting in higher yields of these two products.
5. Conclusion
Limonene ozonolysis with and without addition of NO and cyclohexane (OH• scavenger) was studied using the new derivatization agent, O-tertbutylhydroxylamine hydrochloride (TBOX). The molar yields of the observed single, di- and tricarbonyl reaction products (LimaKet, 76HO, IPOH, 2A5O, and 3A6O) from limonene + O3, limonene + O3 + cyclohexane, and limonene + O3 + NO experiments were also determined. The scavenging of secondary OH• reduced the yields of IPOH and 3A6O highlighting the significance of OH•’s role in the overall limonene oxidation. In the presence of NO, the molar yields for LimaKet and IPOH were not significantly affected; however, the yields of 7H6O, 2A5O, and 3A6O were reduced by > 45% suggesting other possible routes in forming undetected carbonyls or organic nitrates. These studies further highlight the importance NO has on the formation of oxidation products in the gas phase. Future investigations will include the addition of NO2 to study its effect on the ozonolysis of both single and mixtures of terpenes.
Supplementary Material
HIGHLIGHTS.
Aqueous collection and derivatization of gas-phase limonene ozonolysis products.
Multi-functional gas-phase carbonyls detected from limonene ozonolysis.
Hydroxyl radical’s and nitric oxide’s influence on reaction product formation.
Acknowledgments
This work was supported by US Government appropriations.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.atmosenv.2016.03.003.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Alvarez EG, Amedro D, Afif C, Gligorovski S, Schoemacker C, Fittschen C, Doussin JF, Wortham H. Unexpectedly high indoor hydroxyl radical concentrations associated with nitrous acid. Proc Natl Acad Sci U S A. 2013;110:13294–13299. doi: 10.1073/pnas.1308310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschmann SM, Arey J, Atkinson R. OH radical formation from the gas-phase reactions of O3 with a series of terpenes. Atmos Environ. 2002;36:4347–4355. [Google Scholar]
- Atkinson R, Arey J. Atmospheric degradation of volatile organic compounds. Chem Rev. 2003;103:4605–4638. doi: 10.1021/cr0206420. [DOI] [PubMed] [Google Scholar]
- Atkinson R, Aschmann SM, Arey J, Shorees B. Formation of OH radicals in the gas phase reactions of O3 with a series of terpenes. J Geophys Res. 1992;97:6065–6073. [Google Scholar]
- Carslaw N. A mechanistic study of limonene oxidation products and pathways following cleaning activities. Atmos Environ. 2013;80:507–513. [Google Scholar]
- Clausen PA, Wilkins CK, Wolkoff P, Nielsen GD. Chemical and biological evaluation of a reaction mixture of R-(+)-limonene/ozone: formation of strong airway irritants. Environ Int. 2001;26:511–522. doi: 10.1016/s0160-4120(01)00035-6. [DOI] [PubMed] [Google Scholar]
- Criegee R. Mechanism of ozonolysis. Angew Chem Int Ed Engl. 1975;14:745–752. [Google Scholar]
- Donahue NM, Robinson AL, Trump ER, Riipinen I, Kroll JH. Volatility and aging of atmospheric organic aerosol. Top Curr Chem. 2014:97–144. doi: 10.1007/128_2012_355. [DOI] [PubMed] [Google Scholar]
- Ebben CJ, Shrestha M, Martinez IS, Corrigan AL, Frossard AA, Song WW, Worton DR, Petäjä T, Williams J, Russell LM, Kulmala M, Goldstein AH, Artaxo P, Martin ST, Thomson RJ, Geiger FM. Organic constituents on the surfaces of aerosol particles from Southern Finland, Amazonia, and California Studied by vibrational sum frequency generation. J Phys Chem A. 2012;116:8271–8290. doi: 10.1021/jp302631z. [DOI] [PubMed] [Google Scholar]
- EPA. AOPWIN v1.91, AOPWIN 1.91. U.S. Environmental Protection Agency; Washington D.C: 2000. 1.91. http://www.epa.gov/oppt/exposure/pubs/episuite.htm. [Google Scholar]
- Finlayson-Pitts BJ, Pitts JJN. Chemistry of the Upper and Lower Atmosphere. Academic Press; New York: 2000. [Google Scholar]
- Forester CD, Wells JR. Yields of carbonyl products from gas-phase reactions of fragrance compounds with OH radical and ozone. Environ Sci Technol. 2009;43:3561–3568. doi: 10.1021/es803465v. [DOI] [PubMed] [Google Scholar]
- Forester CD, Wells JR. Hydroxyl radical yields from reactions of terpene mixtures with ozone. Indoor Air. 2011;21:400–409. doi: 10.1111/j.1600-0668.2011.00718.x. [DOI] [PubMed] [Google Scholar]
- Hakola H, Arey J, Aschmann SM, Atkinson R. Product formation from the gas-phase reactions of OH radicals and O3 with a series of monoterpenes. J Atmos Chem. 1994;18:75–102. [Google Scholar]
- Ham JE, Jackson SR, Harrison JC, Wells JR. Gas-phase reaction products and yields of terpinolene with ozone and nitric oxide using a new derivatization agent. Atmos Environ. 2015;122:513–520. doi: 10.1016/j.atmosenv.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann F, Winterhalter R, Moortgat GK, Williams J. Hydroxyl radical (OH) yields from the ozonolysis of both double bonds for five monoterpenes. Atmos Environ. 2010;44:3458–3464. [Google Scholar]
- HHS/NIH. Household Products Database: D-limonene. National Library of Medicine. National Institute of Health; 2015. http://householdproducts.nlm.nih.gov/cgi-bin/household/brands?tbl=chem&id=166. [Google Scholar]
- Jenkin ME, Young JC, Rickard AR. The MCM v3.3.1 degradation scheme for isoprene. Atmos Chem Phys. 2015;15:11433–11459. [Google Scholar]
- Jiang L, Xu Y, Yin B, Bai Z. Theoretical study on the reaction mechanism of ozone addition to the double bonds of keto-limonene. J Environ Sci. 2012;24:147–151. doi: 10.1016/s1001-0742(11)60738-9. [DOI] [PubMed] [Google Scholar]
- Larsen BR, Di Bella D, Glasius M, Winterhalter R, Jensen NR, Hjorth J. Gas-phase OH oxidation of monoterpenes: gaseous and particulate products. J Atmos Chem. 2001;38:231–276. [Google Scholar]
- Lee A, Goldstein AH, Keywood MD, Gao S, Varutbangkul V, Bahreini R, Ng NL, Flagan RC, Seinfeld JH. Gas-phase products and secondary aerosol yields from the ozonolysis of ten different terpenes. J Geophys Res Atmos. 2006:111. [Google Scholar]
- Leungsakul S, Jaoui M, Kamens RM. Kinetic mechanism for predicting secondary organic aerosol formation from the reaction of d-limonene with ozone. Environ Sci Technol. 2005;39:9583–9594. doi: 10.1021/es0492687. [DOI] [PubMed] [Google Scholar]
- Nazaroff WW, Cass GR. Mathematical modeling of chemically reactive pollutants in indoor air. Environ Sci Technol. 1986;20:924–934. doi: 10.1021/es00151a012. [DOI] [PubMed] [Google Scholar]
- Nazaroff WW, Weschler CJ. Cleaning products and air fresheners: exposure to primary and secondary air pollutants. Atmos Environ. 2004;38:2841–2865. [Google Scholar]
- Neuenschwander U, Guignard F, Hermans I. Mechanism of the aerobic oxidation of α-Pinene. ChemSusChem. 2010;3:75–84. doi: 10.1002/cssc.200900228. [DOI] [PubMed] [Google Scholar]
- Norgaard AW, Vibenholt A, Benassi M, Clausen PA, Wolkoff P. Study of ozone-initiated limonene reaction products by low temperature plasma ionization mass spectrometry. J Am Soc Mass Spectrom. 2013;24:1090–1096. doi: 10.1007/s13361-013-0648-3. [DOI] [PubMed] [Google Scholar]
- Pan X, Underwood JS, Xing JH, Mang SA, Nizkorodov SA. Photo-degradation of secondary organic aerosol generated from limonene oxidation by ozone studied with chemical ionization mass spectrometry. Atmos Chem Phys. 2009;9:3851–3865. [Google Scholar]
- Pathak RK, Salo K, Emanuelsson EU, Cai C, Lutz A, Hallquist AM, Hallquist M. Influence of ozone and radical chemistry on limonene organic aerosol production and thermal characteristics. Environ Sci Technol. 2012a;46:11660–11669. doi: 10.1021/es301750r. [DOI] [PubMed] [Google Scholar]
- Pathak RK, Salo K, Emanuelsson EU, Cai C, Lutz A, Hallquist ÅM, Hallquist M. Influence of ozone and radical chemistry on limonene organic aerosol production and thermal characteristics. Environ Sci Technol. 2012b;46:11660–11669. doi: 10.1021/es301750r. [DOI] [PubMed] [Google Scholar]
- Sarwar G, Corsi R, Kimura Y, Allen D, Weschler CJ. Hydroxyl radicals in indoor environments. Atmos Environ. 2002;36:3973–3988. [Google Scholar]
- Singer BC, Coleman BK, Destaillats H, Hodgson AT, Lunden MM, Weschler CJ, Nazaroff WW. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmos Environ. 2006;40:6696–6710. doi: 10.1021/es052198z. [DOI] [PubMed] [Google Scholar]
- Wallace JS, Springer GS, Stedman DH. Photochemical ozone and nitric oxide formation in air-nitrogen dioxide mixtures containing sulfur dioxide or chlorine. Atmos Environ (1967) 1980;14:1147–1157. doi: 10.1016/0004-6981(80)90179-1. [DOI] [PubMed] [Google Scholar]
- Waring MS, Wells JR. Volatile organic compound conversion by ozone, hydroxyl radicals, and nitrate radicals in residential indoor air: magnitudes and impacts of oxidant sources. Atmos Environ. 2015;106:382–391. doi: 10.1016/j.atmosenv.2014.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JR, Ham JE. A new agent for derivatizing carbonyl species used to investigate limonene ozonolysis. Atmos Environ. 2014;99:519–526. doi: 10.1016/j.atmosenv.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler CJ, Shields HC. Potential reactions among indoor pollutants. Atmos Environ. 1997;31:3487–3495. [Google Scholar]
- Weschler CJ, Shields HC, Naik DV. Indoor chemistry involving O3, NO, and NO2 as evidenced by 14 months of measurements at a site in Southern California. Environ Sci Technol. 1994;28:2120–2132. doi: 10.1021/es00061a021. [DOI] [PubMed] [Google Scholar]
- Wilson AL, Colome SD, Tian Y, Becker EW, Baker PE, Behrens DW, Billick IH, Garrison CA. California residential air exchange rates and residence volumes. J Expo Anal Environ Epidemiol. 1996;6:311–326. [PubMed] [Google Scholar]
- Youssefi S, Waring MS. Transient secondary organic aerosol formation from limonene ozonolysis in indoor environments: impacts of air exchange rates and initial concentration ratios. Environ Sci Technol. 2014;48:7899–7908. doi: 10.1021/es5009906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





