Abstract
Purpose
To compare self-reported quality of life (QOL) in patients who did versus did not undergo interval secondary cytoreduction after initial surgery and combination chemotherapy for advanced ovarian cancer and to assess the association between baseline QOL scores and survival.
Patients and Methods
Consenting patients participating in a Gynecologic Oncology Group (GOG) phase III treatment trial (GOG 152) completed the Functional Assessment of Cancer Therapy–Ovarian (FACT-O) questionnaire and treatment-specific supplemental questions at the third and sixth chemotherapy cycles and at 6 and 12 months after starting treatment.
Results
For all patients, QOL decreased approximately 1 unit from the first to second assessment. Significant improvement observed at 6 months (P < .001) was sustained at 12 months, with no appreciable between-group difference. The baseline FACT-O score was associated with overall survival (P = .048) but not progression-free survival. Less neurotoxicity was reported among patients who did (38.4%) versus did not (54.0%) undergo interval secondary cytoreduction at the third assessment (P = .005), and older patients experienced more long-term effects.
Conclusion
This is the first multicenter randomized trial in ovarian cancer to longitudinally examine self-reported QOL and establish a predictive value of baseline QOL on survival, attributed primarily to the lowest-scoring quartile. Although interval secondary cytoreduction resulted in no notable long-term difference, a clinically significant improvement was seen in both arms at 6 and 12 months after starting therapy. Interestingly, there were fewer complaints of neurotoxicity at 6 months among patients who did versus did not undergo interval secondary cytoreduction.
INTRODUCTION
Several previous trials have suggested that standard management of advanced ovarian cancer should include initial surgery for staging and debulking of gross tumor, followed by combination chemotherapy with a platinum agent and a taxane.1–3 These studies were primarily concerned with traditional outcomes including tumor response, survival, and physician-rated toxicity. Because of the complex relationship between treatment efficacy and toxicity and the diverse assumptions and expectations for treatment held by patients with cancer, the comprehensive measurement of health status has become an important and appropriate component of many clinical trials. Therefore, outcome evaluation in oncology research has evolved to include a broader, multidimensional quantification of patient functional status, termed quality of life (QOL).4–6
The benefit of interval secondary cytoreduction for suboptimally debulked epithelial ovarian cancer patients is controversial. To prospectively evaluate its clinical impact, the Gynecologic Oncology Group (GOG) conducted a randomized trial (GOG 152) comparing cisplatin (75 mg/m2) and paclitaxel (135 mg/m2) with or without interval secondary cytoreduction including, as a secondary end point, the effect of treatment on patient-reported QOL, which is the subject of this report.
Treatment results of GOG 152 report a median tumor diameter of 2 cm at the time of secondary surgery. Ninety-three percent of patients who were randomly assigned to receive interval secondary cytoreduction actually underwent the procedure. The majority of patients (93% of patients who underwent surgery and 98% of patients who did not undergo surgery) received three additional cycles of chemotherapy. In patients who did receive interval secondary cytoreduction, median time to progression or death and median survival time were 10.5 and 33.9 months, respectively, compared with 10.7 and 33.7 months, respectively, in patients who did not receive interval secondary cytoreduction; thus, secondary surgery did not appreciably increase overall survival after initial maximal surgical cytoreduction. Adverse effects were similar in the two groups, although 16% of patients in the secondary surgery group versus 26% of patients in the no surgery group experienced grade 2 or higher peripheral neuropathy. These results were in contrast to an earlier published trial in which increases in median progression-free survival (PFS) and overall survival were observed with chemotherapy plus interval secondary cytoreduction; however, the authors suggest that the differences could be related to use of dissimilar chemotherapy agents, differing degrees of surgical aggressiveness, or amount of residual disease after primary surgery.6A
Because psychological, physical, and functional disruptions are prominent and frequently enduring,7–9 QOL has been examined in several single-institution studies of patients with advanced ovarian cancer. This literature points out the need to prospectively examine QOL concurrently with treatment to best determine which therapies are most likely to provide meaningful extensions of life. Prospective studies of patients with breast, lung, and mixed advanced cancers indicate that QOL and performance status (PS) can be prognostic.10–13 At the time of this report, these relationships had not been examined in patients with advanced ovarian cancer. To our knowledge, this is the first multicenter study to longitudinally examine QOL as a potential predictor of survival in this patient population. This predictor of survival, examined in a large homogeneous ovarian cancer cohort, can provide valuable information for treatment planning and assessment of relative treatment benefit in future trials.
PATIENTS AND METHODS
The institutional review boards of participating institutions reviewed and approved the treatment and QOL components of the protocol before enrolling patients. All patients provided written informed consent consistent with federal, state, and local requirements before receiving protocol therapy.
Patients and Protocol Treatment
Patients with stage III or IV ovarian carcinoma (confirmed by central pathology review) who had residual tumor exceeding 1 cm in diameter after having received maximal debulking surgery were eligible if they met all other criteria including physician-rated PS of 0 (fully functional) to 2 (requiring rest up to 50% of the day) according to GOG criteria; life expectancy of at least 8 weeks; no history of prior cancer, chemotherapy, or radiotherapy; no infection, hepatitis, or gastrointestinal bleeding; no history of congestive heart failure, myocardial infarction, unstable angina, or abnormal cardiac conduction within the previous 6 months; and laboratory values as described in the protocol. After primary surgical debulking and three cycles of systemic cisplatin (75 mg/m2 intravenous) and paclitaxel (135 mg/m2 intravenous) chemotherapy, patients were randomly allocated to receive either three additional cycles of chemotherapy or a second surgical cytoreduction plus three additional cycles of chemotherapy.14
QOL Assessments
Patients were asked to complete QOL questionnaires after three cycles of chemotherapy, just before random allocation to interval secondary cytoreduction or no interval secondary cytoreduction (midtreatment baseline, first assessment), at the sixth cycle of chemotherapy (second assessment), at 6 months from start of treatment (third assessment), and at 12 months from start of treatment (fourth assessment). The questionnaire was administered by the QOL liaison at each participating institution. An assessment cover sheet, specifying completeness or reasons for a missed assessment, was completed at each scheduled time point by the liaison. Reasons for a missed assessment included institutional error, patient refusal, patient illness, or other.
QOL Measures
QOL was measured using version 2 of the Functional Assessment of Cancer Therapy–Ovarian (FACT-O) instrument, which is a multidimensional questionnaire developed and validated for use by ovarian cancer patients. This version of FACT-O consists of a 33-item Functional Assessment of Cancer Therapy–General (FACT-G) questionnaire, which is targeted to cancer patients generally, and 12 questions specific to issues faced by ovarian cancer patients.15,16
Version 2 of the FACT-G questionnaire includes five sub-scales (physical well-being, social well-being, emotional well-being, relationship with doctor, and functional well-being) that can be analyzed separately or aggregated to produce a total QOL score. The FACT-G has demonstrated reliability, validity, and responsiveness to change over time.17
Twelve items added to the FACT-G reflect issues specific to ovarian cancer. Our initial examination of the FACT-O subscale indicated that the ovarian cancer sample was comparable to the initial FACT-G standardization sample.18 In a later study validating the FACT-O subscale, patients received a second questionnaire either 1 week after baseline to assess the instrument’s test-retest reliability or 2 months after baseline to evaluate its sensitivity to a change in PS. Internal consistency and test-retest reliability of the FACT-O subscale were good.15 The scale correlated with other measures as expected, and all correlations were in the hypothesized direction. In addition, the FACT-O has been found to be sensitive to improvements in QOL experienced by patients responding to platinum and taxane therapy.15,16 An additional five items (GOG supplemental questions) related to incision (three items), numbness and/or tingling (one item), and weight change (one item) were added to assess treatment-specific concerns in this patient population, approximately half of whom would be randomly allocated to receive interval secondary cytoreduction.
The FACT-G, FACT-O subscale, and GOG supplemental questions were scored using a 5-point scale (0 = not at all, 1 = a little bit, 2 = somewhat, 3 = quite a bit, and 4 = very much). Five weighting items (the summary question from each subscale) and the relationship with doctor subscale were not included in the scoring to be consistent with the current FACT-G (version 4). Thus, 26 items were used to calculate the FACT-G score. The scores for the FACT-G and FACT-O subscales and GOG supplemental questions were computed if more than 50% of the subscale items were answered. A subscale score Si with Ni items was calculated as:
where δij is equal to 1 when the jth item has a valid response; otherwise, it is equal to 0. sij is the score of the jth item. The total FACT-G score is the sum of the subscale scores and is considered valid if at least 80% of the items are completed. The maximum scores of FACT-G, FACT-O subscale, and the GOG supplemental subscale are 104, 48, and 20, respectively. Higher scores are associated with better QOL.
Statistical Analysis
The primary QOL objective of this study was to determine whether combined QOL scores as assessed by the FACT-G, FACT-O subscale, and the GOG supplemental subscale changed longitudinally by randomized treatment. A sample size of 400 patients (200 in each arm) was estimated based on the clinical objective. The sample size calculation assumed a 10% mortality rate after 6 months and provided a 90% chance to detect a 6.1-unit difference in the total FACT-G score between treatment groups at a significance level of P = .017 (adjusted from P = .05 because of three planned comparisons).
For purposes of all calculations, midtreatment baseline was defined as the time of randomization. Descriptive statistics were calculated for the FACT-G, FACT-O subscale, and GOG supplemental questions. A mixed linear model with restricted maximum likelihood estimation method and unstructured covariance matrix was fitted for follow-up QOL scores. Because the intervals of two successive time points are not equal, the assessment time points are considered as categoric variables in the model. The interaction between treatment and time points was examined for the constant effect of the interval secondary cytoreduction over time (FACT-G, FACT-O subscale, and GOG supplemental questions).19 The statistical significance level was set at the P = .017 level for each of three measures to control the overall probability of a type I error at the 5% level.
Cox proportional hazard models were fitted with the midtreatment baseline FACT-O score and PS separately to assess their relationship to PFS and overall survival,20 and ties in the failure times were handled with the approximate likelihood of Efron. PFS and overall survival were measured from the date of randomization. PFS was defined as the minimum amount of time until clinical progression, death, or date of last contact. Overall survival was measured to the date of death or, for patients still alive, to the date of last contact.
RESULTS
Patients
Between June 1994 and January 2001, 424 eligible patients were enrolled onto GOG 152. Patient characteristics are listed in Table 1. As indicated, the majority of patients were non-Hispanic white, between the ages of 50 and 69 years, and with stage III disease. The two arms were fairly well balanced with respect to patient characteristics.
Table 1.
Patient Characteristics
| Characteristic | Regimen | |||
|---|---|---|---|---|
|
| ||||
| Interval Secondary Cytoreduction (n = 216) | No Interval Secondary Cytoreduction (n = 208) | |||
|
|
|
|||
| No. of Patients | % | No. of Patients | % | |
| Age | ||||
| <40 years | 11 | 5.1 | 14 | 6.7 |
| 40–49 years | 42 | 19.4 | 38 | 18.3 |
| 50–59 years | 68 | 31.5 | 71 | 34.0 |
| 60–69 years | 60 | 27.8 | 59 | 28.2 |
| ≥70 years | 35 | 16.2 | 26 | 12.4 |
|
| ||||
| Race | ||||
| Black | 10 | 4.6 | 14 | 6.7 |
| White | 198 | 91.7 | 183 | 88.0 |
| Hispanic | 5 | 2.3 | 6 | 2.9 |
| Other | 3 | 1.4 | 5 | 2.4 |
|
| ||||
| Performance status | ||||
| 0 | 83 | 38.4 | 83 | 39.7 |
| 1 | 119 | 55.1 | 108 | 51.9 |
| 2 | 14 | 6.5 | 17 | 8.1 |
|
| ||||
| Cell type | ||||
| Serous adenocarcinoma | 165 | 76.4 | 159 | 76.4 |
| Endometrioid adenocarcinoma | 17 | 7.9 | 11 | 5.3 |
| Mixed epithelial | 20 | 9.3 | 17 | 8.2 |
| Other | 14 | 6.5 | 21 | 10.1 |
|
| ||||
| Measurable disease | 152 | 70.4 | 145 | 69.7 |
|
| ||||
| Stage | ||||
| III | 200 | 92.6 | 200 | 96.2 |
| IV | 16 | 7.4 | 8 | 3.8 |
QOL Completion
All patients who were alive at the scheduled time of assessment were expected to complete the QOL questionnaires and are used as the base in computing the completion rates (Table 2). The initial questionnaire completion rate was acceptable, with 380 (90%) of 424 patients completing the midtreatment baseline assessment; however, four of the questionnaires were completed after the interval secondary cytoreduction and, thus, were excluded from the analyses. Subsequent completion rates remained relatively high, with 349 (83%) of 422 patients, 345 (83%) of 417 patients, and 308 (80%) of 386 patients completing questionnaires at the second, third, and fourth assessment points, respectively. However, completion rates were lower among patients who underwent interval secondary cytoreduction compared with patients who did not (Table 2), especially at the second assessment point (77% v 89%, respectively; χ2 = 13.4; P < .001). Missing data were mainly a result of institutional error.
Table 2.
Quality-of-Life Questionnaire Completion Rates
| Factor | Interval Secondary Cytoreduction | No Interval Secondary Cytoreduction | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||
| Assessment Time Point | Assessment Time Point | |||||||||||||||
|
|
|
|||||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Assessments expected | 216 | 213 | 209 | 189 | 208 | 208 | 207 | 196 | ||||||||
|
| ||||||||||||||||
| Patient deaths | 0 | 3 | 4 | 20 | 0 | 0 | 1 | 11 | ||||||||
|
| ||||||||||||||||
| Assessments received | 190* | 88 | 163 | 77 | 170 | 81 | 144 | 76 | 190 | 91 | 186 | 89 | 175 | 85 | 164 | 84 |
|
| ||||||||||||||||
| Reason for missing assessments | ||||||||||||||||
| Patient illness | 3 | 1 | 1 | 1 | 2 | 1 | 4 | 2 | 1 | 1 | 0 | 0 | 3 | 1 | 4 | 2 |
| Patient refusal | 6 | 3 | 10 | 5 | 11 | 5 | 9 | 5 | 8 | 4 | 5 | 2 | 7 | 3 | 5 | 3 |
| Institutional error | 15 | 7 | 31 | 15 | 18 | 9 | 16 | 8 | 8 | 4 | 11 | 5 | 14 | 7 | 11 | 6 |
| Patient off study | 0 | 0 | 2 | 1 | 1 | 1 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 |
| Other | 2 | 1 | 6 | 3 | 7 | 3 | 13 | 7 | 1 | 1 | 6 | 3 | 8 | 4 | 10 | 5 |
Four patients assessed after interval secondary cytoreduction.
The date ranges for assessment time points were −12 to 0 days at randomization, −7 to +4 days at the sixth cycle, −38 to +60 days at month 6, and −47 to +78 days at month 12. Although the time window widened in the later assessments, the smoothing plot review indicated no consistent association between the variation in the assessment time and variation in the QOL score.
Description of QOL at Randomization
Means and standard deviations (SDs) of the scores are used to describe patient QOL at a given time point. At randomization, both arms reported similar mean scores in all QOL outcomes (Table 3). After adjusting for patient age and PS, a fitted general linear model estimate indicated that none of the differences between the two arms were statistically significant in terms of midtreatment baseline QOL outcomes.
Table 3.
Quality-of-Life Scores at Each Assessment Point
| Assessment Point | Interval Secondary Cytoreduction | No Interval Secondary Cytoreduction | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean Score | SD | Mean Score | SD | |||
| Assessment 1, third chemotherapy cycle | ||||||
| No. of patients | 186 | 190 | ||||
| FACT-G | 78.0 | 14.4 | 79.1 | 13.0 | ||
| Physical well-being subscale | 20.8 | 5.8 | 21.0 | 5.3 | ||
| Social well-being subscale | 23.9 | 4.0 | 24.6 | 3.4 | ||
| Emotional well-being subscale | 15.3 | 3.4 | 15.7 | 3.5 | ||
| Functional well-being subscale | 18.0 | 6.0 | 17.8 | 5.3 | ||
| FACT-O subscale | 35.2 | 5.7 | 35.1 | 5.3 | ||
| GOG supplemental questions | 18.2 | 2.0 | 17.7 | 2.4 | ||
|
| ||||||
| Assessment 2, sixth chemotherapy cycle | ||||||
| No. of patients | 163 | 186 | ||||
| FACT-G | 77.0 | 14.2 | 77.9 | 14.3 | ||
| Physical well-being subscale | 20.2 | 5.3 | 20.1 | 6.0 | ||
| Social well-being subscale | 23.3 | 4.3 | 23.9 | 3.4 | ||
| Emotional well-being subscale | 15.8 | 3.5 | 15.7 | 3.5 | ||
| Functional well-being subscale | 17.7 | 5.8 | 18.2 | 5.5 | ||
| FACT-O subscale | 35.1 | 5.4 | 35.4 | 6.2 | ||
| GOG supplemental questions | 17.4 | 2.6 | 17.2 | 2.4 | ||
|
| ||||||
| Assessment 3, 6 months after starting treatment | ||||||
| No. of patients | 170 | 175 | ||||
| FACT-G | 83.8 | 14.6 | 84.8 | 12.6 | ||
| Physical well-being subscale | 23.0 | 4.7 | 22.9 | 4.4 | ||
| Social well-being subscale | 24.2 | 3.7 | 24.5 | 3.7 | ||
| Emotional well-being subscale | 15.9 | 4.0 | 16.5 | 3.3 | ||
| Functional well-being subscale | 20.6 | 5.8 | 20.8 | 5.2 | ||
| FACT-O subscale | 37.9 | 5.5 | 37.9 | 5.4 | ||
| GOG supplemental questions | 16.5 | 2.8 | 15.9 | 2.8 | ||
|
| ||||||
| Assessment 4, 12 months after starting treatment | ||||||
| No. of patients | 144 | 164 | ||||
| FACT-G | 84.7 | 15.1 | 84.0 | 14.6 | ||
| Physical well-being subscale | 23.3 | 5.2 | 22.3 | 6.0 | ||
| Social well-being subscale | 24.2 | 3.9 | 24.1 | 4.1 | ||
| Emotional well-being subscale | 16.2 | 3.7 | 16.5 | 3.2 | ||
| Functional well-being subscale | 21.3 | 5.8 | 20.9 | 6.1 | ||
| FACT-O subscale | 38.3 | 6.0 | 37.7 | 7.1 | ||
| GOG supplemental questions | 17.1 | 2.9 | 16.9 | 2.3 | ||
Abbreviations: SD, standard deviation; FACT-G, Functional Assessment of Cancer Therapy–General; FACT-O, Functional Assessment of Cancer Therapy–Ovarian; GOG, Gynecologic Oncology Group.
Subsequent QOL Scores
Subsequent QOL mean scores (FACT-G and FACT-O subscale) are also listed in Table 3. After adjustment for midtreatment baseline scores and patient age and PS at randomization, the fixed linear model estimate indicated that there were no interactions between treatment and assessment time according to the QOL scores. However, subsequent QOL scores were significantly associated with the midtreatment baseline score (P < .001) and the time point at which the assessments were completed (P < .001). A marked improvement in QOL scores in the combined groups was observed from the second (sixth cycle of treatment) to the third (6 months after starting treatment) time point of 6.8 units for the FACT-G (effect size: [difference/SDbaseline] = 0.48; P < .001) and 2.5 units for the FACT-O subscale (effect size: [difference/SDbaseline] = 0.43; P < .001). A paired t test was also conducted for 309 patients who completed both assessments and demonstrated similar results. This greater than 5-unit improvement in the FACT-G score was sustained to 12 months after starting treatment and is considered clinically significant.16,21 By examining each FACT-G subscale (Table 3), we found that this improvement was attributed chiefly to improved physical (by 2.8 units) and functional (by 2.7 units) well-being, which together accounted for 81% (5.5 of 6.8 units) of the total increment in the FACT-G score. The QOL scores did not change significantly from 6 months to 1 year after baseline.
Five GOG supplemental questions were aggregated into a total score (GOG supplemental question score, Table 3). A higher score implies a better QOL or less adverse effects. No obvious differences across arms or changes over time were observed in this score. After adjustment, the fitted mixed model estimate indicated no interaction between treatments and assessment time points.
One of the GOG supplemental questions was designed to measure neurotoxicity as an effect of chemotherapy. The patient was asked to rate the amount she was bothered by numbness and tingling (0 = not at all, 1 = a little bit, 2 = somewhat, 3 = quite a bit, and 4 = very much). To explore the difference in reported severity of neurotoxicity between the two arms, the percentages of patients who rated their symptoms as a 3 or 4 on the scale are listed in Table 4. A significant difference was observed at the third assessment point between patients who did versus patients who did not undergo interval secondary cytoreduction (38.4% v 54.0%, respectively; χ2 = 7.9; P = .005).
Table 4.
Patients Reporting Quite a Bit or Very Much Numbness and/or Tingling
| Assessment Point | Interval Secondary Cytoreduction | No Interval Secondary Cytoreduction | P | ||
|---|---|---|---|---|---|
|
|
|
||||
| No. of Patients/Total No. of Patients | % | No. of Patients/Total No. of Patients | % | ||
| Assessment 1, third chemotherapy cycle | 5/171 | 2.92 | 7/170 | 4.12 | .475 |
|
| |||||
| Assessment 2, sixth chemotherapy cycle | 26/150 | 17.33 | 27/172 | 15.70 | .547 |
|
| |||||
| Assessment 3, 6 months after starting treatment | 61/159 | 38.36 | 87/161 | 54.03 | .012 |
|
| |||||
| Assessment 4, 12 months after starting treatment | 38/132 | 28.79 | 49/154 | 31.82 | .542 |
By 12 months from the start of treatment (fourth assessment), approximately one third of all patients reported persistent, bothersome numbness and/or tingling. Again, patients randomly assigned to no interval secondary cytoreduction reported slightly more neurotoxicity. Patients who reported persistent neurotoxicity at the fourth assessment were older than patients who reported less neurotoxicity (median age, 60.7 v 56.7 years, respectively; P = .0024).
Relationship Between Midtreatment Baseline QOL and Survival
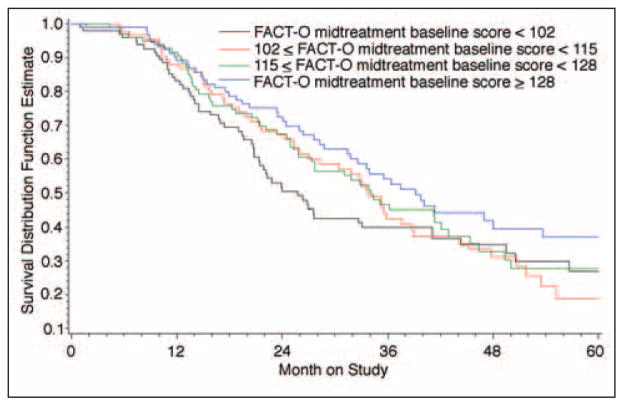
Midtreatment baseline FACT-O scores were calculated by combining FACT-G and the FACT-O subscale. After adjusting for patient age, measurable disease status, and disease stage (III or IV), the fitted Cox proportional hazards model showed that the midtreatment baseline FACT-O score as a continuous variable was associated with overall survival. Patients experienced death rates 8.4% lower for every 10 units of self-reported FACT-O score (hazard ratio [HR] = 0.92 per 10 units; 95% CI, 0.85 to 0.99; P = .025). To further explore this association, FACT-O scores at randomization were classified into four levels according to quartiles. The first level (worst QOL) includes scores of less than 102 (first quartile), the second level includes scores between 102 and 115 (second quartile), the third level includes scores between 115 and 128 (third quartile), and the fourth level (best QOL) includes scores greater than 128 (fourth quartile). After adjustment, this quartile analysis indicates that the association between QOL, as measured by the FACT-O, and survival is attributed primarily to patients whose midtreatment baseline QOL score was in the lowest quartile (FACT-O score of < 102; Fig 1) compared with patients with scores in the highest quartile (FACT-O score of ≥ 128; HR = 0.67; 95% CI, 0.45 to 0.98; P = .04). This relationship was not observed with respect to PFS.
Fig 1.
Overall survival by level of midtreatment baseline Functional Assessment of Cancer Therapy–Ovarian (FACT-O) score.
Relationship Between PS and Survival
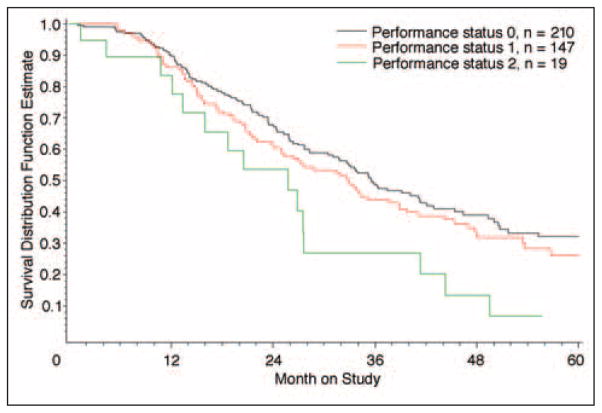
Patient PS was re-evaluated at randomization. After adjusting for age, measurable disease status, and disease stage (III or IV), patients with PS of 0 and 1 experienced death rates 47.3% lower (HR = 0.527; 95% CI, 0.31 to 0.91; P = .025) and 30.1% lower (HR = 0.61; 95% CI, 0.35 to 1.07; not statistically significant), respectively, than patients with a PS of 2 (Fig 2). Similar results were observed with regard to PFS. It is noteworthy that only 19 of the 424 patients assessed at the time of random assignment had a PS of 2.
Fig 2.
Overall survival by performance status at random assignment.
DISCUSSION
The results of GOG 152 demonstrated that interval secondary cytoreduction did not improve survival in patients with advanced suboptimally debulked ovarian cancer treated with cisplatin and paclitaxel.14 The study’s secondary objective was to evaluate between-group differences in patient-reported QOL at four assessment points using several assessment instruments.
Our results show that interval secondary cytoreduction was also not associated with any appreciable change in patients’ health-related QOL. Although between-group differences in QOL scores were not observed at the second assessment (the period most likely to show postsurgical change), it is possible that this time point was too far removed from the event to reflect disruption and/or the assessment measures were not sufficiently sensitive to identify differences. Or, because QOL completion rates were statistically higher at the second assessment among patients not undergoing interval secondary cytoreduction, perhaps the women who did have secondary surgery actually experienced a greater decrease in QOL as a result but were unable or unavailable to complete the questionnaire, thereby masking a potential group difference. Although plausible, this interpretation is inconsistent with the observation that a presumed random cause (ie, neglect to administer questionnaire) was the primary reason for missing data in this trial. In either event, no notable long-term QOL differences associated with interval secondary cytoreduction were observed.
We were able to document the significant toll initial treatment for ovarian cancer exacts, with scores at the first (midtreatment baseline) and second assessment points indicating impaired QOL. The FACT-G mean scores at the first assessment point indicate that these patients experienced QOL disruption similar to a reference group of ovarian cancer patients15 and a normative group of general cancer patients, most of whom were receiving chemotherapy.17 This disruption was most prominent on the physical and functional well-being subscales and was sustained throughout treatment. However, a clinically significant improvement was evidenced once patients completed treatment, as indicated by the change in QOL scores at the third and fourth assessments. It is important to note that these scores surpass those at the midtreatment baseline assessment because random assignment to cytoreduction or no cytoreduction occurred after the initial surgery and the third cycle of chemotherapy. Although it is clear that post-treatment QOL of the surviving patients is superior to QOL as measured at the first (midtreatment baseline) assessment, the absence of the pretreatment assessment prevents us from estimating the extent to which this reflects the impact of acute toxicity versus therapeutic benefit from treatment. There may also be an effect of some overestimation of QOL scores because it was anticipated that missing data would occur in women who had lower scores before dropout because prior literature suggests that missing data may be linked to greater illness burden.22 The FACT subscale data indicate that these changes are generally a result of improved physical and functional well-being. The scales for emotional and social well-being and the ovarian-specific FACT subscale remained generally stable throughout the study.
The GOG supplemental questions were intended to capture treatment-specific concerns via questions related to the effects of surgery or chemotherapy. These supplemental questions revealed a trend in which patients who had interval secondary cytoreduction reported significantly less treatment-related neurotoxicity at the third assessment than patients who did not undergo the surgery. It is possible that patients in the cytoreduction arm may have experienced less neurotoxicity because of the intervening time period when surgery and surgical recovery necessitated time off from chemotherapy. In short, the break between chemotherapy cycles 3 and 4 may have been beneficial in reducing short-term QOL disruption. Although the data at the fourth assessment suggest some recovery from the third assessment, neuropathy did persist in approximately one third of patients, especially among older women. However, neurotoxicity was not measured using a standardized, validated tool; thus, it will be important to do so when planning future treatment regimens where chemotherapy-associated neurotoxicity is likely to be of concern.
Finally, this clinical trial allowed us to examine whether QOL scores can be predictive of PFS and overall survival in patients with advanced ovarian cancer. Although QOL and PS scores obtained in the midst of protocol therapy most likely describe treatment-related disruptions, the data do suggest the potential for detecting disease (ie, recurring or unremitting) activity. This argument is strongest for the data indicating that patients scoring in the lowest QOL quartile at midtreatment baseline experience a greater death rate. These data are consistent with the literature, which suggests that QOL scores can be predictive of survival in advanced cancer patients.10–13
Consequently, this type of nonintrusive assessment can be a useful measure of disease- and treatment-related health status and QOL over the course of a cancer patient’s treatment. The knowledge gained from an assessment of QOL as related to ovarian cancer clinical trials is particularly important when, as is the case in this study, survival time does not differ between treatment arms. The availability of data documenting the effects and longitudinal changes associated with QOL can contribute to the development of future treatment regimens and approaches to clinical care.
Acknowledgments
Supported by National Cancer Institute grant No. CA 27469 to the Gynecologic Oncology Group Administrative Office and grant No. CA 37517 to the Gynecologic Oncology Group Statistical and Data Center.
We thank Caron Modeas for expert assistance in the preparation and editing of this article.
Appendix
The following Gynecologic Oncology Group member institutions participated in this study: University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group, University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University Medical Center, Wake Forest University School of Medicine, Albany Medical College, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke’s Medical Center, SUNY Downstate Medical Center, University of Kentucky, The Cleveland Clinic Foundation, SUNY at Stony Brook, Washington University School of Medicine, Cooper Hospital/University Medical Center, Columbus Cancer Council, M.D. Anderson Cancer Center, University of Massachusetts Medical School, Fox Chase Cancer Center, Medical University of South Carolina, Women’s Cancer Center, University of Oklahoma, University of Virginia, Tacoma General Hospital, Thomas Jefferson University Hospital, Mayo Clinic, Tampa Bay Cancer Consortium, Brookview Research, Inc, and Ellis Fischel Cancer Center.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
Although all authors have completed the disclosure declaration, the following author or their immediate family members has indicated a financial interest. No conflict exists for drugs or devices used in a study if they are not being evaluated as part of the investigation. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
| Authors | Employment | Leadership | Consultant | Stock | Honoraria | Research Funds | Testimony | Other |
|---|---|---|---|---|---|---|---|---|
| Peter G. Rose | Bristol-Myers Squibb (A) | Bristol-Myers Squibb (A) | ||||||
| Dollar Amount Codes (A) < $10,000 (B) $10,000–99,999 (C) ≥ $100,000 (N/R) Not Required | ||||||||
References
- 1.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 2.Piccart MJ, Bertelsen K, James K, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: Three-year results. J Natl Cancer Inst. 2000;92:669–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- 3.Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intra-peritoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 4.Cella DF. Quality of life as an outcome of cancer treatment. In: Groenwald SL, Frogge MH, Goodman M, et al., editors. Cancer Nursing: Principles and Practice. 4. Boston, MA: Jones & Bartlett; 1997. pp. 203–213. [Google Scholar]
- 5.Cella DF, Bonomi AE. Measuring quality of life. In: Pazdur R, Coia LR, Hoskins WJ, et al., editors. Cancer Management: A Multidisciplinary Approach. 2. Huntington, NY: PRR, Inc; 1998. pp. 807–814. [Google Scholar]
- 6.Cella D. Quality of life. In: Holland J, editor. Psycho-Oncology. New York, NY: Oxford University Press; 1998. pp. 1135–1146. [Google Scholar]
- 6A.van der Burg ME, van Lent M, Buyse M, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med. 332:629–634. doi: 10.1056/NEJM199503093321002. [DOI] [PubMed] [Google Scholar]
- 7.Lutgendorf SK, Anderson B, Rothrock N, et al. Quality of life and mood in women receiving extensive chemotherapy for gynecologic cancer. Cancer. 2000;89:1402–1411. [PubMed] [Google Scholar]
- 8.Bodurka-Bevers D, Basen-Engquist K, Carmack CL, et al. Depression, anxiety and quality of life in patients with epithelial ovarian cancer. Gynecol Oncol. 2000;78:302–308. doi: 10.1006/gyno.2000.5908. [DOI] [PubMed] [Google Scholar]
- 9.Kornblith AB, Thaler HT, Wong G, et al. Quality of life of women with ovarian cancer. Gynecol Oncol. 1995;59:231–242. doi: 10.1006/gyno.1995.0014. [DOI] [PubMed] [Google Scholar]
- 10.Coates A, Porzsolt F, Osoba D. Quality of life in oncology practice: Prognostic value of EORTC QLQ-C30 scores in patients with advanced malignancy. Eur J Cancer. 1997;33:1025–1030. doi: 10.1016/s0959-8049(97)00049-x. [DOI] [PubMed] [Google Scholar]
- 11.Eton D, Fairclough D, Cella D, et al. Early change in patient-reported health during lung cancer chemotherapy predicts clinical outcomes beyond those predicted by baseline report: Results from Eastern Cooperative Oncology Group Study 5592. J Clin Oncol. 2003;21:1536–1543. doi: 10.1200/JCO.2003.07.128. [DOI] [PubMed] [Google Scholar]
- 12.Coates A, Gebski V, Signorini D, et al. Prognostic value of quality-of-life scores during chemotherapy for advanced breast cancer: Australian New Zealand Breast Cancer Trials Group. J Clin Oncol. 1992;10:1833–1838. doi: 10.1200/JCO.1992.10.12.1833. [DOI] [PubMed] [Google Scholar]
- 13.Dancey J, Zee B, Osoba D, et al. Quality of life scores: An independent prognostic variable in a general population of cancer patients receiving chemotherapy. Qual Life Res. 1997;6:151–158. doi: 10.1023/a:1026442201191. [DOI] [PubMed] [Google Scholar]
- 14.Rose P, Nerenstone S, Brady M, et al. Phase III randomized trial of secondary surgical cytoreduction in advanced ovarian carcinoma: A Gynecologic Oncology Group study. N Engl J Med. 2004;351:2489–2497. doi: 10.1056/NEJMoa041125. [DOI] [PubMed] [Google Scholar]
- 15.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the Functional Assessment of Cancer Therapy–Ovarian (FACT-O) J Clin Oncol. 2001;19:1809–1817. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- 16.Schink JC, Weller E, Harris LS, et al. Outpatient Taxol and carboplatin chemotherapy for suboptimally debulked epithelial carcinoma of the ovary results in improved quality of life: An Eastern Cooperative Oncology Group phase II study (E2E93) Cancer J. 2001;7:155–164. [PubMed] [Google Scholar]
- 17.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy Scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 18.Cain J, Wenzel LB, Monk BJ, et al. Palliative care and quality of life considerations in the management of ovarian cancer. In: Gershenson DM, McGuire WP, editors. Ovarian Cancer Controversies in Management. New York, NY: Churchill Livingstone, Inc; 1998. pp. 281–307. [Google Scholar]
- 19.SAS Institute, Inc. SAS/STAT User’s Guide, Version 8. Cary, NC: SAS Institute, Inc; 1999. pp. 2083–2227. [Google Scholar]
- 20.SAS Institute, Inc. SAS/STAT User’s Guide, Version 8. Cary, NC: SAS Institute, Inc; 1999. pp. 2569–2659. [Google Scholar]
- 21.Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol. 2002;55:285–295. doi: 10.1016/s0895-4356(01)00477-2. [DOI] [PubMed] [Google Scholar]
- 22.Fairclough DL. Design and Analysis of Quality of Life Studies in Clinical Trials: Interdisciplinary Statistics. Boca Raton, FL: Chapman & Hall/CRC; 2002. [Google Scholar]