Abstract
32P has been available for the treatment of myeloproliferative neoplasms (MPNs) for over seventy years. It was first used in 1938 by John H Lawrence in the treatment of polycythaemia and chronic leukaemias. With the introduction of agents such as hydroxycarbamide, interferon and anagrelide the role of 32P has been diminished. Today, Polycythaemia Rubra Vera (PRV) and Essential Thrombocythaemia (ET) remain the only myeloproliferative conditions in which 32P is indicated.
Materials and Methods
We carried out a retrospective review of all patients who had received 32P in Northern Ireland over a 24 year period. The time to successful response, duration of response, and associated complications were reviewed.
Results
32P was successful in inducing remission in 90% of patients. This remission was sustained following one dose without the need for further therapy in 37% of cases. 47% required repeated doses. 26% required recommencement of alternative therapies. No cases of thrombosis, myelofibrosis or acute leukaemia were observed.
Discussion
We conclude that 32P is a well-tolerated and efficacious treatment option in the elderly. We discuss our results compared with previous work in this area. 32P will continue to be offered to elderly patients in our practice.
Keywords: Polycythemia rubra vera, Essential Thrombocythemia, Radioactive phosphorous
32P IN THE TREATMENT OF MYELOPROLIFERATIVE DISORDERS
32P has been available for the treatment of myeloproliferative neoplasms for over seventy years. It was first used in 1938 by John H Lawrence to treat polycythaemia and chronic leukaemias1, 2. Concerns were however raised over its leukaemogenic potential. It has become a therapeutic modality of the past for chronic leukaemias and with the introduction of other agents such as hydroxycarbamide and interferon in polycythaemia and anagrelide in essential thrombocythaemia, the role of 32P has been diminished. Today, polycythaemia rubra vera (PRV) and essential thrombocythaemia (ET) remain the only myeloproliferative conditions in which 32P is indicated3, 4.
We carried out a retrospective review of all patients who had received 32P in Northern Ireland over a 24-year period.
All patients were under the care of a consultant haematologist. They were referred to a clinical oncologist to receive 32P in a designated nuclear medicine unit. All patients received 185mq of 32P.
METHODS
All patients who received 32P were under the care of a clinical oncologist. A database with records of all patients treated with 32P since 1988 is kept within the nuclear medicine unit of the Northern Ireland Cancer Centre and this was used to identify patients.
Patient characteristics were reviewed including age, diagnosis and previous treatments.
Response was defined as return of blood counts to normal (HCT <0.45 platelets <450x109/l). The time to successful response was reviewed along with the duration of response. Length of follow up was recorded and whether or not 32P was required again. Case notes were reviewed for any signs of complications from 32P.
RESULTS
A total of nineteen patients were identified as having received 32P over a 24-year period (1988-2012). Eight men and eleven women were included. Of these 15 subjects suffered from ET and 4 from PRV.
The median age at the first dose of 32P was 80 years with ages ranging from 68 to 90 years at first dose.
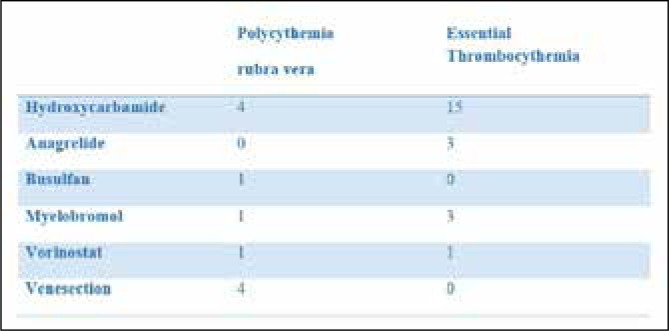
All patients had received treatment with hydroxycarbamide prior to referral for 32P. This was generally stopped due to the development of leg ulcers or anaemia. Of the PRV patients busulfan had also been used in one patient. Myelobromol had also been used in one patient and all four had received venesection as a means of controlling haematocrit prior to referral for 32P. Of the patients with ET, anagrelide had been used in three patients and myelobrommol had also been used in three patients. Two patients, one with ET and one with PRV, had both previously been treated with vorinostat within a clinical trial. (Figure 1)
Fig 1.
Previous treatments prior to 32P.
32P was successful in reducing counts to acceptable levels in all but 2 patients (89% response rate).
Time to response varied between 3 weeks to 8 weeks with a median time to response being five weeks.
Seven patients (37%) required only one dose of 32P to achieve a successful response which, to date has been sustained. These patients have not required any further treatment. The longest duration of response to date in this group is 156 weeks.
However not all patients had a sustained response. In those that went on to relapse, the duration of response varied between 4 weeks to 20 weeks with the median response being 18 weeks. Eight of these patients went on to receive further 32P. Therefore 32P was given more than once in a total of 9 patients with 6 receiving it twice, 2 receiving it three times and one patient receiving it four times. Five patients (26%) required recommencement of alternative therapies.
Length of follow up varied from 7 months to 24 years, with a median follow up of 21 months.
None of the patients in this review who received 32P experienced any adverse haematological effects, in particular there were no cases of myelofibrosis, thrombosis or progression to leukaemia. One patient was later diagnosed with renal cell carcinoma. No other carcinomas were diagnosed post treatment.
DISCUSSION
Myeloproliferative neoplasms (MPN) are a heterogeneous group of disorders characterized by cellular proliferation of one or more haematologic cell lines in the peripheral blood5. They were first recognised by William Dameshek in 1951 and include polycythaemia rubra vera (PRV) and essential thrombocythaemia (ET)5.
32P offers an alternative treatment option. It was first used in 1938 by John H Lawrence for the treatment of polycythaemia and chronic leukaemias1. It is a pure beta emitter with a halflife of 14.3days and is administered as an injection2. It was initially recognised through preliminary work in mice that a large proportion of the injected dose became rapidly localised within the bone marrow and a few days later in bone with smaller quantities seen in the liver, spleen and lymph nodes6. It acts by incorporation of the 32P orthophosphate into the nuclei of rapidly proliferating and protein synthesising cells. It is also taken up by the hydroxyapatite moiety of bone crystals in cortical bone. Radiation protection for staff from a pure beta emitter is straightforward.
PRV is the most extensively studied of the MPNs treated with 32P2. Following Lawrence's work in 1939, the most effective treatment of PRV included phlebotomies combined with 32P5. Concern was however raised in 1948 that patients treated with 32P may develop acute leukemia2.
The Polycythaemia Vera Study Group (PVSG) was organized in 1967 to establish effective diagnostic criteria for polycythaemia rubra vera, to study the natural history of the disease and to define the optimal treatment7. Its first study compared 32P, chlorambucil and phlebotomy in 431 patients in a randomised trial2, 5, 7. It was found that patients treated with chlorambucil carried an excessive risk of acute leukaemia, and therefore this arm was discontinued. Patients treated with phlebotomy alone showed a higher incidence of thrombosis in the first 3 years of treatment5. After 3 to 5 years of study, a considerable number of patients treated with 32P or chlorambucil developed acute leukaemia, lymphoma and carcinomas of the gastrointestinal tract and skin, compared with those treated with phlebotomy alone5. Therefore, patients treated with phlebotomy alone had a better overall median survival of 13.9 years than patients treated with chlorambucil (8.9 years) or 32P (11.8 years)5, 7.
However, rates of acute leukaemia of up to 14% in PRV patients have been reported, which may imply that acute leukaemia is the natural progression for some patients with PRV3. The incidence in randomised trials after 32P alone is 2.5% to 15%3.
The effectiveness of 32P was also demonstrated by Balan et al who examined its use in 259 patients with PRV and ET over a 15 year period8. They found normalisation of counts in 50% patients after a single administration and in 73% after two treatments. With regards to complications; 5.5% developed myelofibrosis, 7.6% developed leukaemia, while other cancers were found in 9% of cases8.
Randi et al also looked specifically at haematological complications post 32P therapy9. Their review included 230 patients with MPNs. None of the patients with ET developed haematological complications. Of the PRV patients, 17% developed complications. Sixty percent of these were classified as minor complications such as transient anaemia or thrombocytopenia. Only 7% had major complications with acute leukaemia developing in 5%.
The use of 32P alone or in combination with hydroxycarbamide has also been examined. Najean et al randomised 461 patients over the age of 65 to receive hydroxycarbamide or no hydroxycarbamide after their first 32P induced remission10. It was found that hydroxycarbamide significantly prolonged the duration of 32P induced remission and reduced the annual mean dose received by a third. The rate of vascular complications was not decreased however. While the leukaemia risk was significantly increased, a significant excess of other carcinomas was also observed.
More recently Bjorkholm et al used data from population based registries in Sweden to conduct a large study examining to what extent cytoreductive therapies contribute to leukaemic and myelodysplastic transformation in patients with MPN11. While the focus of the study was on the effects of hydroxycarbamide, 32P was also reviewed. The risk of AML/MDS transformation was strongly associated with high exposure of 32P and alkylating agents. This equated to cumulative doses of 1,000MBq. Lower exposure to 32P and alkylating agents was not associated with a significantly increased transformation risk. More importantly, 25% of patients with transformation to AML/MDS were never exposed to 32P, hydroxycarbamide or alkylating agents. Furthermore, only 32% of patients with transformed disease were exposed to cumulative doses of 32P and/or alkylating agents. The authors concluded that the risk of transformation to leukaemia or myelodysplasia is mainly associated with the disease itself, with exposure to cytoreductive agents of less importance. This study however may indicate a need for a threshold exposure to 32P given the increased risk of transformation at higher doses.
Due to its increased risk of leukaemic transformation, the British Committee for Standards in Haematology advise that 32P use should be limited to the elderly3, 4.
Our review has shown that 32P was able to induce remission in 90% of cases, whilst 37% of cases required only one dose to provide a sustained remission to date. While some cases required repeated doses of 32P, only 26% required to return to alternative treatments. No adverse haematological complications have been observed to date. Our review is limited by the small number of cases, and although the earliest treatment was in 1988, the median follow up time was 21 months, therefore longer follow up is required.
We recognise that there are many other treatment options in management of PRV and ET. Hydroxycarbamide and anagrelide are two of the commonest cytoreductive agents, however these drugs are not without side effects and limitations. Hydroxycarbamide, an oral anti-metabolite, is often first line in the treatment of PRV and ET, usually in combination with aspirin. It is however associated with nausea, skins rashes, leg ulceration and myelosuppression. Anagrelide hydrochloride is second line in those with high risk ET. It is a phosphodiesterase inhibitor which also blocks megakaryocyte differentiation and proliferation, however it may be associated with headache, dizziness, palpitations and diarrhoea. Anagrelide has also been associated with an increase in reticulin deposition in the bone marrow. The PT-1 trial by Harrison et al, which compared hydroxycarbamide and aspirin to anagrelide and aspirin in high risk ET patients clearly demonstrated higher rates of arterial thrombosis, major haemorrhage and transformation to myelofibrosis in the anagrelide arm12.
Anagrelide is also associated with arrhythmias and cardiomyopathy and therefore may not be suitable for patients with cardiovascular disease, which is often more prevalent in the elderly.
These cytoreductive drugs require regular monitoring accompanied by regular blood counts and dose adjustments. Good compliance is required for these drugs to be effective.
From our review, we are able to conclude that 32P is a welltolerated and efficacious treatment option in the elderly. 32P is cost effective and has the benefit of reducing the monitoring and adjustments needed when on cytoreductive agents. As it is given as an injection, compliance is measurable. It offers an alternative option to controlling blood counts when other cytoreductive methods are ineffective or limited due to their side-effect profile.
32P will continue to be offered to elderly patients in our practice.
The authors declare no conflicts of interest.
REFERENCES
- 1.Lawrence JH. Nuclear physics and therapy: Preliminary report of a new method for the treatment of leukemia and polycythemia. Radiology. 1940;52:51–60. [Google Scholar]
- 2.Berlin NI. Treatment of the myeloproliferative disorders with 32P. Eur J Haematol. 2000;65:1–7. doi: 10.1034/j.1600-0609.2000.9r119.x. [DOI] [PubMed] [Google Scholar]
- 3.McMullin MF, et al. Guidelines for the diagnosis, investigation and management of polycythemia/erythrocytosis. Brit J Haematol. 2005;130:174–195. doi: 10.1111/j.1365-2141.2005.05535.x. [DOI] [PubMed] [Google Scholar]
- 4.Harrison C, Bareford D, Butt N, et al. Guidelines for the investigation and management of adults and children presenting with thrombocytosis. Brit J Haematol. 2010;149:352–375. doi: 10.1111/j.1365-2141.2010.08122.x. [DOI] [PubMed] [Google Scholar]
- 5.Koopmans SM, van Marion AMW, Schouten HC. Myeloproliferative neoplasia: a review of clinical criteria and treatment. Neth J Med. 2012 May;70(4):159–67. [PubMed] [Google Scholar]
- 6.Ledlie EM. Treatment of Polycythemia by 32P. Proc R Soc Med. 1966 Nov;59(11 Pt 1):1095–1100. doi: 10.1177/003591576605911P116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berk PD, Goldberg JD, Donovan PB, et al. Therapeutic recommendations in polycythemia vera based on Polycythemia Vera Study Group protocols. Semin Hematol. 1986;23(2):132–43. [PubMed] [Google Scholar]
- 8.Balan KK. Outcome of 259 patients with primary proliferative polycythemia and idiopathic thrombocythemia treated in a regional nuclear medicine department with phosphorus-32 – a 15 year review. Brit J Radiol. 1997;70:1169–1173. doi: 10.1259/bjr.70.839.9536909. [DOI] [PubMed] [Google Scholar]
- 9.Randi, et al. Haematological complications in polycythaemia vera and thrombocythaemia patients treated with radiophosphorous (32P) Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117(3):461–7. [PubMed] [Google Scholar]
- 10.Najean Y, Rain JD. Treatment of Polycythemia Vera: Use of 32P Alone or in Combination With Maintenance Therapy Using Hydroxyurea in 461 Patients Greater Than 65 Years of Age. Blood. 1997;89:2319–2327. [PubMed] [Google Scholar]
- 11.Bjorkhokm, et al. Treatment-related risk factors for transformation to acute myeloid leukaemia and myelodysplastic syndromes in myeloproliferative neoplasms. Journal Clinical Oncology. 2011;29(17):2410–5. doi: 10.1200/JCO.2011.34.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison, et al. Hydroxycarbamide compared with anagrelide in high risk essential thombocythaemia. N Engl J Med. 2005;353:33–45. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]