Abstract
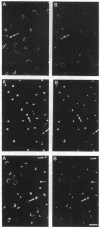
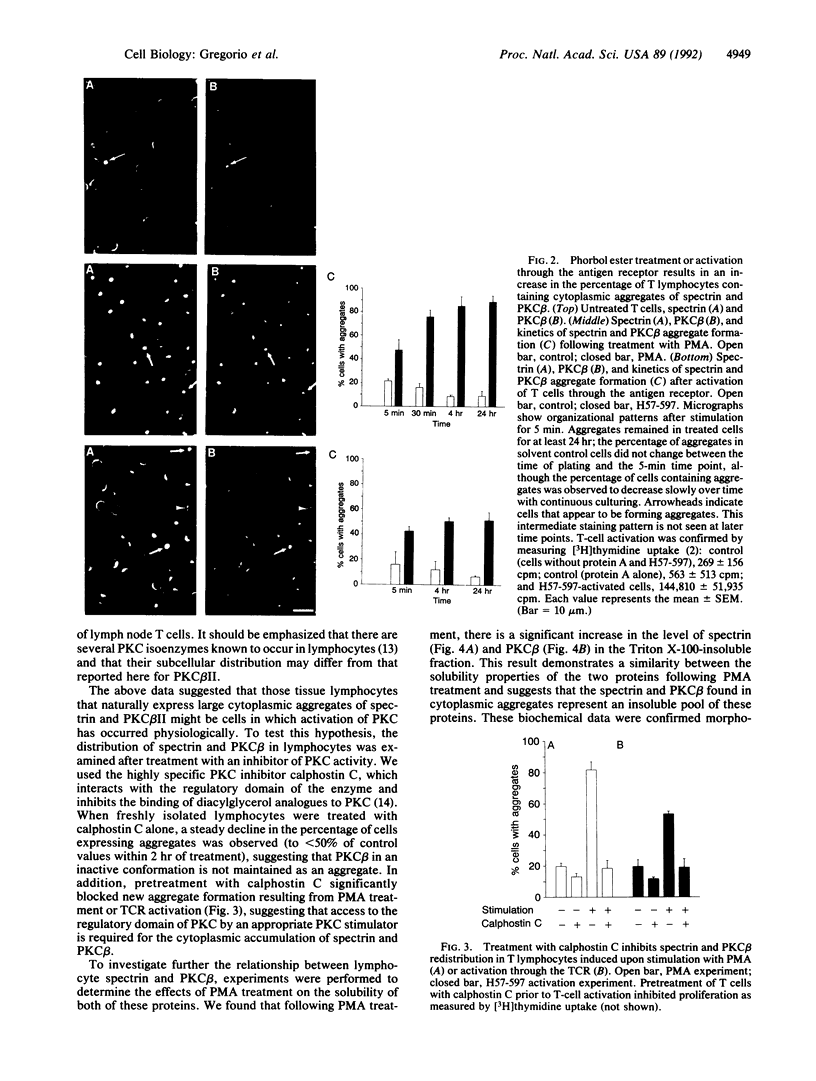
We have previously reported that mammalian tissue lymphocytes exhibit significant heterogeneity with respect to the subcellular distribution of spectrin and that this phenomenon may result from a dynamic behavior of spectrin in response to activation signals. Here, we further characterize the involvement of spectrin in lymphocyte activation by examining its relationship with protein kinase C (PKC). PKC isoenzymes are a family of cytosolic kinases that translocate from the soluble to particulate fraction upon cell stimulation. It is reported here that activation of lymph node T cells through the antigen-specific receptor, or direct activation of PKC by phorbol esters, results in a striking increase in cells expressing a cytoplasmic aggregate of spectrin. Additionally, a concurrent increase in cells expressing aggregates of the beta II isozyme of PKC is observed. Immunofluorescence staining revealed that spectrin and PKC beta II are colocalized in untreated lymphocytes and that these two proteins are coincidently translocated to the same focal aggregate within the cytoplasm following stimulation. This redistribution of spectrin and PKC beta is blocked by pretreatment with calphostin C, a specific inhibitor of PKC. Solubility studies showed that there is an increase of both proteins in the detergent-insoluble fraction of lymphocytes upon activation, and immunoprecipitation studies indicated that the soluble form of these molecules may be associated directly or indirectly as part of a complex of proteins. These data indicate that the positioning of the spectrin-based cytoskeleton is sensitive to activation signals and may play a role in the function or positioning of PKC beta II.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V. Spectrin: a structural mediator between diverse plasma membrane proteins and the cytoplasm. Curr Opin Cell Biol. 1990 Feb;2(1):51–56. doi: 10.1016/s0955-0674(05)80030-4. [DOI] [PubMed] [Google Scholar]
- Berry N., Ase K., Kikkawa U., Kishimoto A., Nishizuka Y. Human T cell activation by phorbol esters and diacylglycerol analogues. J Immunol. 1989 Sep 1;143(5):1407–1413. [PubMed] [Google Scholar]
- Berry N., Nishizuka Y. Protein kinase C and T cell activation. Eur J Biochem. 1990 Apr 30;189(2):205–214. doi: 10.1111/j.1432-1033.1990.tb15478.x. [DOI] [PubMed] [Google Scholar]
- Black J. D., Koury S. T., Bankert R. B., Repasky E. A. Heterogeneity in lymphocyte spectrin distribution: ultrastructural identification of a new spectrin-rich cytoplasmic structure. J Cell Biol. 1988 Jan;106(1):97–109. doi: 10.1083/jcb.106.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn P. Amphitropic proteins: a new class of membrane proteins. Trends Biochem Sci. 1988 Mar;13(3):79–83. doi: 10.1016/0968-0004(88)90043-6. [DOI] [PubMed] [Google Scholar]
- Chen Z. Z., McGuire J. C., Leach K. L., Cambier J. C. Transmembrane signaling through B cell MHC class II molecules: anti-Ia antibodies induce protein kinase C translocation to the nuclear fraction. J Immunol. 1987 Apr 1;138(7):2345–2352. [PubMed] [Google Scholar]
- Greenberg M. E., Edelman G. M. The 34 kd pp60src substrate is located at the inner face of the plasma membrane. Cell. 1983 Jul;33(3):767–779. doi: 10.1016/0092-8674(83)90019-3. [DOI] [PubMed] [Google Scholar]
- Haest C. W. Interactions between membrane skeleton proteins and the intrinsic domain of the erythrocyte membrane. Biochim Biophys Acta. 1982 Dec;694(4):331–352. doi: 10.1016/0304-4157(82)90001-6. [DOI] [PubMed] [Google Scholar]
- Hocevar B. A., Fields A. P. Selective translocation of beta II-protein kinase C to the nucleus of human promyelocytic (HL60) leukemia cells. J Biol Chem. 1991 Jan 5;266(1):28–33. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Arakawa Y. Transport of exogenous fluorescent phosphatidylserine analogue to the Golgi apparatus in cultured fibroblasts. J Cell Biol. 1991 Apr;113(2):235–244. doi: 10.1083/jcb.113.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Lee J. K., Black J. D., Repasky E. A., Kubo R. T., Bankert R. B. Activation induces a rapid reorganization of spectrin in lymphocytes. Cell. 1988 Dec 2;55(5):807–816. doi: 10.1016/0092-8674(88)90136-5. [DOI] [PubMed] [Google Scholar]
- Lee J. K., Repasky E. A. Cytoskeletal polarity in mammalian lymphocytes in situ. Cell Tissue Res. 1987 Jan;247(1):195–202. doi: 10.1007/BF00216562. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Henrich C. J., Cheever L., Khaner H., Simpson P. C. A protein kinase C isozyme is translocated to cytoskeletal elements on activation. Cell Regul. 1990 Aug;1(9):693–706. doi: 10.1091/mbc.1.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Colaço C. A., Lazarides E. Involvement of spectrin in cell-surface receptor capping in lymphocytes. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1626–1630. doi: 10.1073/pnas.80.6.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Dynamics of membrane-skeleton (fodrin) organization during development of polarity in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1986 Nov;103(5):1751–1765. doi: 10.1083/jcb.103.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Schäfer G., Hilz H., Eppenberger H. M. Cyclic-AMP-dependent protein kinase type II is associated with the Golgi complex and with centrosomes. Cell. 1985 Jul;41(3):1039–1051. doi: 10.1016/s0092-8674(85)80084-2. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Perrin D., Langley O. K., Aunis D. Anti-alpha-fodrin inhibits secretion from permeabilized chromaffin cells. Nature. 1987 Apr 2;326(6112):498–501. doi: 10.1038/326498a0. [DOI] [PubMed] [Google Scholar]
- Repasky E. A., Symer D. E., Bankert R. B. Spectrin immunofluorescence distinguishes a population of naturally capped lymphocytes in situ. J Cell Biol. 1984 Jul;99(1 Pt 1):350–355. doi: 10.1083/jcb.99.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski A. F., Terlecki G., Zagon I. S., Goodman S. R. Synapsin I-mediated interaction of brain spectrin with synaptic vesicles. J Cell Biol. 1991 Jul;114(2):313–318. doi: 10.1083/jcb.114.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao N. K., MacDonald R. I., Takeshita K., MacDonald R. C. Characteristics of spectrin-induced leakage of extruded, phosphatidylserine vesicles. Biochim Biophys Acta. 1991 Mar 18;1063(1):147–154. doi: 10.1016/0005-2736(91)90364-e. [DOI] [PubMed] [Google Scholar]