Abstract
Background: The handgrip strength test is widely used by clinicians; however, little has been investigated about its reliability when used in subjects with Parkinson disease (PD). The purpose of this study was to investigate the test-retest reliability of the handgrip strength test for subjects with PD. Methods: The PD group consisted of 15 patients, and the control group consisted of 15 healthy subjects. Each patient performed 3 pain-free maximal isometric contractions on each hand on 2 occasions, 1 week apart. Intraclass correlation coefficient (ICC), standard error of measurement (SEM), and 95% limits of agreement (LOA) were calculated. The 2-way analysis of variance (ANOVA) was conducted to determine the differences between sides and groups. Results: Test-retest reliability of measurements of grip strength was excellent for dominant (ICC = 0.97; P = .001) and non-dominant (ICC = 0.98; P = .001) hand of participant with PD and (ICC = 0.99; P = .001) and (ICC = 0.99; P = .001) respectively, of healthy group. Conclusions: The Jamar hand dynamometer had fair to excellent test-retest reliability to test grip strength in participants with PD.
Keywords: test-retest, Parkinson disease, grip strength, hand
Introduction
Handgrip strength has been found to be a predictor of health status overtime. Individuals with weaker grip are more susceptible to poorer health and have increased difficulty when performing activities of daily living.6,10,14 Because decreased grip strength can predict risk of poor health in advanced age, it is important to determine the reliability of the measurement tool for normal subjects as well as those with different disease states.4,5,9,14,15 Cheng et al suggest that handgrip strength may be a more useful marker of multi-morbidity than chronological age in men.4 Grip strength reliability has been performed on subjects with a variety of diagnoses including rheumatoid arthritis (RA)9 carpometacarpal osteoarthritis,15 symptomatic individuals,5 forearm tendinitis,14 and disabled women.11
Parkinson disease (PD) prevalence increases steadily with age.12 It is more common in the elderly population and can affect as many as 1903 person out of 100 000 individuals over the age of 80 years.12 Many patients with PD undergo rehabilitation procedures in an attempt to slow the progression of the disease and control symptoms. It is important to determine the reliability of grip strength assessment in this population. Also important is to determine what change in grip strength indicates that true change has occurred and the change is not due to measurement error. This information allows clinicians to document important changes in grip strength to help determine the effectiveness of therapeutic interventions. The reliability of the measurement of grip strength is essential for satisfactory data collection for consistent interpretation of the results. In particular, test-retest reliability is clinically important for correct assessment of follow-up results. Good test-retest reliability enables comparisons to be made over a period of time. Reliable results allow the professional to reach conclusions that are minimally affected by external factors, thereby reducing the chances of error. Therefore, the purpose of this study was to investigate the test-retest reliability of the handgrip strength test in subjects with PD.
Methods
Design
This was a prospective observational cross-sectional study.
Participants
A convenience sample of 30 subjects, between the ages of 50 and 90 years, was recruited for the study from the department of physical therapy from June 2014 to October 2014. Fifteen consecutive subjects, 69.5 ± 8.6 years old, who had been diagnosed idiopathic PD by a neurologist according to the UK Brain Bank Parkinson’s criteria were included in the study.8 The healthy control group consisted of 15 consecutive subjects from the same rehabilitation facility between the ages of 67.5 ± 10.2 years. Subjects underwent subjective and physical examination conducted by a physical therapist with 12 years of experience in treating musculoskeletal disorders. All subjects were independent in ambulation and did not use an assistive device.1 All subjects followed their normal medication regimen during testing and the functional evaluation. None of the subjects exhibited dyskinesias. Subjects were excluded if they had parkinsonian disorders as progressive supranuclear palsy, Shy-Drager syndrome, corticobasal degeneration, secondary parkinsonism, familial parkinsonism, a history of dementia as reported by the family or caregiver, or if they were unable to stand unassisted for 5 minutes without an assistive device. In addition, the subjects demonstrated the ability to follow simple instructions, as determined by their responses to questions and instructions. This was assessed during the medical visit and consent process. Exclusion criteria included previous surgical intervention of the hand or the forearm, corticosteroid injection, physical therapy intervention within 6 months prior to the onset of the study, multiple pain diagnoses of the upper extremity, deQuervain’s tenosynovitis, shoulder pathology, cervical radiculopathy, evidence of systemic illness (RA, psoriatic arthritis, systemic lupus erythematosus), or any degenerative or non-degenerative neurological conditions.
Procedure
All subjects performed a standardized warm-up that consisted of two to three preliminary trials for familiarization with the recording procedure and instrumentation, followed by a 10-minute rest period so that maximal grip strength would not be impacted by the preliminary trials. Testing took place between the hours of 9:00 am and 11:30 am. A portable Jamar Hydraulic Hand Dynamometer (Fabrication Enterprises, Inc., Elmsford, New York) was used for handgrip strength measurement in kilograms. The test was performed in the sitting position with the shoulder of tested arm adducted to the side, the elbow flexed at 90°, and the forearm and wrist were positioned in neutral position. The second handle setting of the dynamometer was used in this study.7 The testing protocol consisted of 3 pain-free maximal isometric contractions, held for 3 seconds, performed in a row with both hands, with a 1-minute pause between measurements. Participants were instructed to squeeze the dynamometer as firmly as possible without eliciting pain. Pain was assessed using the verbal analog scale during the testing procedure. If the subject reported pain during testing, the test was re-administered with instruction to stop squeezing before the onset of pain. Each participant performed 3 pain-free maximal isometric contractions on each hand on 2 occasions, 1 week apart. The mean of these 3 trials was used for data analysis.
Data Analysis
Data were analyzed using SPSS version 21.0 (SPSS Inc., Chicago, Illinois). The results are expressed as means, standard deviations, and/or 95% confidence intervals. Test-retest data were analyzed using the intraclass correlation coefficient (ICC). ICC values that are equal or greater than 0.80 are considered high.13 We calculated ICC for single measures using a 2-way random effect model of absolute agreement for the computation of ICC. To assess the absolute reliability, the standard error of measurement (SEM) and the 95% limits of agreement (LOA) were calculated by means of the following equation: SEM = SD × √1 − ICC, and LOA = inter-trial mean difference ± 1.96 SD of the between-trial difference. The SEM expresses measurement error in the same units as the original measurement, and it is not influenced by variability among patients. The inter-trial agreement was also examined graphically by plotting the difference between test and retest against their mean, according to the Bland and Altman approach to calculate LOA.2 Agreement between 2 methods of measurement can be inappropriately assessed using correlation that assesses the relationship of the measurements on a straight line. The LOA calculation plots the differences between test and retest against their mean on both a horizontal and vertical axis. The plot enables a visual inspection of the association between the difference in measurements and the magnitude of grip strength.
Results
Demographic and Clinical Data of Participants
Between June 2014 and October 2014, 15 patients (7 males, 69.5 ± 8.6 years old) who presented with idiopathic PD satisfied all the eligibility criteria and agreed to participate in the study. Fifteen subjects, healthy controls (6 males, 67.5 ± 10.2 years old) were also included. No participants dropped out during the different phases of the study, and no adverse effects were detected after the application of the measurement. None of the participants began drug therapy during the course of the study. The results indicated non-significant differences between test and retest handgrip strength values (see Table 1).
Table 1.
Baseline Demographics for Both Groups.
| Parkinson (n = 15) | Healthy (n = 15) | P valuea | |
|---|---|---|---|
| Age, y | 69.5 ± 8.6 | 67.5 ± 10.2 | .49 |
| Male gender | 7 (46.7%) | 6 (40.0%) | — |
| Right handed | 13 (86.7%) | 14 (93.3%) | — |
| Height, cm | 163.0 ± 9.5 | 163.5 ± 6.3 | .81 |
| Weight, kg | 70.1 ± 14.3 | 67.7 ± 9.4 | .84 |
| BMI | 26.4 ± 5.3 | 25.3 ± 3.0 | .84 |
| Tinetti score | 22.9 ± 5.4 | 27.5 ± 0.9 | .3 |
| UPDRS | 8.8 ± 5.9 | — | — |
| Hoehn and Yahr | 1.9 ± 0.9 | — | — |
| Rankin | 2.1 ± 1.0 | — | — |
| Length of time in years of PD diagnosis | 5.29 ± −5.13 | — | — |
Note. Data are expressed as n (%) or mean ± standard deviation. BMI, body mass index (kg/m2); UPDRS, Unified Parkinson’s Disease Rating Scale; PD, Parkinson disease.
P < 0.05 was considered statistically significant.
Test-Retest Reliability
The relative reliability between test and retest was very high. Test-retest reliability was calculated to be for the PD dominant hand (ICC = 0.97; P = .001) and non-dominant hand (ICC = 0.98; P = .001) as well as between the dominant (ICC = 0.99; P = .001) and non-dominant (ICC = 0.99; P = .001) hand of the healthy subjects. For both the PD and healthy groups, the ICC between test and retest reliability was high for the dominant hands and the non-dominant hands and ranged between 0.92 and 0.99 (see Figures 1 and 2 and Tables 2 and 3).
Figure 1.
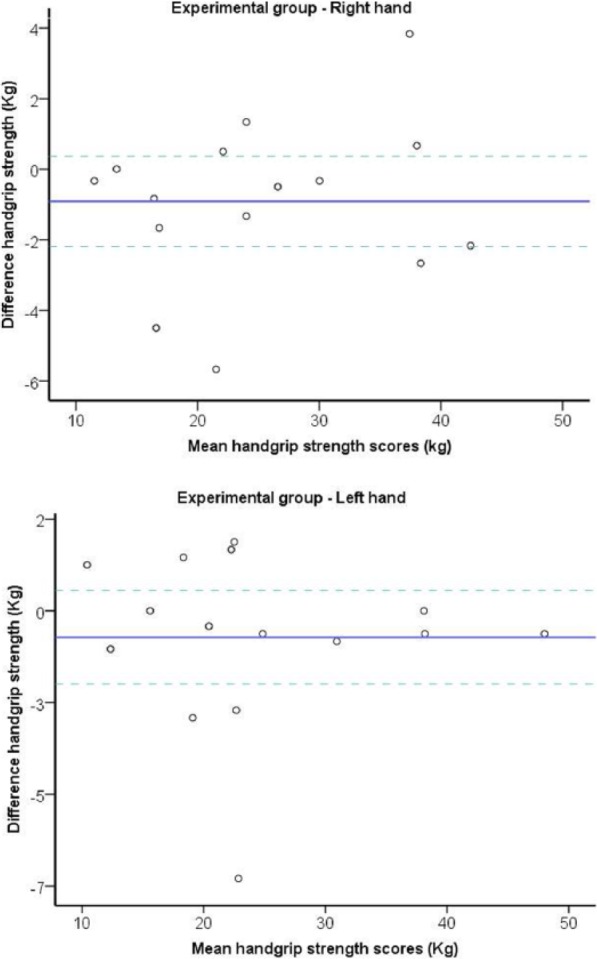
Bland-Altman plots of the handgrip strength test for experimental group in subjects with PD.
Note. The central line characterizes the mean difference between test and retest values; the upper and lower lines characterize the upper and lower 95% limits of agreement (LOA = inter-trial mean difference ± 1.96 SD of the inter-trial difference), respectively. PD, Parkinson disease; LOA, limits of agreement.
Figure 2.
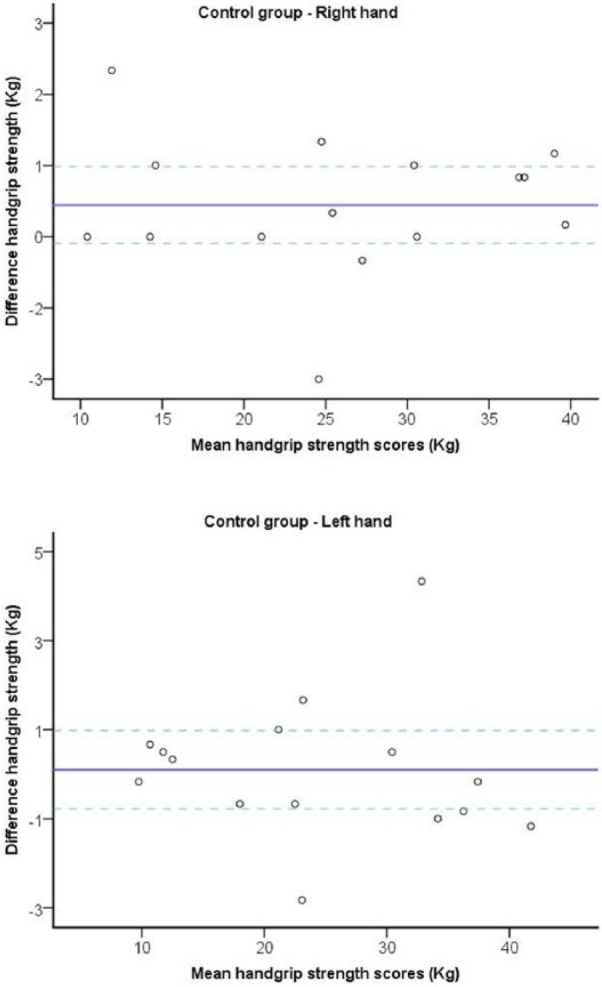
Bland-Altman plots of the handgrip strength test for control group in subjects with PD.
Note. The central line characterizes the mean difference between test and retest values; the upper and lower lines characterize the upper and lower 95% limits of agreement (LOA = inter-trial mean difference ± 1.96 SD of the inter-trial difference), respectively. PD, Parkinson disease; LOA, limits of agreement.
Table 2.
Test and Retest Values and Index of Relative and Absolute Reliability of Grip Strength in Patients With Parkinson Disease.
| 95% LOA, kg |
||||||||
|---|---|---|---|---|---|---|---|---|
| Grip pinch | Side | Test, kg | Retest, kg | Difference, kg | ICC (95% CI) | Lower | Upper | SEM, kg |
| Parkinson group | Dominant hand | 24.8 ± 10.2 | 25.7 ± 9.7 | 0.9 | 0.97 | −2.19 | 0.36 | 0.05 |
| (0.92-0.99) | ||||||||
| Non-dominant hand | 23.8 ± 10.3 | 24.9 ± 10.4 | −1.1 | 0.98 | −2.10 | −0.06 | 0.05 | |
| (0.95-0.99) | ||||||||
| Healthy group | Dominant hand | 25.8 ± 9.9 | 25.8 ± 9.9 | 0.1 | 0.99 | −0.59 | 0.48 | 0.24 |
| (0.98-0.99) | ||||||||
| Non-dominant hand | 24.4 ± 10.5 | 24.3 ± 10.6 | 0.1 | 0.99 | −0.78 | 0.98 | 0.80 | |
| (0.97-0.99) | ||||||||
Table 3.
Mean (SD) for Outcomes at All Study Visits for Each Group, Mean (SD) Difference Within Groups, and Mean (95% CI) Difference Between Groups.
| Groups |
Difference within groups |
Difference between groups |
||||||
|---|---|---|---|---|---|---|---|---|
| Parkinson |
Healthy |
Parkinson group (n = 15) | Healthy group (n = 15) | Right side (n = 30) | Left side (n = 30) | |||
| Outcome | Right (n = 15) | Left (n = 15) | Right (n = 15) | Left (n = 15) | ||||
| Grip strength, kg | 25.3 | 24.4 | 25.9 | 24.4 | 0.8 | 1.5 | −0.6 | 0.0 |
| (9.9) | (10.3) | (9.9) | (10.6) | (−1.4 to 3.1) | (−0.8 to 3.8) | (−8.0 to 6.8) | (−7.8 to 7.8) | |
| Visual analogue scale | 0.4 | 0.0 | 0.1 | 0.0 | 0.4 | 0.1 | 0.3 | 0.0 |
| (0.7) | (0.0) | (0.3) | (0.0) | (0.1 to 0.7) | (−0.2 to 0.4) | (−0.1 to 0.7) | (0.0 to 0.0) | |
Note. CI, confidence interval.
Discussion
Our study demonstrated that maximum handgrip strength could be measured reliably, using the Jamar hand dynamometer, for subjects with PD. In this study, test-retest reliability was excellent for the PD dominant hand (ICC = 0.97; P = .001) and non-dominant hand (ICC = 0.98; P = .001). These findings are in agreement with the previous studies that demonstrated excellent test-retest reliability for the evaluation of one trial of grip strength in symptomatic persons and populations with painful conditions.5,9,11,15 The SEMs in this study were small, and the mean was 0.046 kg for grip with the dominant and non-dominant hand of the PD group. This is similar to small SEM values reported in a study of a population with RA.9 Clinically, this implies that a change as small as ±0.092 kg (2 SEM) is indicative of a true change in pain-free grip strength in subjects with PD.1 This information indicates that changes in grip strength of patients with PD can be accurately and reliably measured with a Jamar dynamometer. The clinician can determine whether change in physical capacities has occurred.
We found that the mean grip strength of the PD population was 23.89 kg for the non-dominant hand and 24.81 kg for the dominant hand in our population that ranged in age from 50 to 90 years. It has been reported that mean grip strength for adults between the ages of 50 and 75+ ranged from 16.4 kg to 30.9 kg.3 The values that we found fall within this range.
Future research is necessary to determine the relationship between handgrip strength as a predictor of health status in this population and to further determine grip strength values that indicate poor health.
Limitations
We did have a variety of ages represented in our study, but we did not stratify the data from different age groups and calculated the differences between groups using the entire sample.
Conclusions
The results of our study indicate that reliable grip strength testing of subjects with PD can be performed with the Jamar dynamometer if the Jamar is calibrated properly and standardized testing procedures are followed. Grip strength values are useful in research and in the clinic to determine the effect of interventions on improving grip.
Acknowledgments
The authors thank Pietro Uberti (P.U.) for his assistance.
Footnotes
Ethical Approval: The ethical committee approved the protocol of the study.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: Informed consent was obtained from all participants, and all procedures were conducted according to the Declaration of Helsinki.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bissolotti L, Gobbo M, Villafañe JH, Negrini S. Spinopelvic balance: new biomechanical insights with clinical implications for Parkinson’s disease. Eur Spine J. 2014;23(3):576-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307-310. [PubMed] [Google Scholar]
- 3. Bohannon RW, Peolsson A, Massy-Westropp N, Desrosiers J, Bear-Lehman J. Reference values for adult grip strength measured with a Jamar dynamometer: a descriptive meta-analysis. Physiotherapy. 2006;92:11-15. [Google Scholar]
- 4. Cheung CL, Nguyen US, Au E, Tan KC, Kung AW. Association of handgrip strength with chronic diseases and multimorbidity: a cross-sectional study. Age. 2013;35(3):929-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coldham F, Lewis J, Lee H. The reliability of one vs. three grip trials in symptomatic and asymptomatic subjects. J Hand Ther. 2006;19(3):318-326. [DOI] [PubMed] [Google Scholar]
- 6. Cooper R, Hardy R, Aihie Sayer A, et al. Age and gender differences in physical capability levels from mid-life onwards: the harmonisation and meta-analysis of data from eight UK cohort studies. PLoS ONE. 2011;6(11):e27899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fess E, Moran C. Clinical Assessment Recommendations. Garner: American Society of Hand Therapists; Chicago, IL; 1981. [Google Scholar]
- 8. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kennedy D, Jerosch-Herold C, Hickson M. The reliability of one versus three trials of pain-free grip strength in subjects with rheumatoid arthritis. J Hand Ther. 2010;23(4):384-390. [DOI] [PubMed] [Google Scholar]
- 10. Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57(10):B359-B365. [DOI] [PubMed] [Google Scholar]
- 11. Nitsche J, McMeeken J, Burry H, Matyas T. When is a change a genuine change? a clinically meaningful interpretation of grip strength measurements in healthy and disabled women. J Hand Ther. 1999;12:25-30. [PubMed] [Google Scholar]
- 12. Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1583-1590. [DOI] [PubMed] [Google Scholar]
- 13. Sanderson WC, Scherbov S. Measuring the speed of aging across population subgroups. PLoS ONE. 2014;9(5):e96289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stratford P, Levy D, Gauldie S, Levy K, Miseferi D. Extensor carpi radialis tendonitis: a validation of selected outcome measures. Physiother Can. 1987;39:250-255. [Google Scholar]
- 15. Villafañe JH, Valdes K, Vanti C, Pillastrini P, Borboni A. Reliability of handgrip strength testing in elderly subjects with unilateral thumb carpometacarpal osteoarthritis. Hand. 2015;10(2):205-209. doi: 10.1007/s11552-014-9678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


