Abstract
Background: We compare the ability of 3 diagnostic tests to reproduce the pain of basilar joint arthritis (BJA): the grind test, the lever test (grasping the first metacarpal just distal to the basal joint and shucking back and forth in radial and ulnar directions), and the metacarpophalangeal extension test. Methods: Sixty-two patients with thumb BJA were enrolled. The 3 tests were performed in a random order on both hands of each patient. Prior to testing, patients reported their typical pain level and subsequently rated their pain after each test on a 0 to 10 scale, also specifying the extent to which the test reproduced their thumb pain (fully, partially, not at all). All patients had radiographs that displayed basal joint arthritis. A test was defined as positive for BJA if pain produced was greater than 0. Sensitivity and specificity for each test were calculated using the patients’ history of pain localized to the basal joint and BJA diagnosis on radiographs as the gold standard. Results: The lever test produced the greatest level of pain and best reproduced the presenting pain. The lever test also had the highest sensitivity, high specificity, and the lowest false-negative rate. The grind test had the lowest sensitivity, highest specificity, and highest false-negative rate. Conclusions: The lever test was the diagnostic test that best reproduced the pain caused by thumb basal joint osteoarthritis. We recommend using the lever physical examination test when evaluating the patient with suspected basal joint osteoarthritis. The often-quoted grind test is of limited diagnostic value.
Keywords: arthritis, basal joint, carpometacarpal, osteoarthritis, thumb
Introduction
Arthritis of the first carpometacarpal (CMC) joint is a common condition seen by hand surgeons and primary care providers alike. The prevalence of thumb CMC osteoarthritis, or basilar joint arthritis (BJA), has been reported to be as high as 33% among postmenopausal women and 8% to 12% in the general population.1,10,11
Physical examination findings contribute to the diagnosis of BJA, but it remains unknown whether symptom severity correlates with specific physical exam findings. The grind test is perhaps the most commonly referenced diagnostic tool to evaluate and confirm osteoarthritis.2-4,6,14,15,17 To perform the grind test, the examiner applies axial compression along the plane of the thumb metacarpal and simultaneously rotates the thumb metacarpal base (Figure 1). The test is positive if it reproduces pain in the joint.8,13,16 Merritt et al have shown the sensitivity of the grind test for 2 examiners to be 42% and 53% and the specificity to be 80% and 93%, and Choa et al have shown 30% sensitivity and 96.7% specificity.5,12
Figure 1.
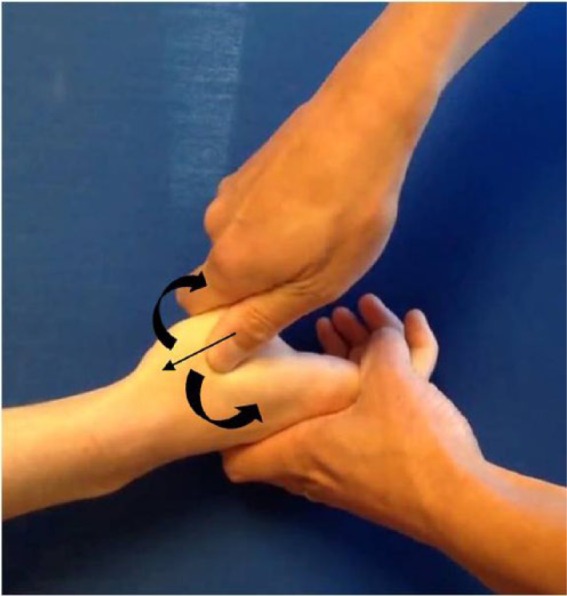
The grind test.
Note. The examiner applies axial compression along the plane of the metacarpal bone and rotates the thumb metacarpal base.
We describe the “lever test,” performed by grasping the first metacarpal just distal to the basal joint and shucking back and forth in radial and ulnar directions (Figure 2). For the metacarpophalangeal (MP) extension test, the patient attempts to extend the thumb while the examiner provides resistance against extension on the proximal phalanx (Figure 3). Palpation is fundamental to any physical examination but has not been studied relative to these provocative maneuvers. Examiners also performed direct palpation of the thumb basal joint on the palmar side in addition to the 3 physical examination maneuvers.
Figure 2.
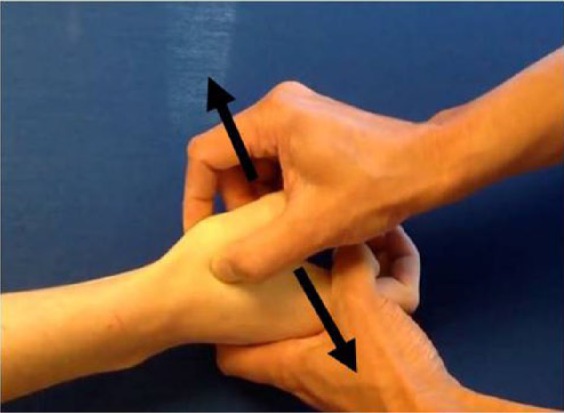
The lever test.
Note. The examiner puts his or her thumb and index finger on both sides of the thumb basal joint and levers the first metacarpal joint radially and ulnarly to their endpoints at the basal joint.
Figure 3.
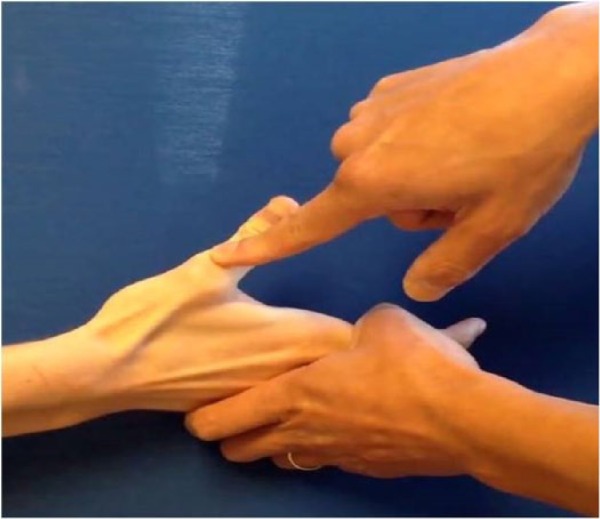
The MP extension test.
Note. The patient tries to extend the thumb while the examiner provides resistance against extension by placing 1 finger on the thumb IP joint. MP, metacarpophalangeal; IP, interphalangeal.
The purpose of the study is to compare the effectiveness of these 3 physical examination maneuvers as a diagnostic tool.
Materials and Methods
Sixty-two consecutive patients (21 male, 41 female) who presented to 4 hand surgeons between April and August 2014 with the inclusion criteria of pain at the base of the thumb were enrolled in this prospective cohort study. This study received hospital institutional review board approval, and written informed consent was obtained from all patients for being included in the study. The mean age was 62 ± 8 years (range, 45-86 years). A total of 39 patients were bilaterally symptomatic and 23 were unilaterally symptomatic. Hands with traumatic injury to the thumb or inflammatory arthritis were excluded, which led to the exclusion of 1 symptomatic hand from the bilateral cohort and 2 asymptomatic hands from the unilateral cohort. De Quervain’s tenosynovitis was ruled out in each case using the Eichhoff test.7 A total of 121 hands were included in the analysis: 100 symptomatic hands and 21 asymptomatic hands from the patients with unilateral pain that were used as a baseline for comparison.
During the evaluation, the grind, lever, and MP extension tests were performed on both hands of each patient. The order in which the tests were performed was randomized for each patient prior to patient enrollment. Randomization was done to eliminate any effect from the order in which the tests were administered.12 All investigators were trained on the tests with instructional videos and one-on-one training to create test uniformity across investigators. First patients verbally reported their pain in each hand on a scale of 0 (no pain) through 10 (worst pain) averaged over the previous week, which we define as presenting pain; then they were asked to rate their pain on a scale of 0 through 10 after each test was performed on both hands. For each test, patients were asked to define the extent to which the test reproduced their chief complaint (no, partially, yes). Direct palpation of the basal joint on the palmar side was also performed on each patient to confirm pain localized to the basal joint, and patients were asked to report their pain after palpation. Radiographic evidence of BJA was recorded. The literature is unclear about a gold standard for positive BJA diagnosis, though investigators typically use a combination of both physical and radiographic evidence for diagnosis.2 We define a positive diagnosis of BJA to be a history of pain localized to the basal joint and radiographic reading of BJA.
Descriptive statistics were used and are displayed as means and standard deviations for continuous variables (patient-reported pain scores) and frequencies and percentages for categorical variables (pain reproduced no/partially/yes, and BJA diagnosis). Differences in the level of pain produced by the 3 tests were assessed with Kruskal-Wallis tests. When significant, post hoc tests were conducted pairwise, and a Bonferroni correction was performed for multiple tests. To assess how well each test reproduced the presenting pain, intraclass correlation (ICC) coefficients and 95% confidence intervals were calculated. An ICC coefficient closer to 1 signals a higher level of agreement between the pain produced by the tests and the presenting pain. The effectiveness of the 3 tests was examined by calculating sensitivity, specificity, positive and negative predictive values, and false-negative rates. For this analysis, a positive test result was defined as a patient reporting pain greater than 0 for the test, and a negative test result was defined as a patient reporting 0 pain for the test. Each test was compared with the diagnosis of BJA based on history of pain and radiographic reading of BJA. Statistical analyses were performed for all hands in SAS 9.3 (Cary, North Carolina) with a level of significance of α = .05. Given the sample size of the study, we had greater than 80% power to detect a difference of 15% in pain scores between tests.
Results
The efficacy of each test was determined based on how well it reproduced the presenting pain. The pain levels produced by the lever test and direct joint palpation did not differ from the presenting pain (P = .4 and P = 1, respectively), whereas the grind and MP tests produced pain levels significantly lower than the presenting pain (P < .001 for each vs. everyday pain). Pain produced from the lever test was greater than that produced by the grind test (P < .001) and MP extension test (P < .001; Table 1). In unaffected hands, the mean presenting pain as well as pain reported with each test approached zero.
Table 1.
Which Test Produced the Most Pain in Symptomatic Hands?
| Variable | n | Mean | SD | Overall P value |
|---|---|---|---|---|
| Presenting pain | 98 | 4.87 | 2.65 | <.001 |
| MP pain | 100 | 2.57 | 2.52 | |
| Lever pain | 100 | 4.17 | 3.08 | |
| Grind pain | 100 | 1.21 | 1.95 | |
| Palpation pain | 100 | 4.44 | 2.87 |
Note. MP, metacarpophalangeal.
The lever test and joint palpation most closely reproduced BJA pain (Table 2). Joint palpation completely or partially reproduced patients’ pain 86% of the time, and the lever test completely or partially reproduced patients’ pain 79% of the time, whereas the grind test and MP test had only 39% and 57% affirmative responses, respectively (Table 3).
Table 2.
Pairwise Post Hoc Tests to See Which Differed.
| Presenting pain | MP pain | Lever pain | Grind pain | Palpation pain | |
|---|---|---|---|---|---|
| Presenting pain | <0.001 | 0.40 | <0.001 | >0.99 | |
| MP pain | 0.001 | <0.001 | <0.001 | ||
| Lever pain | <0.001 | >0.99 | |||
| Grind pain | <0.001 | ||||
| Palpation pain |
Note. MP, metacarpophalangeal.
Table 3.
Patient-Reported Reproduction of Presenting Pain After Each Test.
| Reproduce pain? | MP extension |
Lever |
Grind |
Palpation |
|---|---|---|---|---|
| % | % | % | % | |
| No | 43 | 21 | 61 | 14 |
| Partially | 10 | 12 | 14 | 14 |
| Yes | 47 | 67 | 25 | 72 |
Note. MP, metacarpophalangeal.
Sensitivity measures the proportion of patients who are correctly identified as having BJA, and specificity measures the proportion of patients who are correctly identified as not having the disease. The lever test and joint palpation had both high sensitivity and high specificity, and the lowest false-negative rates. The grind test had the lowest sensitivity, highest specificity, and highest false-negative rate (Table 4).
Table 4.
Sensitivity and Specificity Analysis.
| MP | Lever | Grind | Palpation | |
|---|---|---|---|---|
| Sensitivity | 0.65 | 0.82 | 0.41 | 0.91 |
| Specificity | 0.95 | 0.81 | 1.00 | 0.76 |
| Positive predictive value | 0.98 | 0.95 | 1.00 | 0.95 |
| Negative predictive value | 0.36 | 0.49 | 0.26 | 0.64 |
| False positives (%) | 0.8 | 3 | 0 | 4 |
| False negatives (%) | 29 | 15 | 49 | 7 |
Note. MP, metacarpophalangeal.
Discussion
Our study indicates that, of the 3 provocative tests, the lever test was the most sensitive for BJA. As expected, direct palpation of the joint was highly provocative of tenderness and mirrored the patient’s symptoms. Sensitivity, specificity, and pain reproduction values for joint palpation were high, and joint line tenderness is expected for an arthritic joint. The value of the lever test is that the maneuver is similar to palpation but with the added manipulation of the CMC joint. The lever tests produce a high level of pain, as well as a high correlation with patients’ arthritic pain symptoms. The MP extension test produces significantly less pain, and the grind test elicits the least pain. The lever test is the more sensitive test for BJA, whereas the grind and MP extension tests are less sensitive tests. Although the grind test had a higher specificity than the other tests, indicating a lower probability of false-positive results, we believe that its low sensitivity and therefore high false-negative rate result in inferior clinical utility to both joint palpation and the lever test for diagnosis of BJA.
Other studies have also questioned the utility of the grind test. The study by Merritt et al concluded that a positive grind test has low sensitivity and high specificity and that a negative grind test does not rule out BJA.12 The false-negative rate for the grind test in our study was 49%, which is consistent with the assessment by Merritt et al. Choa et al found the grind test to have inferior specificity and sensitivity to the traction-shift test, a newly described diagnostic test in which the examiner provokes subluxation and then relocation of the joint.5 However, 33% of patients in the study with confirmed radiographic BJA did not experience any pain with either test, which is indicative of a high false-negative rate. The study also did not randomize the order of the tests to ensure that pain from the first test did not affect pain from the second test.
Because patients presenting with BJA often have bilateral symptoms, a limitation of this study is the lack of an internal control for each enrolled patient. Consequently, 100 arthritic hands were analyzed, compared with 21 asymptomatic hands serving as a baseline. One solution for future studies involving BJA may be to only enroll patients with unilateral BJA. Another limitation of this study is that only symptomatic patients were enrolled; therefore, these maneuvers may be less discriminatory for BJA on asymptomatic patients. A third limitation is the use of patient-reported pain scores rather than examiner-determined crepitus in the joint during the test, which would have been complicated by the variability among data collectors in their ability to detect crepitus.
Patients enrolled in the study presented to 4 different hand surgeons; therefore, variations between examiners in performing each diagnostic test, such as the amount of pressure applied, may have influenced patient-reported pain scores. We attempted to reduce this variation with instructional videos and one-on-one training for each investigator. In addition, pain is inherently subjective, and non-uniform ceiling effects in interpreting maximum pain limit the utility of the pain scale to quantify pain.9 Patients may also be subject to recall bias, in which they remember the most painful episodes over the past week.
Despite these limitations, the results of this study demonstrate that the lever test and joint palpation best reproduce the pain caused by thumb basal joint osteoarthritis. A physical examination test sensitive for BJA optimizes efficiency while seeing patients. Although we use the criteria of history of pain and positive x-ray reading of BJA as the gold standard in this study, those criteria were used because we were testing physical examination maneuvers. We believe that a combination of all 3 is necessary for diagnosis: history of pain at the basal joint, pain with a physical examination maneuver sensitive for BJA, and positive radiographic reading. We recommend using the lever physical examination test and joint palpation when evaluating a patient with suspected basal joint arthritis. The often-quoted grind test is of limited value.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent: Informed consent was obtained from all patients for being included in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J Hand Surg Br. 1994;19(3):340-341. [DOI] [PubMed] [Google Scholar]
- 2. Barron OA, Catalano LW. Thumb basal joint arthritis. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Elsevier/Churchill Livingstone; 2011:407-425. [Google Scholar]
- 3. Barron OA, Glickel SZ, Eaton RG. Basal joint arthritis of the thumb. J Am Acad Orthop Surg. 2000;8(5):314-323. [DOI] [PubMed] [Google Scholar]
- 4. Carr MM, Freiberg A. Osteoarthritis of the thumb: clinical aspects and management. Am Fam Physician. 1994;50(5):995-1000. [PubMed] [Google Scholar]
- 5. Choa RM, Parvizi N, Giele HP. A prospective case-control study to compare the sensitivity and specificity of the grind and traction-shift (subluxation-relocation) clinical tests in osteoarthritis of the thumb carpometacarpal joint. J Hand Surg Eur Vol. 2014;39(3):282-285. [DOI] [PubMed] [Google Scholar]
- 6. Dias R, Chandrasenan J, Rajaratnam V, Burke FD. Basal thumb arthritis. Postgrad Med J. 2007;83(975):40-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eichhoff E. Pathogenese der tendovaginitis stenosans. Bruns Breitage z klin Chir. 1927(136):745-755. [Google Scholar]
- 8. Glickel SZ. Clinical assessment of the thumb trapeziometacarpal joint. Hand Clin. 2001;17(2):185-195. [PubMed] [Google Scholar]
- 9. Gonzalez-Fernandez M, Ghosh N, Ellison T, McLeod JC, Pelletier CA, Williams K. Moving beyond the limitations of the visual analog scale for measuring pain: novel use of the general labeled magnitude scale in a clinical setting. Am J Phys Med Rehabil. 2014;93(1):75-81. [DOI] [PubMed] [Google Scholar]
- 10. Haara MM, Heliovaara M, Kroger H, et al. Osteoarthritis in the carpometacarpal joint of the thumb: prevalence and associations with disability and mortality. J Bone Joint Surg Am. 2004;86-A(7):1452-1457. [DOI] [PubMed] [Google Scholar]
- 11. Lawrence JS, Bremner JM, Bier F. Osteo-arthrosis. Prevalence in the population and relationship between symptoms and x-ray changes. Ann Rheum Dis. 1966;25(1):1-24. [PMC free article] [PubMed] [Google Scholar]
- 12. Merritt MM, Roddey TS, Costello C, Olson S. Diagnostic value of clinical grind test for carpometacarpal osteoarthritis of the thumb. J Hand Ther. 2010;23(3):261-267. [DOI] [PubMed] [Google Scholar]
- 13. North ER, Eaton RG. Degenerative joint disease of the trapezium: a comparative radiographic and anatomic study. J Hand Surg Am. 1983;8(2):160-166. [DOI] [PubMed] [Google Scholar]
- 14. Polatsch DB, Paksima N. Basal joint arthritis: diagnosis and treatment. Bull NYU Hosp Jt Dis. 2006;64(3-4):178-184. [PubMed] [Google Scholar]
- 15. Pomerance JF. Painful basal joint arthritis of the thumb. Part I: Anatomy, pathophysiology, and diagnosis. Am J Orthop. 1995;24(5):401-408. [PubMed] [Google Scholar]
- 16. Poole JU, Pellegrini VD., Jr Arthritis of the thumb basal joint complex. J Hand Ther. 2000;13(2):91-107. [DOI] [PubMed] [Google Scholar]
- 17. Swanson AB, de Groot Swanson G. Osteoarthritis in the hand. Clin Rheum Dis. 1985;11(2):393-420. [PubMed] [Google Scholar]


