Abstract
Background: Local anesthetics are routinely used in hand surgery for procedures such as trigger finger releases (TFRs). However, little is known as to the difference in efficacy and patient experience with various local anesthetics. We prospectively evaluated the efficacy of Lidocaine (L), Marcaine (M), and Exparel (E) to elucidate differences in pain scores and opioid consumption between these groups. Methods: All consecutive TFR performed over a 6-month period in 2014 at our institution were divided to receive Lidocaine, Marcaine, or Marcaine with postoperative Exparel. Pain levels, daily opioid consumption, and adverse reactions were recorded and analyzed for postoperative day (POD) 0-3. Results: A total of 154 patients were enrolled (L:53, M:50, E:51). The Lidocaine group reported the highest pain levels for POD 0-1. Marcaine pain levels were similar to Exparel on POD 0 but higher on POD 1. Opioid consumption on POD 0-1 was significantly different with E:27%, M:58% and L:59% as was the number of pills consumed (E:0.70, M: 1.08 and L:1.62). In addition, 50% of Exparel patients required no pain medications and experienced significantly less adverse reactions (E:4%, M:10%, L:13%). By POD 2-3, there were no statistical differences between the 3 groups. Conclusions: Patients treated with Marcaine attain better pain control than Lidocaine on POD 0-1but only patients who received Exparel maintained the lowest pain levels through POD 0-3 while using little-to-no opioid medications and with less adverse reactions than Lidocaine or Marcaine alone.
Keywords: trigger finger, wide awake, Lidocaine, Marcaine, Exparel
Introduction
Trigger finger is one of the most common conditions treated by hand surgeons with a lifetime risk estimated at 2.6% in the general population and 4% to 10% in patients with diabetes.7,8,13,17 It is generally characterized by pain, swelling, limitation of finger motion, and a triggering sensation caused by thickening of the A1 pulley and/or localized thickening of the tendon with resultant entrapment of the flexor tendons.14
Conservative treatments are typically attempted first, including activity modification, splinting, physical therapy, nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroid injections, all of which have demonstrated varying degrees of success.13,14 If conservative management fails, surgical treatment is recommended that can result in up to a 100% success rate.3 Surgery is traditionally performed with or without sedation and a tourniquet, via a small incision at the level of the volar aspect of the metacarpal head followed by exposure and longitudinal release of the A1 pulley.
A number of local anesthetics are readily available and utilized in hand surgery including but not limited to ropivacaine, Lidocaine and Marcaine. Recently, Exparel (Pacira, Parsippany, New Jersey) has been introduced which is an extended-release liposomal Marcaine-based analgesic that was granted FDA approval in 2011 for postsurgical analgesia through single-dose local administration into the surgical wound.15 The extended-release formulation consists of a microscopic, spherical, lipid-based delivery system, which allows for diffusion of Marcaine over an extended period, resulting in purported pain relief for up to 96 hours after surgery. Since its introduction, there have been numerous reports of successfully achieving prolonged pain relief with Exparel after various procedures such as breast augmentation, bunionectomy, hernia repairs, and total knee arthroplasty.1,9,21 However, currently there are no studies on Exparel in hand surgery.
In this study, we prospectively evaluated the efficacy Lidocaine, Marcaine, or Marcaine with postoperative Exparel in controlling postoperative pain, opioid usage, and adverse reactions following trigger finger release (TFR) surgery performed wide awake without sedation or a tourniquet. The study hypothesis was that Exparel would result in lower postoperative pain and opioid consumption than with Lidocaine and Marcaine.
Materials and Methods
After obtaining institutional review board approval, all consecutive patients scheduled to undergo single digit TFR surgery over a 6-month period in 2014 were invited to participate. All procedures were performed under local anesthesia without sedation or tourniquet by 1 of 7 orthopedic board-certified fellowship-trained hand surgeons. Informed consent was obtained by all interested patients before participation in the study. Inclusion criteria were single digit TFR surgery in any patient above the age of 18. Exclusion criteria included multiple digit TFRs, concomitant hand surgical procedures (ie, carpal tunnel release), and revision TFR surgery. Patients requesting surgery under sedation, patients requiring opioid analgesia for other complaints preoperatively, and those with known allergies to either Lidocaine, Marcaine, or Exparel were also excluded.
All of the injections were performed in a predetermined similar manner. The technique for injection was that of a single volar injection at the level of the A1 pulley with a volume of 5 to 10 mL of the local anesthetic delivered subcutaneously superficial to the flexor tendon sheath. The injectate formulation consisted of either 1% Lidocaine or 0.5% Marcaine injected into the closed surgical site. All formulations of both Lidocaine and Marcaine included 1:100 000 epinephrine, as well as 8.4% bicarbonate (mixed 10 mL:1 mL). Patients in the Exparel group received an injection of 5 to 10 mL of 0.5% Marcaine with 1:100 000 epinephrine and 8.4% bicarbonate (mixed 10 mL:1 mL) prior to skin incision, followed by injection of 5 cc of Exparel into the surgical site upon closure as recommended by the manufacturer. This technique is in line with the manufacturer’s recommendations which indicate that Exparel can be injected following Marcaine but not following other anesthetics such as Lidocaine as the latter has a conflicting effect with their formulation. Participating surgeons were assigned to 1 of the 3 groups (L, M, or E) and subsequently only utilized that injection type for all of their enrolled patients.
Surgeries were all performed in a similar fashion with an approximately 1.0- to 1.5-cm incision followed by complete longitudinal release of the A1 pulley of the operative finger. Complete release of the pulley and resolution of triggering was confirmed by active motion by the awake patient. Patients received no systemic anesthesia and had no tourniquet applied. Postoperatively, all patients were given a standardized script for an opioid (Percocet, Vicodin, or Tylenol #3) of their choice. Patients were also instructed to record their medication use, their pain levels using a visual analog scale (VAS) scoring system, and any adverse reactions experienced such as dry mouth, nausea, vomiting, feeling drowsy, trouble sleeping, feeling bloated, constipation, trouble urinating, itching, dizziness, sweating, coughing, and lack of energy. Patients were contacted at home after postoperative day (POD) 3 to determine their pain on a VAS, daily opioid consumption, and any adverse reactions.
An analysis of variance was used to detect significant differences between groups, and subsequent pairwise comparisons were performed. The 3 arms were compared using Kruskal-Wallis or analysis of variance for continuous variables, depending on parametric fit of the data. For continuous data, study arms were directly compared with each other with a Mann-Whitney U test. The chi-square test was utilized for categorical variables. Statistical analyses were performed using SPSS version 20 (IBM, Armonk, New York).
Results
The study consisted of a total of 163 patients (85 women and 78 men), with only 9 patients lost to follow-up for an overall attrition rate of 5.5%. After excluding patients lost to follow-up, the Marcaine group included 50 patients with an average age of 61, the Lidocaine group included 53 patients with an average age of 65, and the Exparel group included 51 patients with an average age of 64. Age weakly correlated with pain on POD 1 (R = −0.17; P = .03) and number of narcotic pills on POD 1 (R = −0.205; P = .01) and POD 2 (R = −0.19; P = .016).
VAS Pain Scores
Patients were contacted after POD 3 and asked about their pain levels over POD 0-3 (Figure 1). On POD 0, patients in the Lidocaine group reported the most pain, which was statistically higher than both the Marcaine and Exparel groups. The VAS score for the Lidocaine group was 4.40 as compared with the Marcaine group with 2.54 (P = .011) and Exparel group with 2.46 (P < .0001). On POD 1, this difference was maintained with the Lidocaine group at 3.73 as compared with the Marcaine and Exparel groups at 2.90 (P = .116) and 2.33 (P = .003), respectively. Only the Exparel group maintained significance on POD 1. In contrast, on POD 2 and POD 3, the differences were more subtle and did not reach statistical significance. The Lidocaine group continued to report decreasing pain at 2.39 for POD 2 and 1.69 for POD 3, as compared with the Marcaine group that reported pain scores 2.50 and 1.96, and the Exparel group reporting 1.94 and 1.78, respectively.
Figure 1.
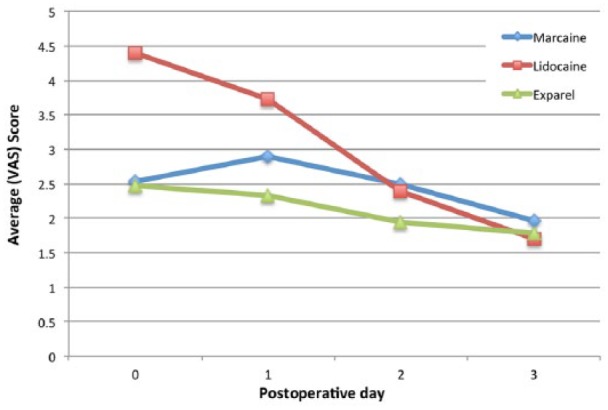
Patient reported pain as a VAS score during the first 3 days postoperatively for each of the anesthetic agents administered.
Note. VAS, visual analog scale.
Interestingly, pain scores remained low through the study period with no significant change within the Exparel group from POD 0-3 (R = −0.087, P = .22), as opposed to Marcaine (R = −0.115, P = .13) and Lidocaine (R = −0.398, P ≤ .001) groups that demonstrated a gradual decrease in pain reported reaching the pain levels of the Exparel group by POD 3.
Opioid Consumption
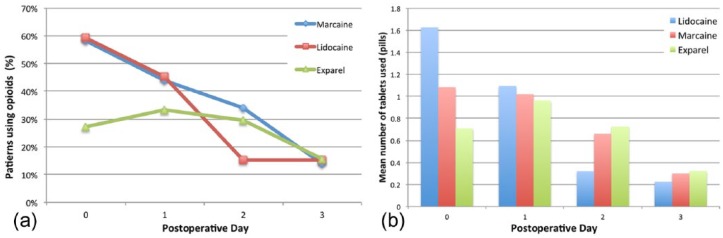
Patients were also asked to keep a record of the medication type as well as the number of pills used postoperatively (Figure 2a). On POD 0, 58% (P = .01) and 59% (P = .004) of patients who received Marcaine and Lidocaine, respectively, used opioids for pain control as compared with 27% of patients in the Exparel group. By POD 1, the percentage of Exparel patients utilizing opioids showed a statistically insignificant increase to 33.3%, while still maintaining lower opioid consumption than the Marcaine and Lidocaine groups, where 44% (P = .271) and 45% (P = .213) of the patients, respectively, used opioids. At POD 2, the percentage of patients using opioids continued to decrease in all groups and converged to about 15% by POD 3.
Figure 2.
(a) Percentage of patients in each anesthetic group that required opioid medications during the first 3 days after surgery. (b) Average number of pills consumed by the subset of patients requiring opioid medications during the postoperative period.
A similar trend is seen when the average number of opioid pills consumed by these groups is analyzed (Figure 2b). The only statistically different pill consumption was observed on POD 0 where opioid users in the Lidocaine group consumed an average of 1.62 pills as compared with 1.08 (P = .214) and 0.70 (P = .013) pills in the Marcaine and Exparel groups, respectively. Total pill consumption on POD 1-3 was similar in all groups.
Pain Control and Medication Use
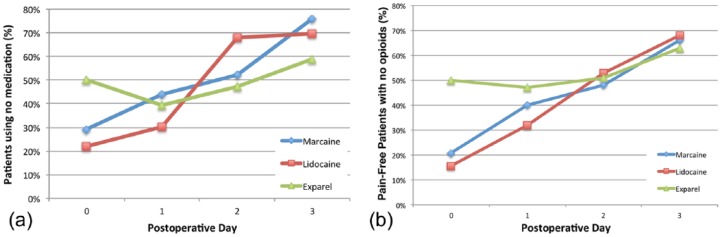
The percentage of patients who required no pain medication (opioids or otherwise) was also recorded for POD 0-3 (Figure 3a). On POD 0, 50% of patients in the Exparel group required no pain medications as compared with the Lidocaine and Marcaine groups where 22% (P = .011) and 29% (P = .092), of patients were medication free. By POD 1, this percentage decreased in the Exparel group to 39% whereas it increased to 30% and 44% in the Lidocaine and Marcaine groups, respectively, without reaching statistical difference. For POD 2 and POD 3, the percentage of people not requiring any pain medication continued to increase in all groups and reached an average of 68% by POD 3.
Figure 3.
(a) Percentage of patients in each anesthetic group that required no medications (opioid or otherwise) during the first 3 days after surgery. (b) Percentage of patients who report being pain-free (VAS score < 3) while not taking any opioid medication.
Note. VAS, visual analog scale.
An analysis of patients who were deemed pain-free (defined as a VAS score ≤ 2) while also not using any opioid medication was also performed (Figure 3b). On POD 0, 50% of patients who received Exparel were pain-free without requiring opioids, which was statistically higher than Lidocaine at 16% (P = .002) and Marcaine at 21% (P = .017). This trend continued on POD 1 with Exparel at 47% (P = .474), Marcaine at 40%, and Lidocaine at 32% (P = .118). By POD 2-3, the percentage of patients in this category converged and there were no statistical differences between them.
Adverse Reactions
All adverse reactions experienced by patients in the first 3 days after surgery were recorded (Table 1). The percentage of patients reporting any adverse reaction at any time in the first 3 days after surgery was significantly lower in the Exparel group as compared with the Marcaine and Lidocaine groups. Only 4% of the Exparel patients reported adverse reactions as opposed to 13% (P = .017) and 10% (P = .133) of patients in the Lidocaine and Marcaine groups, respectively (Figure 4). The most common reactions reported included dry mouth, nausea, lack of energy, and itching, whereas the least common reactions were dizziness, coughing and a sensation of bloating. There were no adverse reactions requiring emergency room visits, hospitalization, or reoperation in any group.
Table 1.
Summary of AEs.
| Lidocaine (n = 53) |
Marcaine (n = 50) |
Exparel (n = 51) |
Total (n = 154) |
|
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Patients with one or more AE | 7 (13.2) | 5 (10) | 2 (3.9) | 14 (9.1) |
| Dry mouth | 4 (7.5) | 2 (4.0) | 0 (0) | 6 (3.9) |
| Nausea | 5 (9.4) | 0 (0) | 1 (2.0) | 6 (3.9) |
| Vomiting | 3 (5.7) | 0 (0) | 1 (2.0) | 4 (2.6) |
| Feeling drowsy | 2 (3.8) | 2 (4.0) | 0 (0) | 4 (2.6) |
| Trouble sleeping | 2 (3.8) | 0 (0) | 0 (0) | 2 (1.3) |
| Feeling bloated | 1 (1.9) | 0 (0) | 0 (0) | 1 (0.6) |
| Constipation | 1 (1.9) | 1 (2.0) | 0 (0) | 2 (1.3) |
| Trouble urinating | 1 (1.9) | 1 (2.0) | 0 (0) | 2 (1.3) |
| Itching | 3 (5.7) | 3 (6.0) | 0 (0) | 6 (3.9) |
| Dizziness | 1 (1.9) | 0 (0) | 0 (0) | 1 (0.6) |
| Sweating | 0 (0) | 2 (4.0) | 0 (0) | 2 (1.3) |
| Coughing | 0 (0) | 0 (0) | 1 (2.0) | 1 (0.6) |
| Lack of energy | 4 (7.5) | 1 (2.0) | 1 (2.0) | 6 (3.9) |
Note. AEs, adverse events.
Figure 4.
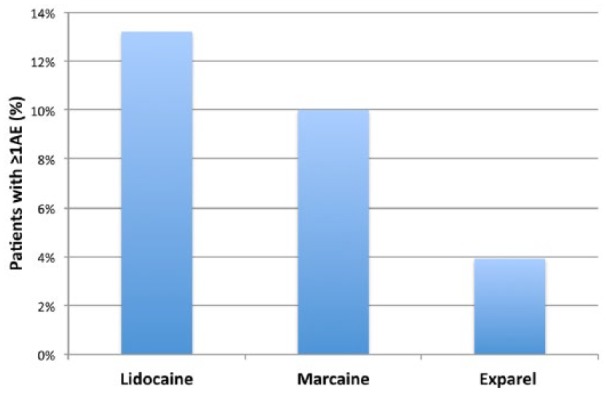
Patient reported percentage of adverse reactions based on the anesthetic group.
Note. AE, adverse event.
Discussion
Effective postsurgical pain control is an essential prerequisite for procedures that are performed on an ambulatory basis, particularly in hand surgery. The most effective pain management is achieved with a multimodal strategy involving regional, intravenous, and local infiltration of anesthetics perioperatively, and typically a combination of opioid medication and NSAIDs postoperatively. Despite the use of these strategies, it is estimated that up to 30% to 40% of ambulatory surgical patients suffer from moderate to severe pain during the first 24 to 48 hours after their discharge.11 Pain often times will interfere with sleep and daily functioning and remains the most common reason for recurrent general practitioner office visits and unanticipated hospital admissions.2,12,20 This is especially true in patients undergoing hand surgery. Chung et al4 prospectively studied 1008 consecutive ambulatory surgical patients across 8 surgical specialties and found that in the recovery room, orthopedic patients (including hand surgical cases) had the highest incidence of pain, more so than urologic, general surgery, and plastic surgery patients. Furthermore, a survey study administered by Rawal et al12 revealed that 37% of hand surgery patients will suffer from moderate to severe pain postoperatively, affecting their function and quality of life in the immediate postoperative period.
Achieving a balance between adequate analgesia and safety can be a challenging task. Increasing the intake of opioids might be an attractive solution for patients but the well-known opioid-induced adverse effects such as respiratory depression, urinary retention, nausea, vomiting, central nervous system depression, pruritus, and constipation make this option often impractical and even dangerous.19 However, local anesthetics once carefully administered tend to have a safer profile and predominantly have minimal systemic effects postoperatively. However, despite attempts to prolong their action through the use of adjuvants, vehicles, and gel formulations of classic analgesics and anesthetics, the typical duration of local anesthetic pain control has been approximately 24 hours.5 The introduction of new-generation extended-release local anesthetics, such as Exparel, for postoperative pain control has the potential of extending the anesthetics effect postoperatively.
Even though multiple studies have been conducted in recent years, looking at the efficacy of Exparel in a number of surgical specialties such as plastic surgery and joint arthroplasty, to the best of our knowledge this study is the first report on the efficacy of Exparel in hand surgery. We compare the efficacy of Lidocaine, Marcaine, and Marcaine followed by a postoperative injection of Exparel, for patients undergoing single TFR surgery in a wide awake fashion without sedation or a tourniquet. In our series, the patients who received Lidocaine reported the most pain by the end of POD 0 when compared with the other two groups, suggesting that its anesthetic effects wear off the earliest. In contrast, both the Marcaine and Exparel groups reported similarly low pain scores indicating that the Marcaine received in the operating room by the patients in the 2 groups provided adequate pain relief though the end of the operative day. By POD 1, the Marcaine effects appear to wear off and patient discomfort increased as compared with the Exparel group where pain control continues. By POD 2 and more so by POD 3, difference in pain scores diminished between the 3 groups indicating that either the pain levels for this procedure have reached their baseline values or that all 3 anesthetic agents lost their efficacy within the first 2 days. Pain improvement in the Marcaine and Exparel groups follows a very similar slope for POD 1-3, but Exparel shows a consistently lower score throughout the study period. In contrast, the Lidocaine group had a steady and steeper decrease in the pain scores over POD 0-3.
This pattern and duration of pain relief is similar to what has previously been described in the literature. Thomson and Lalonde18 performed a randomized double-blind comparison of duration of anesthesia among Lidocaine and Marcaine in digital nerve block and report that Marcaine provided significantly longer digital anesthesia time with average of 24.9 hours as compared with 2% Lidocaine that averaged 4.9 hours. In addition, in a double-blind randomized study by Spivey et al,16 patients undergoing minor surgery in the emergency department reported significant pain 1 hour after closure of their wound when Lidocaine was used, but not until 6 hours later if they received Marcaine.
Perhaps more important than the absolute pain scores is patient utilization of opioids to achieve adequate pain control. A significantly lower percentage of patients in the Exparel group required opioids to control their pain on POD 0, when compared with the patients in the Marcaine group. Furthermore, the small percentage of Exparel patients who reported taking narcotics consumed on average less opioid tablets. These trends continued on POD 1, but by POD 2-3 there were no differences in opioid consumption between the groups. In addition, the Exparel group also comprised the highest percentage of patients requiring no pain medication (opioid or otherwise) on POD 0. Given that the 3 groups required varying amounts of pain medications to attain a comfortable postoperative pain control, a subgroup analysis was performed to separate the effects of local anesthetics from those of oral opioids. Half of all the patients in the Exparel group did not require any opioids on POD 0, while also reporting that they were pain-free (VAS score ≤ 2). This was statistically different than both the Marcaine and Lidocaine groups, of which only about 20% were able to achieve pain control with no opioids. This trend continued on POD 1 but was undetectable by POD 2 and 3. By then, most patients, regardless of anesthetic used, do not have much pain from TFR surgery and have no need for narcotics.
From our data, it appears that Exparel makes a difference in pain perception the first 1 to 2 days after surgery, which is in agreement with what has been reported in other series. In a recent randomized, multicenter, double-blind phase 3 clinical study,6 Exparel was compared with placebo for the prevention of pain after bunionectomy. Using a numeric rating scale (NRS) for pain, scores were significantly lower in patients treated with Exparel as compared with patients receiving placebo at 24 and 36 hours. They also found that more patients in the Exparel group avoided use of opioid rescue medication during the first 24 hours and were pain-free up to 48 hours after surgery. Furthermore, in our patient population, patients who received Exparel postoperatively reported significantly less adverse reactions when compared with the patients receiving Lidocaine or just Marcaine. In the same study by Golf et al6 looking at pain control after bunionectomy, they found that fewer adverse events were reported by patients treated with Exparel (59.8%) versus placebo (67.7%). Portillo et al10 just completed their systematic review of prospective studies on the use of Exparel and the analysis of the incidence of reported adverse effects when compared with conventional Marcaine or placebo in knee arthroplasty, hemorrhoidectomy, augmentation mammoplasty, bunionectomy, and healthy volunteers. They found that Exparel used in therapeutic doses was well tolerated, and showed a favorable safety profile compared with Marcaine and controls.
Despite these considerations, some thought must be given to the cost of these anesthetic agents. Exparel is significantly more expensive than either Lidocaine or Marcaine and therefore its cost must be balanced against the cost of utilization versus the value of extended pain relief for the specific procedure being performed.
Our study had some limitations. First, the patients were followed prospectively in cohorts but not randomized. Second, any study that depends on patient reporting, as ours did for opioid consumption, can suffer from recall bias. Similarly, patients were permitted to utilize over-the-counter medication such as acetaminophens and NSAIDs postoperatively that could have affected ultimate opioid consumption. Third, it is difficult to discern whether the adverse events experienced postoperatively were due to the local anesthetic or opioid use.
Overall, pain following TFR surgery performed wide awake and without a tourniquet is low. To the best of our knowledge, this study was the first to examine the efficacy of Exparel in hand surgery and we identified longer pain relief, decreased opioid consumption, and a better adverse reaction profile with Exparel from POD 0-2, compared with Lidocaine and Marcaine. By POD 3, the differences between the Lidocaine, Marcaine, and Exparel groups were minimal. More studies are needed to validate both the efficacy and cost of Exparel versus other local anesthetic agents in patients undergoing more extensive and painful hand and other orthopedic procedures.
Acknowledgments
The authors wish to thank Mitchell Maltenfort, PhD, for his support and contribution with the statistical analysis.
Footnotes
Authors’ Note: All work described in this article was performed at the Rothman Institute at the Thomas Jefferson University Hospital, Department of Orthopaedic Surgery.
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent: Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with human or animal subjects.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The research represented in this study was supported through an unrestricted educational grant from Pacira.
References
- 1. Baxter R, Bramlett K, Onel E, Daniels S. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studies. Clin Ther. 2013;35(3):312-320.e5. [DOI] [PubMed] [Google Scholar]
- 2. Beauregard L, Pomp A, Choinière M. Severity and impact of pain after day-surgery. Can J Anaesth. 1998;45(4):304-311. [DOI] [PubMed] [Google Scholar]
- 3. Bonnici AV, Spencer JD. A survey of “trigger finger” in adults. J Hand Surg Br. 1988;13(2):202-203. [DOI] [PubMed] [Google Scholar]
- 4. Chung F, Ritchie E, Su J. Postoperative pain in ambulatory surgery. Anesth Analg. 1997;85(4):808-816. [DOI] [PubMed] [Google Scholar]
- 5. Colombo G, Padera R, Langer R, Kohane DS. Prolonged duration local anesthesia with lipid-protein-sugar particles containing bupivacaine and dexamethasone. J Biomed Mater Res A. 2005;75(2):458-464. [DOI] [PubMed] [Google Scholar]
- 6. Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28(9):776-788. [DOI] [PubMed] [Google Scholar]
- 7. Griggs SM, Weiss AP, Lane LB, Schwenker C, Akelman E, Sachar K. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20(5):787-789. [DOI] [PubMed] [Google Scholar]
- 8. Gurley DJ, Lucas GL. Diabetes and trigger finger. J Hand Surg Br. 1997;22(5):682. [DOI] [PubMed] [Google Scholar]
- 9. Lambrechts M, O’Brien MJ, Savoie FH, You Z. Liposomal extended-release bupivacaine for postsurgical analgesia. Patient Prefer Adherence. 2013;7:885-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Portillo J, Kamar N, Melibary S, Quevedo E, Bergese S. Safety of liposome extended-release bupivacaine for postoperative pain control. Front Pharmacol. 2014;5:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rawal N. Postoperative pain treatment for ambulatory surgery. Best Pract Res Clin Anaesthesiol. 2007;21(1):129-148. [DOI] [PubMed] [Google Scholar]
- 12. Rawal N, Hylander J, Nydahl PA, Olofsson I, Gupta A. Survey of postoperative analgesia following ambulatory surgery. Acta Anaesthesiol Scand. 1997;41(8):1017-1022. [DOI] [PubMed] [Google Scholar]
- 13. Sahu R, Gupta P. Experience of percutaneous trigger finger release under local anesthesia in the Medical College of Mullana, Ambala, Haryana. Ann Med Health Sci Res. 2014;4(5):806-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sampson SP, Badalamente MA, Hurst LC, Seidman J. Pathobiology of the human A1 pulley in trigger finger. J Hand Surg Am. 1991;16(4):714-721. [DOI] [PubMed] [Google Scholar]
- 15. Saraghi M, Hersh EV. Three newly approved analgesics: an update. Anesth Prog. 2013;60(4):178-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spivey WH, McNamara RM, MacKenzie RS, Bhat S, Burdick WP. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16(7):752-757. [DOI] [PubMed] [Google Scholar]
- 17. Strom L. Trigger finger in diabetes. J Med Soc N J. 1977;74(11):951-954. [PubMed] [Google Scholar]
- 18. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118(2):429-432. [DOI] [PubMed] [Google Scholar]
- 19. Tong YC, Kaye AD, Urman RD . Liposomal bupivacaine and clinical outcomes. Best Pract Res Clin Anaesthesiol. 2014;28(1):15–27. [DOI] [PubMed] [Google Scholar]
- 20. Wu CL, Berenholtz SM, Pronovost PJ, Fleisher LA. Systematic review and analysis of postdischarge symptoms after outpatient surgery. Anesthesiology. 2002;96(4):994-1003. [DOI] [PubMed] [Google Scholar]
- 21. Youha Al S, Lalonde DH. Update/review: changing of use of local anesthesia in the hand. Plast Reconstr Surg Glob Open. 2014;2(5):e150. [DOI] [PMC free article] [PubMed] [Google Scholar]