Abstract
Background: The purpose of this case report is to describe the findings of a neuroma within an allograft, highlight the unique opportunity to evaluate the allograft (following human engraftment) ex vivo histologically, to reinforce an effective treatment strategy, and review outcomes in peripheral nerve surgery regarding gap defect distance. Method: A 55-year-old, right hand dominant man suffered a workplace injury 37 years ago resulting in lacerations and crush injury of the palm and lacerations of the left index finger requiring multiple neuroma excisions and eventual ray amputation. In an attempt to address stump neuroma pain and restore sensation of the radial digital nerve of the middle finger, which was lost after the ray amputation, a neuroma was resected and reconstructed with a 45-mm bioabsorbable allograft (AxoGen, Inc, Alachua, Florida). After the inciting injury in 1977, the patient initially presented to our clinic in 2013 with return of pain at the palm and numbness along the distribution of the common digital nerve and radial nerve of the middle finger prompting surgical exploration. A recurrent common digital nerve neuroma was identified at the proximal aspect of the allograft measuring 20 mm and was resected along with the remaining allograft. Results: A 50-mm reversed superficial peroneal interpositional nerve graft was used for reconstruction resulting in progressive resolution of pain. On 6-month follow-up, the patient regained indiscriminate sensation with moving 2-point discrimination at the pulp of the middle finger with improved grasp function. Conclusion: In the setting of recalcitrant neuromas and intractable pain following multiple neuroma excisions, allografts may be suboptimal in reconstruction of larger gap defects. Autologous reconstruction with porcine submucosa extracellular matrix, as in this case, can avoid tethering, local ischemia, and nerve traction to optimize outcomes.
Keywords: Axogen, neuroma, sural, nerve, common digital nerve
Introduction
Following the implementation of collagen-derived nerve conduits, the advent of allogeneic processed nerve graft in 2009 gained momentum as a viable option in reconstructing absent or damaged nerve segments.6 The preserved, decellularized, extracellular matrix architecture, which is present grossly from the epineureum down to the microscopic level of endoneural tubes, fosters host cellular ingrowth and targets neuronal sprouting in an organized manner.13 The conservative application of Avance® Nerve Graft (AxoGen, Inc, Alachua, Florida) for defects less than 20 mm boasted encouraging results both from motor and sensory outcomes and has subsequently led to its application in larger defects.6 For larger nerve defects, autologous nerve grafting remains the gold standard for which biologic material graft performance is compared with.
It remains unclear what role allografts serve in an unfavorable environment plagued by multiple excisions and failed excisional procedures. Recommendations for implementing allograft versus autograft nerve are constantly in flux with regard to the defect gap distance, but the surgical site and history may aid in selecting an optimal approach. Herein we present a 55-year-old male with a recalcitrant neuroma having undergone more than 17 surgeries over the span of 37 years, recently failing allograft nerve reconstruction and ultimately was reconstructed with autologous peroneal nerve to achieve a successful outcome. The purpose of this case is to report the findings of a neuroma within the allograft, highlight the unique opportunity to evaluate the allograft ex vivo and histologically, reinforce an effective treatment strategy, and review outcomes in peripheral nerve surgery regarding gap defect distance.
Case
A 55-year-old, right hand dominant, Caucasian male sustained a workplace machinery injury in 1977 resulting in multiple lacerations of zone II of the left index finger with zone III palmer laceration and crush injury. Over the next 12 years, the patient developed progressive hypersensitivity ultimately failing transcutaneous nerve stimulation therapy, oral analgesia, and multiple scar and neuroma excisions. This ultimately led to ray amputation of the left index finger in 1989, which resolved symptoms for 4 years. He subsequently developed stabbing pain at the previous surgical site prompting further surgical interventions, including 2 neuronal stump resections in 1993 resolving his symptoms for 6 to 7 years. The stabbing pain returned, and in 2013, he underwent excision of a recalcitrant neuroma of the common digital nerve of the second digital web space and radial digital nerve of the middle finger with nerve repair using a 45-mm bioabsorbable allograft (Avance® Nerve Graft; AxoGen, Inc, Alachua, Florida). The patient did not find relief following surgery, and his stabbing pain continued with impaired grasp function due to pain ultimately reducing his quality of life.
At this point, the patient presented to our clinic for further treatment. A diagnostic and therapeutic nerve block over the distribution of the common digital nerve and radial digital nerve of the middle finger provided relief for several hours. A history of cervical spine fusion and cervical spinal stenosis precluded candidacy for placement of a spinal nerve stimulator, and he also did not desire a forearm median nerve stimulator. Approximately 37 years following his initial injury and 17 hand surgeries in the interim, the patient underwent surgical exploration. A large 20-mm neuroma of the common digital nerve was identified in the proximal graft (Figure 1a). The neuroma and bioabsorbable nerve graft were excised proximal to the common digital nerve coaptation and distal to the radial digital nerve coaptation (Figures 1b and 1c). A 50-mm segment of reversed autologous interpositional nerve graft, harvested from the left superficial peroneal nerve, was used to reconstruct the common digital nerve at the takeoff of the median nerve to the radial digital nerve of the middle finger (Figure 2). The proximal and distal nerve coaptations were wrapped with porcine submucosa extracellular matrix (AxoGard® Nerve Protector; AxoGen, Inc, Alachua, Florida). There were no postoperative complications, and the patient experienced progressive improvement of pain following surgery. On 6-month follow-up, the patient regained indiscriminate sensation with moving 2-point discrimination at the pulp of the middle finger and exhibited pain relief of the palm and middle finger. Improved grasp function was also observed.
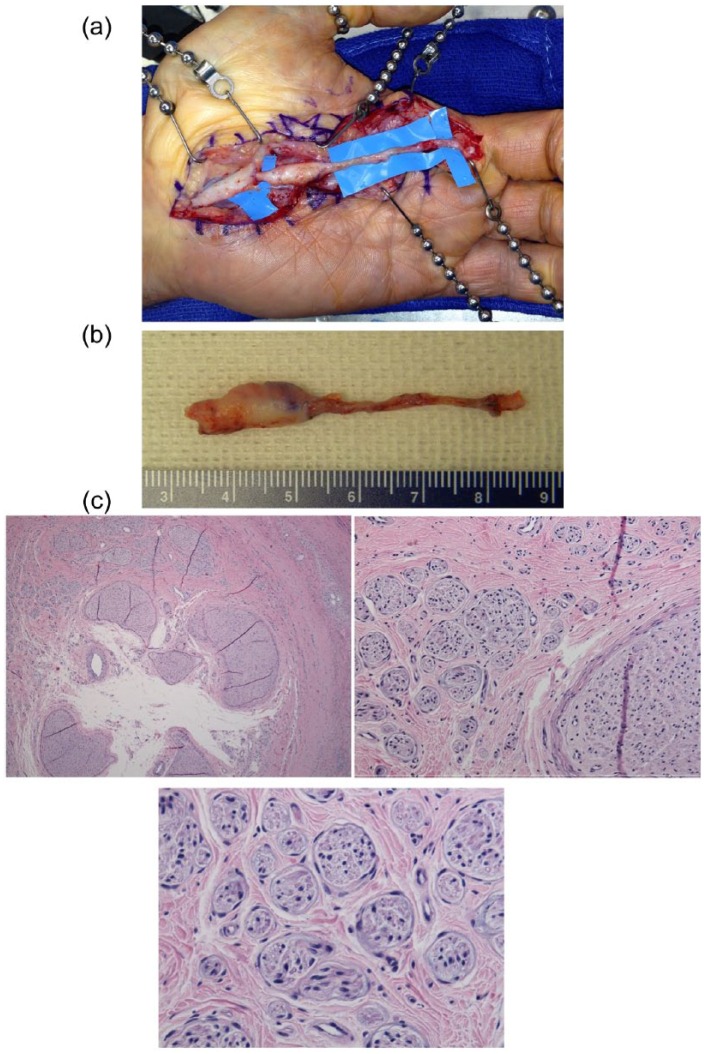
Figure 1.
(a) Exposure of the previously failed surgery reveals the allograft with a proximal neuroma of the common digital nerve. (b) Recalcitrant neuroma ex vivo. (c) A cross-section hematoxylin and eosin stain of neuroma. Low-power field demonstrating fascicles within the lumen of the allograft (upper left). High-power field magnification of fascicles varying in size, characteristic of neuromas (upper middle and lower).
Figure 2.
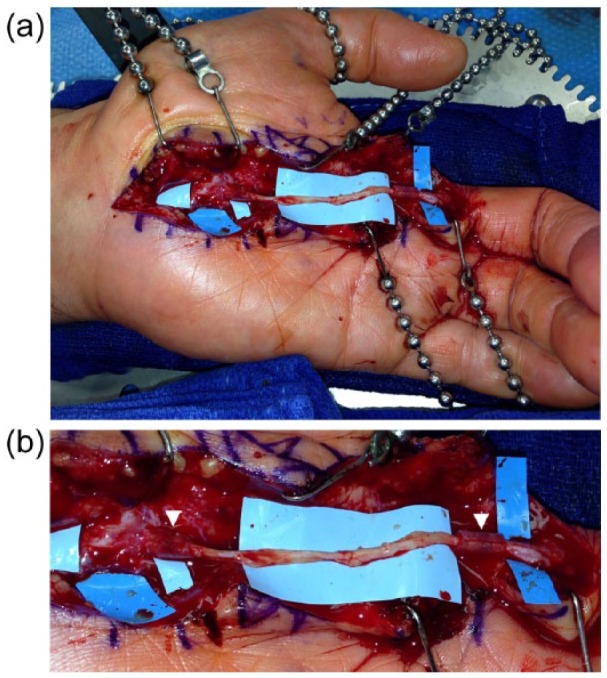
(a) The reversed interposition superficial peroneal nerve graft is anastomosed proximally to the common digital nerve takeoff of the median nerve and distally to radial digital nerve of the middle finger. (b) Closer magnification shows the porcine submucosa extracellular matrix nerve protector wrapping the nerve coaptations (arrow heads).
Discussion
The treatment of recurrent neuromas can be challenging for the surgeon but also poses a particularly arduous and prolonged process for the patient. The cohort of patients with unresolved pain following neuroma excision is more likely to undergo multiple excisional procedures.10 Following digit amputation, patients have a significantly increased risk of neuroma formation with the incidence varying from 7.8% to 30%.9,10 Leaving a nerve defect may result in sensory dysfunction with secondary motor deficits leading to a decreased quality of life. This case report demonstrates the pervasiveness of recalcitrant neuroma, its resistance to multiple treatment strategies, and the impact of long-term pain on hindering specific hand function.
Multiple strategies for effective means of nerve repair include primary end-to-end nerve coaptation, utilizing an allograft nerve conduit, and implementing autologous conduits. When a digital nerve defect is too large for end-to-end neurorrhaphy, determining the type of nerve reconstruction should be based on multiple factors, including length of nerve defect, magnitude of sensory defect, available donor nerves, caliber contrast of donor and recipient nerve, and ease of harvest. Typically, autologous nerve grafts are reserved for lengthy nerve gaps, but allogeneic bioabsorbable grafts are better suited for nerve gaps less than 20 mm.3 Shorter nerve grafts using processed nerve allografts provide pre-degenerated, decellularized human nerve tissue that provides a conducive environment for axon regeneration.3 However, this case confirms that such conduits may be ineffective within longer gaps. This is inconsistent with the Registry Study of Avance® Nerve Graft Evaluating Outcomes in Nerve Repair (RANGER) data, which support the use of nerve allograft in segment defects up to 50 mm, even in the setting of chronic injury.6 It remains unclear why such findings deviate from analogous allograft nerve studies. Wangensteen and Kalliainen reported effective use of collagen tubes in 43% of nerve injuries for defects up to 20 mm.11 Segmental nerve defects of greater than 30 mm are more prone to adhesions, pressure ischemia, and loss of sensory discrimination.8 In fact, Liodaki et al have published a series of unsuccessful nerve conduit requiring explantation, all of which were found to have histologic evidence of excessive inflammatory reactions with disorganized neuronal growth.7 However, favorable outcomes for bioabsorbable nerve conduits, tension-free end-to-end nerve coaptation, and autografts for shorter gaps are comparable yet vary from 60% to 90%.1,7,8,11,12 Despite novel conduits gaining momentum in the clinical realm, they remain alternatives to the gold standard of autologous nerve grafting, especially when faced with nerve defects greater than 30 mm.
Autologous nerve interposition repairs are limited by the availability of a suitable sensory donor nerve grafts.4 However, autologous nerve grafts have several drawbacks, including donor site sensory loss, an extra incision site, scar formation, and the potential for developing of a painful neuroma. The sural nerve is a purely sensory nerve innervating the lateral aspect of the ankle and is usually the nerve of choice for repair of nerves in the hand or brachial plexus. In addition, it is able to provide the greatest available length (up to 40 cm) for repair.5 Higgins et al determined that the sural nerve is the most appropriate donor nerve for the common digital nerve proximal to the bifurcation, while the lateral antebrachial cutaneous nerve is the best match from the fingertip trifurcation to the common digital nerve bifurcation.5 Another suitable option includes the superficial peroneal nerve, which innervates the peroneus longus and brevis muscles and provides sensation to the dorsal foot and lateral aspect of the lower extremity. This graft provides adequate length in addition to sensory and motor functions and has been described as an alternative comparable with the sural nerve.2 Selecting the superficial peroneal nerve as a primary rather than alternative donor nerve may be favored due to its predictable anatomic course, few branch points, ease of dissection, suitable diameter for coaptation, and maintaining supine intraoperative positioning, precluding the need for any intraoperative positional changes.2 In addition, the porcine submucosa extracellular matrix (AxoGard® Nerve Protector; AxoGen, Inc, Alachua, Florida) was used because the surgical site had already proven to be prone to scarring and because the coaptations were located in more confined anatomic spaces in a re-operated site. The decision to select the superficial peroneal nerve was driven by the author’s (R.A.D.) preference due to good size match with the recipient nerve and an easy harvest precluding the need to rotate the leg as it is required for sural nerve harvest. In addition, the distal superficial peroneal nerve fibers are sensory fibers innervating non-critical sensation of the dorsum of the foot. Our patient had no donor nerve site complaints.
Given the multitude of treatment options, the chosen technique should be tailored to each individual patient. In the setting of recalcitrant neuromas and intractable pain following multiple neuroma excisions, it is prudent to use autologous reconstruction if the nerve defect is at least 30 mm. Furthermore, the surgeon should expect a previously operated surgical site to be prone to adhesions and may elect to protect each nerve coaptation with porcine submucosa extracellular matrix, as in this case, to avoid tethering, local ischemia, and nerve traction. Although the available literature suggests allografts can be successfully utilized in 30- to 50-mm gap defects, it remains unclear whether this approach is comparable in primary neuroma excision, recalcitrant neuroma excision, or both. More clinical studies are needed to investigate the different types of nerve grafts, length of nerve defects, and types of neuromas (primary or recalcitrant) to determine superior treatment modalities.
Footnotes
Ethical Approval: Ethical Approval was not necessary for this case report.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent: Informed consent was obtained from all patients for being included in the study. No patient identifiers have been used in this article. However, patient informed consent was obtained for the publication of this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Brooks DN, Weber RV, Chao JD, et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32:1-14. [DOI] [PubMed] [Google Scholar]
- 2. Buntic RF, Buncke HJ, Kind GM, Chin BT, Ruebeck D, Buncke GM. The harvest and clinical application of the superficial peroneal sensory nerve for grafting motor and sensory nerve defects. Plast Reconstr Surg. 2002;109:145-151. [DOI] [PubMed] [Google Scholar]
- 3. Cho MS, Rinker BD, Weber RV, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am. 2012;37:2340-2349. [DOI] [PubMed] [Google Scholar]
- 4. Guse DM, Lawrence S. Outcomes of the surgical treatment of peripheral neuromas of the hand and forearm: a 25-year comparative outcome study. Ann Plast Surg. 2013;71:654-658. [DOI] [PubMed] [Google Scholar]
- 5. Higgins JP, Fisher S, Serletti JM, Orlando GS. Assessment of nerve graft donor sites used for reconstruction of traumatic digital nerve defects. J Hand Surg. 2002;27:286-292. [DOI] [PubMed] [Google Scholar]
- 6. Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y). 2009;4:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liodaki E, Bos I, Lohmeyer JA, et al. Removal of collagen nerve conduits (NeuraGen) after unsuccessful implantation: focus on histological findings. J Reconstr Microsurg. 2013;29:517-522. [DOI] [PubMed] [Google Scholar]
- 8. Lohmeyer JA, Siemers F, Machens HG, Mailänder P. The clinical use of artificial nerve conduits for digital nerve repair: a prospective cohort study and literature review. J Reconstr Microsurg. 2009;25:55-61. [DOI] [PubMed] [Google Scholar]
- 9. Nelson AW. The painful neuroma: the regenerating axon versus the epineural sheath. J Surg Res. 1977;23:215-221. [DOI] [PubMed] [Google Scholar]
- 10. van der Avoort DJJC, Hovius SER, Selles RW, van Neck JW, Coert JH. The incidence of symptomatic neuroma in amputation and neurorrhaphy patients. J Plast Reconstr Aesthet Surg. 2013;66:1330-1334. [DOI] [PubMed] [Google Scholar]
- 11. Wangensteen KJ, Kalliainen LK. Collagen tube conduits in peripheral nerve repair: a retrospective analysis. Hand (N Y). 2010;5:273-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber RA, Breidenbach WC, Brown RE, Jabaley ME, Mass DP. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1036-1045. [DOI] [PubMed] [Google Scholar]
- 13. Whitlock EL, Tuffaha SH, Luciano JP, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009;39:787-799. [DOI] [PubMed] [Google Scholar]