Abstract
Neurophysiological changes in the basal ganglia thalamo-cortical circuit associated with the development of parkinsonian motor signs remain poorly understood. Theoretical models have ranged from those emphasizing changes in mean discharge rate to increased oscillatory activity within the beta range. The present study characterized neuronal activity within and across the internal and external segments of the globus pallidus as a function of motor severity using a staged, progressively severe 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinsonism in three rhesus monkeys. An increase in coherence between neuronal pairs across the external and internal globus pallidus was present in multiple frequency bands in the parkinsonian state; both the peak frequency of oscillatory coherence and the variability were reduced in the parkinsonian state. The incidence of 8-20 Hz oscillatory activity in the internal globus pallidus increased with the progression of the disease when pooling the data across the three animals; however it did not correlate with motor severity when assessed individually and increased progressively in only one of three animals. No systematic relationship between mean discharge rates or the incidence or structure of bursting activity and motor severity was observed. These data suggest that exaggerated coupling across pallidal segments contribute to the development of the parkinsonian state by inducing an exaggerated level of synchrony and loss of focusing within the basal ganglia. These data further point to the lack of a defined relationship between rate changes, the mere presence of oscillatory activity in the beta range and bursting activity in the basal ganglia to the motor signs of Parkinson's disease.
Keywords: Basal Ganglia, Pathophysiology, Parkinson's disease, oscillations, synchronization
INTRODUCTION
Parkinson's disease is a neurodegenerative disorder characterized by bradykinesia, rigidity, tremor, and postural abnormalities. Its histopathological hallmark is the progressive, asymmetric loss of nigrostriatal dopaminergic neurons in the substantia nigra pars compacta and other midbrain nuclei. Although various hypotheses have been advanced to explain how this progression of neuroanatomical and related physiological changes within the basal ganglia BGTC circuit lead to the presence of motor signs, a clear answer remains elusive amid a field of often conflicting data.
Several models of basal ganglia dysfunction have been postulated to account for the development of Parkinson's disease motor signs. The “rate model”, put forth in the late 1980's and early 1990's, posited that the cardinal motor signs of Parkinson's disease were the result of changes in the overall mean discharge rate of neurons within key nodal points of the BGTC circuit (Albin et al., 1989; DeLong, 1990; Miller and DeLong, 1988). Although supported by lesion studies in both humans and nonhuman primates (Vitek et al., 2003), more recent studies in nonhuman primates have failed to show increased rates in GPi in Parkinsonism (Raz et al., 2000; Soares et al., 2004). Furthermore, pallidotomy in patients with Parkinson's disease has been demonstrated to reduce, rather than enhance, dyskinesias (Baron et al., 1996; Vitek et al., 2003), while STN DBS has been shown to increase rates in GPi coincident with improvement in motor signs (Hashimoto et al., 2003). These observations have cast doubt on the validity of the rate model and have led to the development of alternate theories including the development of bursting and synchronized oscillations in particular spectral frequency ranges within and across key nodal points of the basal ganglia (Brown, 2007; Kuhn et al., 2009; Ray et al., 2008; Vitek and Giroux, 2000; Wichmann et al., 1999). Oscillatory synchronization in the 8-30 Hz (beta) band has been observed in the STN, GPi and primary motor cortex in humans undergoing DBS surgery (Kuhn et al., 2009), in the MPTP non-human primate model of Parkinson's disease (Devergnas et al., 2014), and in rats treated with 6-hydroxydopamine (Gradinaru et al., 2009). Other studies however, have questioned a causative role of beta band oscillations in the pathogenesis of Parkinson's disease finding that such oscillations occur in the naïve state (Connolly et al., 2015b; Courtemanche et al., 2003; Leventhal et al., 2012; Murthy and Fetz, 1992), that motor signs may precede the occurrence of oscillatory activity in the GPi and STN (Leblois et al., 2007), and that these oscillations are not found in all Parkinson's disease patients (Rosa et al., 2011). Thus, the precise nature of changes in neuronal activity within the basal ganglia and their role in the development of Parkinson's disease motor signs remains unclear.
The goal of the current study was to characterize the changes in neuronal activity within the pallidum as a function of motor severity using a staged, progressively severe, MPTP model of Parkinsonism. Oscillatory neuronal activity occurred early and across multiple lower frequency bands and generally increased in the parkinsonian state, but did not consistently correlate with increasing severity of motor signs. There was a consistent shift in the low beta coherence peak from higher (16.1 Hz) to a lower frequency range (12.7 Hz) in the parkinsonian state and a noted shift in the number of paired cells in GPi and between GPi and GPe that showed significant coherence in the post-MPTP state. These data, while supportive of a role for synchronized oscillations, also point to the marked variability across subjects and spectral bands. While it is unlikely that any one feature can account for the overall severity of motor signs in the parkinsonian state, these data draw attention to the potential impact of synchronized activity that occurs within and across nodal points in the BGTC in the development of the motor signs associated with Parkinson's disease.
EXPERIMENTAL PROCEDURES
Animals
All procedures were performed under a protocol approved by the Institutional Animal Care and Use Committee and complied with United States Public Health Service policy on the humane care and use of laboratory animals. Three adult rhesus monkeys (macaca mulatta) were used: NHP B (female, 5.2 kg); NHP T (male; 9.4 kg); and NHP C (female, 7.4 kg). Animals were acclimated to the laboratory environment and to passive manipulation of the limbs using positive reinforcement techniques. Multi-channel extracellular neuronal recordings were made in the naïve (i.e., pre-MPTP) state followed by three levels of parkinsonian severity as indexed by a modified version of the Unified Parkinson's disease Rating Scale for use with the non-human primate.
Surgical procedures
Recording chamber placement
Pre-operative cranial CT and MRI were merged using Cicerone (Miocinovic et al., 2007) for surgical planning of cephalic chamber placement. The recording hardware was implanted in an aseptic surgical procedure under isoflurane anesthesia as detailed previously (Hashimoto et al., 2003). Briefly, once sedated, the animal's head was secured in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA), the skin was prepped and draped, and the scalp was incised at midline. Following partial blunt dissection of the skin and temporalis muscle bilaterally, craniotomies were made under stereotactic guidance leaving the underlying dura intact. Cephalic recording chambers were aligned over the cranial opening and secured in place using a combination of surgical bone screws and dental acrylic. In NHP B, a single small chamber was targeted at the GPi and the external segment of the globus pallidus (GPe). In NHP T, a single large chamber was used to facilitate recording of the pallidum and thalamus. In NHP C two chambers were implanted: one targeting the thalamus, STN and pallidum and the other targeting the primary motor areas. Buprenorphine was administered prophylactically for pain management (0.01-0.03 mg/kg IM BID for 2 days then PRN) in combination with meloxicam (0.1 – 0.3 SQ every 24 hours on days 2-4). Prophylactic antibiotics were given intra-operatively and continued post-operatively for 7-10 days with veterinary oversight.
MPTP administration
Once data collection was complete for the naïve state, the animal was made progressively hemi-parkinsonian through staged, unilateral intra-carotid injections of the neurotoxin MPTP (0.2-0.8 mg/kg, 1mg/ml solution, 15-minute infusion). Administration was performed during aseptic surgical procedures under isoflurane anesthesia. Prophylactic antibiotics were administered and post-operative pain management was provided using buprenorphine.
Experimental model
Multiple injections of the neurotoxin were made in each animal to move it into the desired level of severity and maintain it within that range. The subjects differed in their susceptibility to the neurotoxin and hence, the number of injections varied between the subjects and between states for each subject. The level of severity was determined behaviorally using the modified UPDRS rating scale (see below) and was monitored by serial mUPDRS assessments during the treatment and recording phases to insure we kept each animal within the predetermined mUPDRS range for each level of severity. Additional injections were made when necessary to keep each animal within the predetermined severity level and subsequently to advance it to the next level for the next series of recordings. Recordings were obtained once the animal was out of quarantine, had recovered from the MPTP surgery and the expression of PD symptoms had stabilized (~ 10-14 days). For example in NHP B, the recordings in the mild state were performed over 2 months. Two MPTP injections were required to keep the animal within the pre-defined mUPDRS range for the mild state. Eighteen behavioral assessments were made distributed around the recording days. A total of three MPTP injections were made in the mild state and the animal transitioned to the moderate state after the third treatment.
Behavioral assessment
Motor severity was assessed with the mUPDRS, which was used to rate rigidity, bradykinesia, akinesia, and tremor of the upper and lower extremities bilaterally as well as six global motor features of Parkinsonism (i.e., gait, posture, balance, turning, reaction and food retrieval). Scoring (0-3) was performed during observation of spontaneous behavior, investigator interaction, and passive limb manipulation. The maximum possible score was 42; composite scores within the ranges of 3-13, 18-28, and 32-42 were used to define the mild, moderate, and severe parkinsonian states, respectively. These ranges included a buffer between parkinsonian states to assign stronger distinctions amongst the states. Behavioral assessments were conducted at least twice per week to ensure the level of severity was stable over each week of recording. While PD symptoms may vary in patients and the same may occur with the nonhuman primate, we were able to ensure that the overall level of severity was stable and within the predetermined mUPDRS range over the time the recordings were made.
Multi-channel neuronal recording
Recording sessions were generally 2-3 hours long. All of the data were collected in the awake, but resting state, with the animals sitting in a primate chair with their head and arms restrained. The NHPs were visually monitored by the investigator collecting the data to ensure that they were awake, relaxed and not fighting the restraints or exhibiting any other overt movements during recordings. A total of 525, 738 and 340 recordings were made in the three animals divided among the four different conditions normal, mild, moderate and severe. The numbers reflect all the recordings made in the subjects regardless of the location within specific nuclei.
The locations of the GPe and GPi were mapped using extracellular microelectrode techniques. Dual, epoxylite-insulated tungsten microelectrodes (impedance ≈1.0 MΩ at 1 KHz; FHC Inc., Bowdoin, ME USA) were advanced independently using a multi-channel microdrive system attached to the recording chamber (Alpha EPS, Alpha Omega, Nazareth Illit, Israel). Neuronal activity was transduced acoustically, allowing on-line evaluation of isolated neurons for somatosensory responsiveness through passive limb manipulation. Neuronal activity was amplified (×10k), filtered (300 Hz – 6 kHz) and digitized (25 kHz) for off-line analysis (Alpha Lab, Alpha Omega, Nazareth Illit, Israel). Using the dual electrode approach, individual as well as paired, synchronized recordings were made from neurons within and across the GPi and the GPe. A 3-dimensional electrophysiological map of the subcortical targets was constructed by classifying neuronal activity using techniques similar to those applied during human functional neurosurgery (Vitek et al., 1998). Recording locations were additionally confirmed histologically (see below).
Spike data analysis
Spike activity for sample epochs with sustained neuronal activity across a minimum of 30-seconds was discriminated off-line using a combination of principal component analysis and clustering (Offline Sorter v. 2.8.7, Plexon, Inc.). Waveform size and shape and the inter-spike interval (ISI) were used to further ensure isolation of single units from the recordings. Consecutive spikes with ISI less than 1.5 ms were eliminated. 89% of the units included in the analysis had an overlap of less than 0.3% of the total number of spikes and the rest had <0.7% overlap. No more than 10-15 spikes were eliminated from the spike train of a single unit, which given the firing rate of pallidal neurons would not affect the outcome of any of the analysis reported here. Timestamps were exported for subsequent analysis using NeuroExplorer (v.4. Nex Technologies, MA, USA) and custom algorithms coded in Matlab (Mathworks, Natick, MA). The effect of behavioral severity on spontaneous discharge rate, bursting and oscillatory activity, and coherence was examined separately for each nucleus.
Bursts were identified using the Poisson surprise method described by Legendy and Salcman (Legendy and Salcman, 1985), which identifies bursts as low probability events assuming that the spike times within a train of action potentials have a Poisson distribution. The minimum surprise value and inter-spike interval for identification of bursts were set at 4.6 (p = 0.01) and half of the mean inter-spike interval, respectively; the minimum number of spikes in a burst was set at three.
The presence, power and spectral content of oscillatory neuronal activity were examined within several predefined bands (3-8 Hz [theta], 8-20 Hz [alpha + low beta], 20-30 Hz [high beta], 30-58 Hz [low gamma], and 65-200Hz [high gamma]). Power spectral density in the 0.5-200 Hz range was estimated with 2 s windows, no overlap and a Hanning window of the same duration using Welch's periodogram. Distortions in the power spectrum resulting from the refractory period were corrected as described by Rivlin-Etzion et al (Rivlin-Etzion et al., 2006). Peaks in the PSD were considered significant if they were greater than the threshold, defined as mean plus three times the standard deviation (3σ) of the PSD in the >0.5-200 Hz frequency range for the given unit; median absolute deviation of the power spectrum was used to remove outliers in the data prior to calculating the standard deviation. Coherence between pairs of cells was calculated using the multi-taper method (4 sec windows, 3 tapers, and 0.25 Hz resolution). The 95 % confidence level, corrected for multiple comparisons, was used to estimate the significance of the peak. Two consecutive peaks had to cross the threshold to be considered significant for both the coherence and power spectrum.
Statistical analysis
Statistical analysis was performed using Matlab. None of the variables analyzed here were normally distributed; hence, the non-parametric one-way ANOVA, the Kruskal-Wallis test K (DoF), was used to test for the main effect of severity. Medians and interquartile range are reported in the place of mean and standard deviation. Whenever possible, analysis was performed on the pooled data set as well as for individual subjects in order to examine consistency across subjects. Post-hoc analyses were performed using Dunn's all pair test. Changes in the percent of units with significant peaks in the power spectra and coherence, with disease progression, were inspected using the likelihood ratio test (χ2(DoF)). A significance level of 0.05 was used in all cases, with the Bonferroni correction applied for multiple comparisons.
Histology
Once the experimental endpoints were met, each animal was deeply anesthetized with sodium pentobarbital and perfused transcardially with perfusion wash (1222SK, Electron Microscopy Sciences) followed by 10% formalin perfusion fixative (1223SK, Electron Microscopy Sciences). Tissue sections were blocked bilaterally, with slices (50 μm thick) through the pallidum taken in the coronal plane, while the more medial brain regions, including the substantia nigra, were cut in the parasagital plane. Alternate slices were stained using acetylocholinesterase, Nissl and parvalbumin. Nissl and AChE staining allowed for identification and reconstruction of the recording tracks. Neurons were localized to the GPe or the GPi using cytoarchitectonic and chemoarchitectonic criteria (DeLong and Coyle, 1979; DeLong et al., 1985).
RESULTS
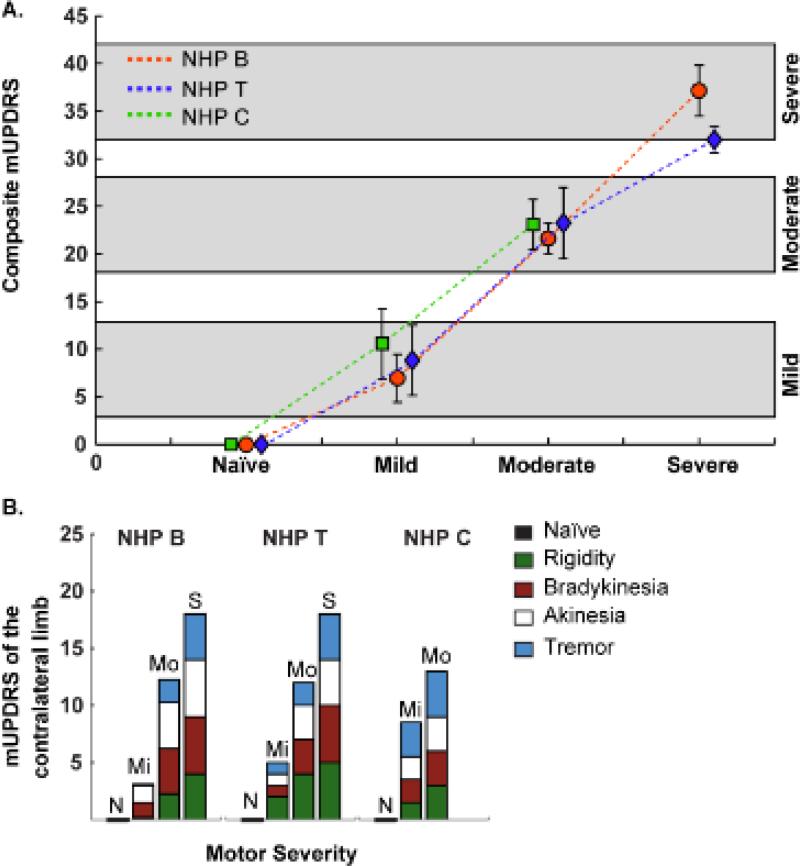
A total of 482 cells from GPe and 246 cells from GPi were recorded across four behavioral states (Table 1). Composite mUPDRS scores (± SD) for the three animals were 7.8 ± 3.2, 22.6 ± 2.7, and 37.5 ± 4.2 for the mild, moderate and severe parkinsonian states, respectively (Figure 1A). For NHPs B, T and C, the scores at each stage were 7 ± 2.5, 8.8 ± 3.7 and 10.6 ± 3.7 (Mild), 21.6 ± 1.6, 23.3 ± 3.7 and 23.1 ± 2.6 (Moderate) and 37 ± 2.1 and 32 ± 2.1 (severe), respectively; animal C was lost to follow-up for health related reasons that arose between the moderate and severe state, hence no severe data are presented for that animal. Each animal exhibited rigidity, bradykinesia and akinesia, with gait and balance problems more prominent in the moderate and severe states. Although tremor was present in the wrist and ankle, it was more readily apparent during posture and movement than when the animal was at rest. The mUPDRS scores by symptom and motor severity are shown in Figure 1B.
Table 1.
Pallidal units by subject and motor severity
| GPe | GPi | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Naïve | Mild | Moderate | Severe | Total | Naïve | Mild | Moderate | Severe | Total | |
| NHP B | 112 | 54 | 26 | 19 | 211 | 12 | 20 | 15 | 28 | 75 |
| NHP T | 105 | 36 | 29 | 43 | 213 | 56 | 32 | 10 | 24 | 122 |
| NHP C | 26 | 15 | 17 | - | 58 | 20 | 14 | 15 | - | 49 |
| Pooled | 243 | 105 | 72 | 62 | 482 | 88 | 66 | 40 | 52 | 246 |
Figure 1. Modified UPDRS Scores.
Three non-human primates (NHP) were assessed using a modified UPDRS (mUPDRS) protocol (max score = 42). Composite scores within the ranges of 3-13, 18-28, and 32-42 were used to define three different levels of parkinsonian severity: mild, moderate, and severe parkinsonian states, respectively. A. Mean+/−Standard deviation of the composite mUPDRS for the three subjects. B. Median mUPDRS score for the limb contralateral to the intra-carotid injections, separated by symptoms.
Incidence of oscillatory activity changed with motor severity in both pallidal nuclei
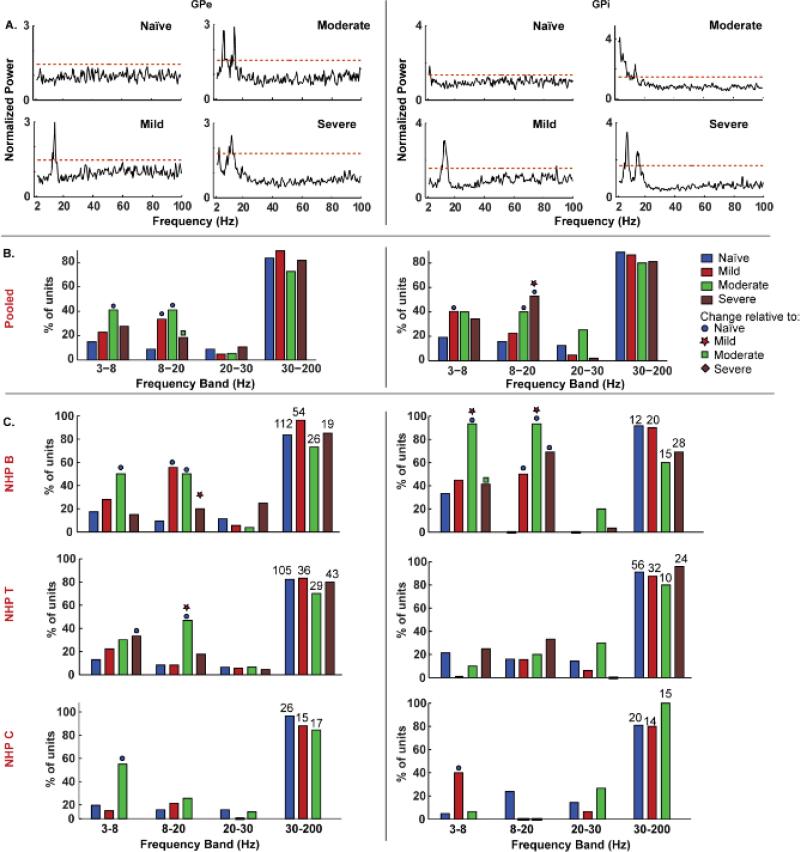
Example traces of the spectral content of GPe and GPi neurons are presented in Figure 2 A, for each level of severity; the bar graphs in Figure 2B summarize the incidence of peaks within each frequency band in the pooled data as a function of behavioral severity for each pallidal segment. Table 2 details the number of neurons exhibiting significant power within each frequency band by behavioral state. Alpha + low-beta band activity was examined together as this provided a more consistent change with severity than either band alone. The most notable change in the pooled data was the progressive increase in the incidence of alpha + low-beta activity with severity of disease. Figure 2C displays the spectral content for each individual animal and illustrates the variability in the changes in oscillatory activity across subjects.
Figure 2. Oscillatory activity varied with motor severity in specific frequency bands within the pallidal segments.
A. Example plots illustrating power spectral density of units in the GPe and GPi with increasing severity. The normalized power spectrum of each unit was computed by dividing by the mean in the 0.5-200 Hz range. The red dashed line indicates the threshold used to identify significant peaks, which was set as three standard deviations above the mean in the 0.5-200 Hz range. A peak within a pre-defined frequency band was considered significant only if two consecutive bins crossed the threshold. B. Bar graphs show the percentage of units pooled across all subjects with oscillatory activity within each frequency band and disease state. C. The percentage of units with theta, 8-20 Hz and high beta activity to those with gamma activity as a function of motor severity for each animal. The numbers of units used in the analysis, for each level of severity and NHP, is indicated over the bars. Filled circle, star, square and diamond symbols indicate significant change relative to naïve, mild, moderate and severe states at an alpha of 0.05 with Bonferroni correction.
Table 2.
The number and per cent of cells with significant oscillations in GPe and GPi by motor severity and frequency band.
| GPe | GPi | |||||||
|---|---|---|---|---|---|---|---|---|
| 3-8 Hz | 8-20 Hz | 20-30 Hz | 30-200 Hz | 3-8 Hz | 8-20 Hz | 20-30 Hz | 30-200 Hz | |
| Naïve | 37 (15%) | 22 (9%) | 22 (9%) | 208 (83.5%) | 17 (19%) | 14 (16%) | 11 (12.4%) | 79 (89%) |
| Mild | 24 (23%) | 35 (33.4%) | 5 (5) | 94 (89.5%) | 27 (40%) | 15 (22.4%) | 3 (4.5%) | 58 (87%) |
| Moderate | 30 (41%) | 30 (41%) | 4 (5.5%) | 53 (72.6%) | 16 (40%) | 16 (40%) | 10 (25%) | 32 (80%) |
| Severe | 18 (28%) | 12 (18.5%) | 7 (11%) | 53 (81.5%) | 18 (34%) | 28 (53%) | 1 (2%) | 43 (81%) |
Pooled data
In GPe, the incidence of oscillatory activity in the 3-8 Hz (theta) (likelihood ratio; χ2(1)= 15.6, p = 0.001) and 8-20 Hz (alpha + low beta) (χ2(1) = 40, p<0.0001) bands increased post MPTP (Figure 2B left, Table 2). Post hoc testing revealed an increased proportion of cells with theta band activity in the moderate (p<0.0001) compared to the naïve state. The percent of cells with a peak in the alpha + low-beta band increased in the mild (p<0.0001) and moderate (p<0.0001) states, and although it remained increased in the severe state, it was reduced compared to the moderate condition.
In GPi, oscillatory activity in the 3-8 Hz (χ2(1) = 9.6, p=0.002) and 8-20 Hz (χ2(1) = 12.3, p=0.0004) bands changed significantly in the parkinsonian state (Figure 2B right and Table 2); the proportion of units with theta and alpha+low-beta activity increased in the mild (theta: p = 0.004), moderate (8-20 Hz: p = 0.003), and severe (8-20 Hz: p<0.0001) states. Although theta activity was also increased in the moderate and severe states, it was not significant; likely due to the smaller sample size in these conditions. Gamma band activity tended to decrease in the parkinsonian state, however these changes were not significant compared to the naïve condition.
Individual subjects
Contrary to the changes observed in the pooled data, individual subjects showed inconsistent changes in the incidence of oscillatory activity with disease progression in both nuclei (Figure 2C). The principle changes observed for each individual animal, however, still tended to occur within the low frequency (i.e., theta and alpha+low-beta) bands, similar to the pooled data.
In GPe, NHP B showed trends similar to the pooled data in the incidence of units with theta and alpha+low-beta band activity. In both bands, the percent of units with a significant peak increased in the mild (8-20: p<0.0001) and moderate (theta: p = 0.0004; 8-20: p<0.0001) state relative to the naïve, but not in the severe state. NHP T had a linear increase in the proportion of units with theta activity with motor severity; however, only the difference between the naïve and severe states (p = 0.004) was significant. A large increase in 8-20 Hz activity was seen only in the moderate state in the GPe (p<0.0001). NHP C had a larger percentage of GPe units with theta activity in the moderate state compared to the naïve state (p = 0.009) and a non-significant increase in the percentage of GPe units with 8-20 Hz activity in the mild and moderate states.
For neuronal activity in the GPi, two of the three subjects showed a significant increase in the proportion of units with theta activity in one parkinsonian state compared to the naïve state and only one of the three NHPs demonstrated a significance increase in 8-20 Hz activity after induction of Parkinsonism compared to the naïve state. None of the subjects showed a systematic increase with progression of disease in oscillatory activity in any one frequency band. A large increase in the incidence of theta activity was seen in the moderate state in NHP B (p = 0.001) and mild state in NHP C (p=0.008). Alpha+low-beta band activity was not seen in the naïve state in NHP B, but a large percentage of units in the mild (p=0.003), moderate (p < 0.0001) and severe (p = 0.0001) states had 8-20 Hz activity. NHP T had small, but insignificant increments in the incidence of alpha+low-beta activity in the severe state and 8-20 Hz peaks were seen only in the naïve state in NHP C.
The peak oscillatory frequency and variability was reduced in the parkinsonian state
Pooled data
Peak frequency in the alpha+low-beta band shifted to lower frequencies in the parkinsonian state and the variability was reduced (data not plotted). In the GPi, the peak frequencies in the naïve state were distributed widely over the 8-20 Hz band (median: 16.1, IQR: 4.3 Hz; Kruskal Wallis: K (3) = 9.4, p = 0.02; two sample F test: F= 2.0, p = 0.06); induction of Parkinsonism caused the median to shift to 12.7 (2.6) Hz, which was sustained in the moderate (13.0 (3.4) Hz) and severe (12.7 (2.1) Hz, p = 0.004) states. Although the shift in median peak frequency with increasing motor severity was smaller in the GPe, the variability was lower in the parkinsonian state (F= 2.7, p = 2.3e-4); the median peak frequencies were 13.7 (6.6), 13.2(1.9), 12.4(2.9) and 13.2(2.8) Hz in the naïve, mild, moderate and severe states, respectively.
Individual subjects
Similar to the pooled data, the peak frequencies in the GPe in the 8-20 Hz band did not change significantly with severity for all three subjects, but the variability was reduced for NHPs B (IQR: 4.9, 1.9, 1.9, and 1.2 Hz; two sample F test: F = 3.1, p = 9.2e-4) and T (IQR: 5.4, 4.1, 4.4, 2.9 Hz; F = 2.9, p = 0.02). NHP C had low variability (IQR) in the naïve (0.5 Hz) and moderate (2.4 Hz) states, but not in the mild state (10.7 Hz). Only NHP T (F = 3.8, p = 0.02) had units with 8-20 Hz activity in all four disease states in the GPi; the median (IQR) peak frequency reduced in the parkinsonian state from 16.1 (4.9) Hz in the naïve to 12.2 (1.6), 13.2 (9.8) and 12.4 (1) Hz in the mild, moderate and severe states. The median peak frequencies for the mild, moderate and severe states for NHP B were 13.4 (2.4), 13 (2.2) and 12.7 (4.4) Hz.
Oscillatory coherence within and across pallidal nuclei increased in the parkinsonian state
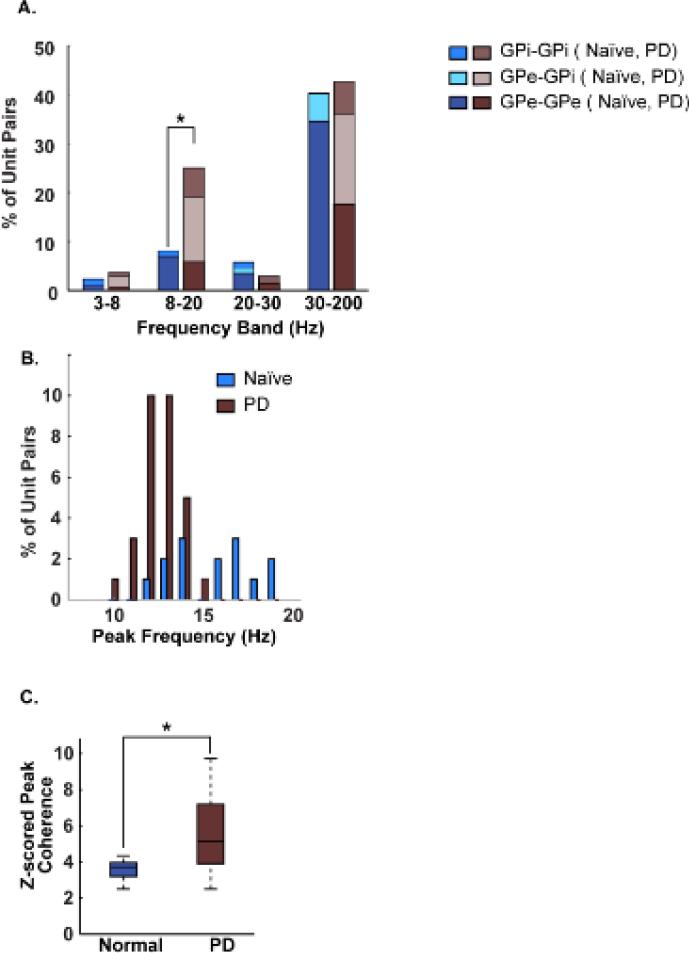
Data from all inter- and intra- nuclei pairs were pooled for this analysis due to an insufficient number of cell pairs for separate analysis of the main effects of motor severity, subject, and nucleus. The percentage of all cell pairs with synchronized oscillatory activity for the naïve and parkinsonian state, separated by location within the GPe or GPi are illustrated in Figure 3A, while the distribution of peak frequency and normalized peak amplitude of coherence in the 8-20 Hz range are shown in Figures 3 B-C.
Figure 3. Oscillatory coherence within and across nuclei increased from the normal to the parkinsonian state in the alpha+low-beta band.
A. Bar graph of the percentage of unit-pairs with oscillatory coherence separated by frequency band, naïve and Parkinson's disease states, and by recording nucleus (i.e., GPi-GPi, GPe-GPi and GPe-GPe). B. Distribution of the peak coherence frequency in the normal and parkinsonian states across all subjects and inter- and intra- nuclei pairs. C. Boxplot shows the normalized amplitude of oscillatory coherence in the alpha+low-beta band in the normal and parkinsonian states pooled across all subjects and inter- and intra- nuclei pairs (*p<0.05). The coherence values were normalized using the mean and standard deviation of surrogate coherence data. The surrogate data consisted of the coherence spectrum computed after randomly shuffling the spike timestamps of one of the two units; the shuffling was repeated a 100 times.
Analysis of the pooled data from all three subjects revealed a significant increase in both the percent of correlated cell pairs (likelihood ratio; χ2(1)=11.2, p=0.0008; Figure 3A) and the strength of correlated activity (Wilcoxon rank rum: Z = −2.3, p = 0.02; Figure 3C) in the 8-20 Hz band in the parkinsonian state relative to the naïve condition. Although none of the other frequency bands showed any significant change from the naïve to the parkinsonian state, the distribution of cell pairs with significant coherence was different in the parkinsonian condition with greater coherence occurring across nuclei (i.e. GPe-GPi) in both the alpha-low beta and high gamma frequency bands. Similar to the reduction in peak frequencies for oscillatory activity in individual neurons in the GPi, the median peak frequency for paired units was also shifted to lower frequencies (13.2 (1.5) Hz; Wilcoxon rank sum: p = 0.0002) in the parkinsonian state, compared to the naïve condition 16.5 (3.2) Hz where coherence peaks were distributed over the entire 8-20 Hz range (Figure 3B). The variability in the peak frequencies was also significantly reduced in the parkinsonian condition, as can be seen from the IQRs in the naïve (3.2 Hz) vs. parkinsonian (1.5 Hz) state.
Changes in neuronal mean discharge rate varied across pallidal subnuclei
GPe
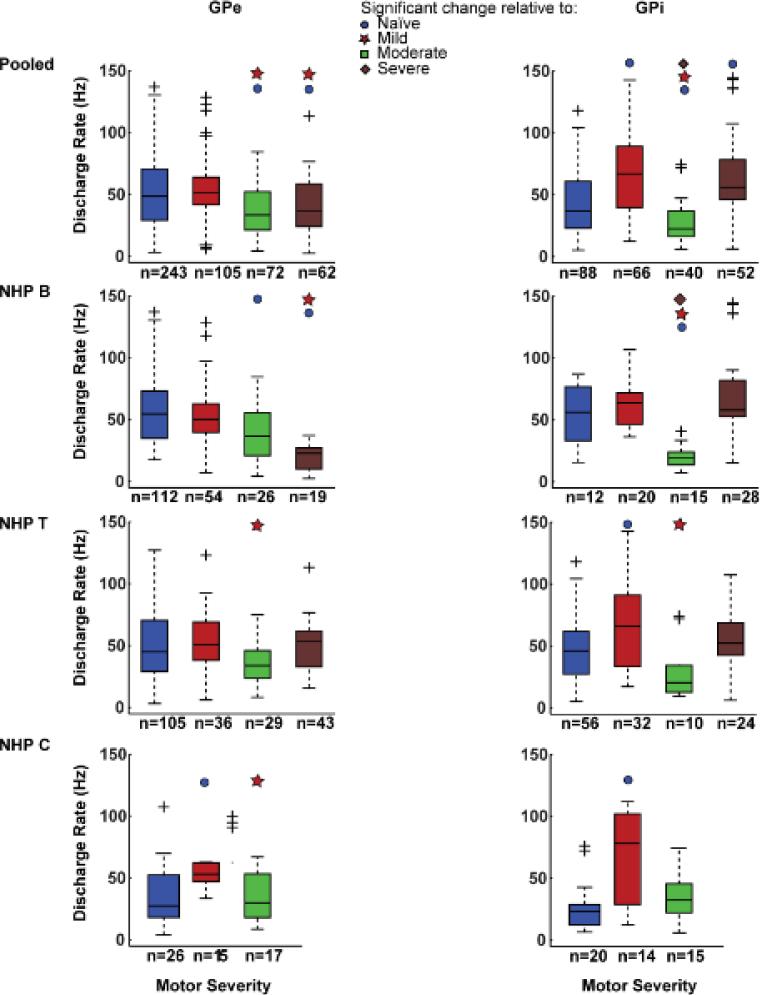
Pooling data across subjects demonstrated a main effect of parkinsonian severity on mean discharge rate within GPe (Kruskal Wallis: K (3) = 30.7, p = 9.8 e-7; Figure 4 top left). Post-hoc comparison with Dunn's all pair test, revealed a reduction in the median discharge rate in the moderate (34 Hz; p=0.0005) and severe (36.3 Hz; p = 0.01) states compared to naïve state (48.0 Hz), whereas the mild state (51.1 Hz) did not differ relative to the naïve condition. However, this pattern was not observed consistently across the individual subjects (Figure 4 left rows 2-4). NHP B exhibited a monotonically decreasing trend in the median discharge rate with increasing severity; the median firing rate in the mild (50.3 Hz), moderate (36.8 Hz; Dunn's all pair: p=0.04) and severe (22.8 Hz; p<0.0001) states were reduced relative to the pre-MPTP (54.6 Hz) condition. Although there was a main effect of severity on median discharge rate in both NHPs T and C, this was primarily due to a significant change in only one post-MPTP state. In the GPe of NHP T, the median of the discharge rates increased in the mild (51.4 Hz) and severe (50 Hz) states and reduced in the moderate (34 Hz) state, but none of the changes relative to the pre-MPTP condition (45.2 Hz) was significant. In NHP C, the median of the discharge rate increased in the mild state (53.2 Hz; p = 0.01), but returned towards the pre-MPTP (27.5 Hz) level in the moderate state (29.9 Hz).
Figure 4. Spontaneous firing rates in the GPe and GPi did not exhibit a systematic change with motor severity.
Box and whisker plots characterizing the firing rate for neurons within the GPe (left) and GPi (right) pooled across all subjects (top row). Rows 2-4: the trends seen in the pooled data were not replicated in the data from individual subjects. Filled circle, star, square and diamond symbols indicate significant change relative to naïve, mild and moderate states at alpha = 0.05 with Bonferroni correction and the + sign indicates outliers.
GPi
Similar to GPe, when data from all three subjects was pooled, there was a significant main effect of parkinsonian severity on mean discharge rate in the GPi (K (3) = 56.8, p = 2.9 e-12; Figure 4 top right). Post MPTP, the median discharge rate in the mild (68.6 Hz; Dunn's all pair; p<0.0001) and severe (56.4 Hz; p = 0.0005) states increased, but reduced in the moderate state (22.6 Hz; p=0.04), compared to the naïve condition (37.1 Hz).
This same trend was observed in the discharge rate of NHPs B and T (Figure 4 rows 2-3 right). The median of the discharge rates increased in the mild and severe states (NHP B: 63.8 and 60.2 Hz; NHP T: 69.7, p = 0.02 and 52.2 Hz), but was reduced in the moderate state (NHP B: 19.1 Hz, p = 0.002; NHP T: 20.4 Hz), relative to the pre-MPTP condition (NHP B: 56.2; NHP T: 46 Hz). In NHP C however, the median of the discharge rates in the mild (78.3 Hz; p =0.0008) and moderate (32.5 Hz) states were higher than in the naïve (23.3 Hz) state (Figure 4 row 4 right).
These results are similar to that observed for oscillatory activity; pooled data showed clear trends as a function of changing parkinsonian severity while the data from the individual subjects revealed significant variability in changes in mean discharge rate without a clear relationship to increasing severity of motor signs.
Bursting increased post MPTP, but varied inconsistently with motor severity and across nuclei
Pooled burst characteristics as a function of motor severity are shown in Table 3. In GPi there was an increase in the incidence of bursting in the parkinsonian state as reflected by the increase in the number of bursts/minute and a corresponding change in the percent of spikes within bursts. There was a significant main effect of motor severity on median burst frequency (bursts/minute) (K (3) = 25.6, p = 1.2e-5). Post MPTP the median incidence of bursting increased in the mild (p =0.03), moderate (p = 0.004) and severe (p <0.0001) states relative to the naïve state. For individual subjects the change in burst frequency with severity was similar to the pooled data, with the exception of the moderate state in NHP T (see Table 3B). The percent of spikes within bursts of individual subjects did not change systematically with disease progression and was not consistent with the pooled data (see Table 3C).
Table 3.
A: Median burst duration (milliseconds) by subject and motor severity
| GPe | GPi | |||||||
|---|---|---|---|---|---|---|---|---|
| Naïve | Mild | Moderate | Severe | Naïve | Mild | Moderate | Severe | |
| Pooled | 78.5 | 66.1* | 56.6* | 85.8 | 70.8 | 65.9 | 54.6 | 53.8 |
| NHP B | 72.4 | 48.8* | 46.3* | 86.2 | 52.3 | 44.2 | 41.2 | 43.0 |
| NHP T | 82 | 72.8 | 58.9* | 85.4 | 70.6 | 73.4 | 87.7 | 77.6 |
| NHP C | 83.1 | 121.1* | 88 | - | 83.0 | 96.0 | 60.0 | - |
| Table 3 B: Median of the burst frequency (bursts/min) by subject and motor severity | ||||||||
|---|---|---|---|---|---|---|---|---|
| GPe | GPi | |||||||
| Naïve | Mild | Moderate | Severe | Naïve | Mild | Moderate | Severe | |
| Pooled | 54.8 | 72.7 | 63.5 | 50.3 | 18.4 | 37.4* | 65.7* | 63.2* |
| NHP B | 57.2 | 81.4 | 83.4 | 11.4* | 46.1 | 90.3 | 106.1 | 98.7 |
| NHP T | 52.5 | 79.2* | 63.6 | 73.0 | 17.2 | 27.6 | 16.0 | 51.8* |
| NHP C | 47.0 | 45.2 | 21.8 | - | 12.7 | 24.0 | 30.7 | - |
| Table 3 C: Median of the percent of spikes within bursts by subject and motor severity | ||||||||
|---|---|---|---|---|---|---|---|---|
| GPe | GPi | |||||||
| Naïve | Mild | Moderate | Severe | Naïve | Mild | Moderate | Severe | |
| Pooled | 20.6 | 23.8 | 20.9 | 21.5 | 6.1 | 12.5* | 24* | 18.7* |
| NHP B | 20.2 | 23.2 | 25.2 | 5.5* | 14.4 | 21.8 | 56.0* | 20.6 |
| NHP T | 20.5 | 27.0 | 28.0 | 26.9 | 4.5 | 8.7 | 10.7 | 16.4* |
| NHP C | 21.3 | 22.9 | 11.3 | - | 5.9 | 8.2 | 16.5 | - |
p<0.05 relative to naïve state
In GPe the incidence of bursting did not change systematically with motor severity, but there was a significant main effect of severity on burst duration (Kruskal Wallis: K (3) = 25.2, p = 1.4e-5). Post hoc comparison with Dunn's all pair test revealed significant changes in the mild and moderate states; however, these changes were not monotonically increasing. The median burst duration in the mild (p = 0.04) and moderate (p=0.0003) states decreased compared to naïve, but was similar to the pre-MPTP condition in the severe state. Burst frequency post MPTP was highly variable and exhibited changes that were the inverse of those in burst duration; hence, the percent of spikes within bursts did not change significantly in the post-MPTP conditions. Examination of data from individual subjects revealed trends similar to the pooled data in the burst duration and frequency of NHP B and T, but not in NHP C (see Table 3 A-C).
DISCUSSION
Oscillations
The results of the current study follow on our previous report of local field potential (LFP) activity in the basal ganglia with increasing severity of parkinsonian motor signs (Connolly et al., 2015a; Connolly et al., 2015b) by examining changes in single and paired spike activity within and between the GPe and GPi. In the naïve primate, we demonstrated that the majority of spectral content present in GPe and GPi units was in the gamma band, however, following induction of parkinsonism we observed an increase in low-frequency activity, as has been reported by other groups (Devergnas et al., 2014; Kuhn et al., 2009). However, contrary to prior reports suggesting low frequency oscillatory activity in the beta band develops only in severely parkinsonian animals (Leblois et al., 2007), we observed alpha and low beta oscillations in naïve animals and across all three stages of parkinsonism. An increase in the incidence of oscillatory activity in the 8-20 Hz range in the parkinsonian state is consistent with previous reports in non-human primates (Devergnas et al., 2014) and humans (Kuhn et al., 2009). The relationship of beta activity to severity however was not consistent and varied significantly across animals, in spite of the fact that the presence and severity of individual motor signs were similar across animals, suggesting that while beta oscillations may correlate with the severity of disease in some cases, this is not a consistent finding across all subjects. Previous studies supporting the role of beta activity in Parkinson's disease, in particular bradykinesia (Brown, 2007; Kuhn et al., 2009; Pogosyan et al., 2009; Ray et al., 2008), collected LFP's from patients in more advanced stages of disease and combined data across multiple patients. Pooling data in this way could mask the absence of any relationship of beta to Parkinson's disease severity observed in some by a stronger correlation in others.
Thus, although assigning a significant role for beta oscillations in the pathogenesis of Parkinson's disease might be compelling in some studies (e.g., pooled data), arguments that such activity reflects the parkinsonian state remains problematic given 1) oscillations in the beta band are present in naïve and non-symptomatic states as demonstrated in this study and reported previously by others (Connolly et al., 2015a; Connolly et al., 2015b; Murthy and Fetz, 1992); 2) beta oscillations are not present in all Parkinson's disease patients (Rosa et al., 2011); and 3) while some evidence in Parkinson's disease patients suggests that beta band activity in the STN contributes to akinesia (Kuhn et al., 2004; Ozkurt et al., 2011) others have failed to find a strong correlation between clinical impairments and STN beta-band activity (Kuhn et al., 2009; Ray et al., 2008). This does not exclude one from using beta activity as a biomarker for closed-loop systems as demonstrated by Little et al (Little et al., 2013), nor does it exclude a potential role for exaggerated beta activity in the pathophysiology of Parkinson's disease; it does however, suggest that the presence of beta activity by itself is not pathognomonic of Parkinson's disease, cannot by itself explain the increasing severity of Parkinson's disease motor signs, and while sufficient for some individuals, is not sufficient for all (Bergman and Deuschl, 2002; Brown, 2003; Connolly et al., 2015b).
Rate changes and Bursting
Changes in rate or burst activity have played a significant role in models of Parkinson's disease, yet the precise nature of their relationship to the development and severity of Parkinson's disease motor signs remains ill-defined. According to the rate model, the firing rate of GPe neurons decreases while GPi rates increase (Albin et al., 1989; DeLong, 1990). Although pooled data from the three subjects in this study did show trends in support of this hypothesis, similar to our findings with regards to beta activity, the changes were inconsistent across animals. Specifically, although median GPi firing rates in the mild and severe states were increased relative to the naïve state, the overall trend was not monotonic. Such variability could account for the discrepancies reported across previous studies (Albin et al., 1989; DeLong, 1990; Raz et al., 2000; Wichmann et al., 1999) and supports recent suggestions that rate changes associated with Parkinsonism (at least in the GPi) may be an epiphenomenon that is not directly responsible for the onset and progression of motor signs. However, it does not rule out a contribution of these changes to the parkinsonian state, rather that these changes cannot by themselves account for motor sign development. This is similar to previous reports in the human where oscillations and burst characteristics were combined to account for 60% of the variance in bradykinetic-rigid axial motor scores (Litvak et al., 2011; Moran et al., 2011; Palmer et al., 2009; Sharott et al., 2014).
The consistent reduction in population discharge rate in the moderate state observed in 2 of the 3 animals was surprising, and suggests a possible compensatory process may occur in some animals (Figure 4B) that helps to maintain homeostasis until dopamine loss reaches a critical mass. Bezard et al reported an increasing trend in the discharge rate of GPi and STN based on multi-unit activity recordings and no change in GPe discharge rates with increasing motor severity beyond the pre-symptomatic phase (Bezard et al., 2001). Their explanation for the observed trend was that non-dopaminergic compensatory mechanisms, which underlie the pre-symptomatic phase were no longer able to maintain homeostasis in the BG circuit, which resulted in elevated GPi and STN firing rates (Bezard et al., 2001).
Computational modeling studies of the BGTC network have shown that transmission error by the thalamo-cortical relay cells increases with irregularity of GPi output as a result of increased bursting (Guo et al., 2008). Thus the increase in bursting activity as shown here and by others (Boraud et al., 1998; Wichmann et al., 1999), combined with the loss of receptive field specificity (Filion et al., 1988; Goldberg et al., 2002; Pessiglione et al., 2005), could also contribute to the development of the motor signs in Parkinson's disease. Supporting this hypothesis are reports that improvements in bradykinesia during STN or GPi DBS are correlated with regularization of pallidal activity (Dorval et al., 2010; Guo et al., 2008; Hashimoto et al., 2003).
Role of correlation across pallidal segments in Parkinson's disease
It is well known that nodes in the BGTC circuit demonstrate altered patterns of neuronal activity in the parkinsonian state; however few, if any, studies have explored the changes that occur in inter-nuclear signal processing. We observed a significant change in coherence between neurons in GPe and GPi that occurred across multiple frequency spectra in the parkinsonian state. Although the increase in incidence of coherence occurred predominately in low frequency bands, even in the absence of a significant change within a given pallidal segment, there was a marked and significant increase in paired neurons coherent in the gamma range across segments. This cross-segment increase in coherence was a predominant feature in both low and high frequency bands and is consistent with our previous observations that information transmitted from GPe to GPi increases with symptom severity (Dorval et al., 2015). Taken together these findings suggest that in the parkinsonian state the direct and indirect pathways receive input from a common source and lose independence (Dorval et al., 2015; Mink, 1996). This loss of functional segregation leads to a loss of specificity, altered temporal spatial processing of information flow within the pallidum, and disruption in information transmission throughout the BGTC network.
While more detailed and complex means of assessing signal processing in the BGTC circuit are likely to evolve over the coming years, based on these and other data it is clear that changes in the activity of one frequency band (beta) or one physiological parameter (rate, bursting) are not able to fully account for the development and progression of parkinsonian motor signs. The question is whether we are looking at the wrong physiological parameters or whether the answer may simply require a combination of parameters, e.g. multiple frequency bands, coupling across frequency bands or nodal points in the circuit, bursting characteristics, or connectivity and coherence changes, to explain the motor signs of Parkinson's disease. We previously reported a correlation between the amplitude of high frequency oscillations and the phase of beta activity, phase amplitude coupling (PAC), to the severity of Parkinson's disease in the nonhuman primate(Connolly et al., 2015a). Similarly, PAC across M1 and STN has been demonstrated to occur in Parkinson's disease and is reduced during STN DBS (de Hemptinne et al., 2013; de Hemptinne et al., 2015). These studies provide compelling evidence for a role in cross frequency coupling of neuronal activity in Parkinson's disease pathophysiology. Similarly we would propose that changes in the presence and distribution of synchronized oscillations within and across pallidal segments in different spectral bands as observed in the present study likely provide a strong modulatory effect on the timing and depth of modulation of oscillatory rhythms (Jenkinson and Brown, 2011; Leventhal et al., 2012) important in motor control and whose dysfunction contribute to development of the parkinsonian state. Future studies exploring the relationship of such changes to the development and progression of motor signs together with deciphering their role in normal motor control will be critically important to understanding the pathophysiology of Parkinson's disease and developing new neuromodulatory treatment approaches.
STUDY LIMITATIONS
In this study we have reported changes in pallidal neuronal activity with increasing motor severity measured using the modified UPDRS. The lack of a consistent trend in the reported physiological characteristics with increasing motor severity, while consistent with many previous reports in the literature, could have been influenced in part by the small sample size in some monkeys at different levels of severity, and/or to inter-subject differences in the relative location of the recordings sites with some sample sites occurring outside of the sensorimotor territory. These data however are further supported by our previously published study examining the change in LFP activity in different power spectrums at different levels of severity in the same animals where there was also no correlation between any single power spectrum to increasing severity of motor signs (Connolly et al., 2015b). Last, measures of bradykinesia are assessed during movement, while our recordings were made in the awake animal in the resting state. In spite of these limitations, the lack of consistent changes in beta activity, bursting and mean discharge rates within each subject in relationship to overall motor severity suggest a more complex relationship between physiological changes in the basal ganglia and expression of motor signs. Future studies will need to focus on the amalgam of changes that occur, include features such as coherence, cross frequency coupling and the relationship of changes that occur within and across nodal points in the motor circuit.
HIGHLIGHTS.
-
(1)
Synchronization between units within and across pallidal nuclei increased significantly in the parkinsonian state across multiple frequency bands.
-
(2)
Oscillations occur early and across multiple frequency bands in the parkinsonian state, suggesting they are present at the onset of motor signs.
-
(3)
Loss of dopamine decreases the spectral frequency of beta oscillations and the variability in the frequency highlighting the similarity of synaptic inputs to neurons.
-
(4)
Neither the rate nor the incidence of beta oscillations from individual animals was correlated linearly with motor severity. This observation points to a potential problem with previous human studies supporting a relationship between beta oscillatory activity and disease severity, as those data are pooled across multiple subjects.
ACKNOWLEDGEMENTS
The study was supported by NINDS R01 058945 (J.L.V.) and 037019 (J.L.V.) and a Postdoctoral Fellowship for Basic Scientists awarded to A.M. by Parkinson's Disease Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Baron MS, Vitek JL, Bakay RA, Green J, Kaneoke Y, Hashimoto T, Turner RS, Woodard JL, Cole SA, McDonald WM, DeLong MR. Treatment of advanced Parkinson's disease by posterior GPi pallidotomy: 1-year results of a pilot study. Ann Neurol. 1996;40:355–366. doi: 10.1002/ana.410400305. [DOI] [PubMed] [Google Scholar]
- Bergman H, Deuschl G. Pathophysiology of Parkinson's disease: from clinical neurology to basic neuroscience and back. Mov Disord 17 Suppl. 2002;3:S28–40. doi: 10.1002/mds.10140. [DOI] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, Crossman AR, Bioulac B, Brotchie JM, Gross CE. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease. J Neurosci. 2001;21:6853–6861. doi: 10.1523/JNEUROSCI.21-17-06853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Guehl D, Bioulac B, Gross C. Effects of L-DOPA on neuronal activity of the globus pallidus externalis (GPe) and globus pallidus internalis (GPi) in the MPTP-treated monkey. Brain Res. 1998;787:157–160. doi: 10.1016/s0006-8993(97)01563-1. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol. 2007;17:656–664. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Connolly AT, Jensen AL, Baker KB, Vitek JL, Johnson MD. Classification of pallidal oscillations with increasing parkinsonian severity. J Neurophysiol. 2015a;114:209–218. doi: 10.1152/jn.00840.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly AT, Jensen AL, Bello EM, Netoff TI, Baker KB, Johnson MD, Vitek JL. Modulations in oscillatory frequency and coupling in globus pallidus with increasing parkinsonian severity. J Neurosci. 2015b;35:6231–6240. doi: 10.1523/JNEUROSCI.4137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci. 2003;23:11741–11752. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, Ostrem JL, Galifianakis NB, Starr PA. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110:4780–4785. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, Starr PA. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson's disease. Nat Neurosci. 2015;18:779–786. doi: 10.1038/nn.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Devergnas A, Pittard D, Bliwise D, Wichmann T. Relationship between oscillatory activity in the cortico-basal ganglia network and parkinsonism in MPTP-treated monkeys. Neurobiol Dis. 2014;68:156–166. doi: 10.1016/j.nbd.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval AD, Kuncel AM, Birdno MJ, Turner DA, Grill WM. Deep brain stimulation alleviates parkinsonian bradykinesia by regularizing pallidal activity. J Neurophysiol. 2010;104:911–921. doi: 10.1152/jn.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval AD, Muralidharan A, Jensen AL, Baker KB, Vitek JL. Information in pallidal neurons increases with parkinsonian severity. Parkinsonism Relat Disord. 2015 doi: 10.1016/j.parkreldis.2015.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion M, Tremblay L, Bedard PJ. Abnormal influences of passive limb movement on the activity of globus pallidus neurons in parkinsonian monkeys. Brain Res. 1988;444:165–176. doi: 10.1016/0006-8993(88)90924-9. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson's disease. J Neurosci. 2002;22:4639–4653. doi: 10.1523/JNEUROSCI.22-11-04639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Rubin JE, McIntyre CC, Vitek JL, Terman D. Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. J Neurophysiol. 2008;99:1477–1492. doi: 10.1152/jn.01080.2007. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011;34:611–618. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, Schneider GH, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215:380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Leblois A, Meissner W, Bioulac B, Gross CE, Hansel D, Boraud T. Late emergence of synchronized oscillatory activity in the pallidum during progressive Parkinsonism. Eur J Neurosci. 2007;26:1701–1713. doi: 10.1111/j.1460-9568.2007.05777.x. [DOI] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53:926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73:523–536. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, Foltynie T, Limousin P, Ashkan K, Fitzgerald J, Green AL, Aziz TZ, Brown P. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 2013 doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain. 2011;134:359–374. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- Miller WC, DeLong MR. Parkinsonian symptomatology. An anatomical and physiological analysis. Ann N Y Acad Sci. 1988;515:287–302. doi: 10.1111/j.1749-6632.1988.tb32998.x. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Zhang J, Xu W, Russo GS, Vitek JL, McIntyre CC. Stereotactic neurosurgical planning, recording, and visualization for deep brain stimulation in non-human primates. J Neurosci Methods. 2007;162:32–41. doi: 10.1016/j.jneumeth.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran RJ, Mallet N, Litvak V, Dolan RJ, Magill PJ, Friston KJ, Brown P. Alterations in brain connectivity underlying beta oscillations in Parkinsonism. PLoS Comput Biol. 2011;7:e1002124. doi: 10.1371/journal.pcbi.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25-to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci U S A. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkurt TE, Butz M, Homburger M, Elben S, Vesper J, Wojtecki L, Schnitzler A. High frequency oscillations in the subthalamic nucleus: a neurophysiological marker of the motor state in Parkinson's disease. Exp Neurol. 2011;229:324–331. doi: 10.1016/j.expneurol.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Eigenraam L, Hoque T, McCaig RG, Troiano A, McKeown MJ. Levodopa-sensitive, dynamic changes in effective connectivity during simultaneous movements in Parkinson's disease. Neuroscience. 2009;158:693–704. doi: 10.1016/j.neuroscience.2008.06.053. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Guehl D, Rolland AS, Francois C, Hirsch EC, Feger J, Tremblay L. Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci. 2005;25:1523–1531. doi: 10.1523/JNEUROSCI.4056-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogosyan A, Gaynor LD, Eusebio A, Brown P. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr Biol. 2009;19:1637–1641. doi: 10.1016/j.cub.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Wang S, Holland P, Brittain JS, Joint C, Stein JF, Aziz T. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson's disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol. 2008;213:108–113. doi: 10.1016/j.expneurol.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Ritov Y, Heimer G, Bergman H, Bar-Gad I. Local shuffling of spike trains boosts the accuracy of spike train spectral analysis. J Neurophysiol. 2006;95:3245–3256. doi: 10.1152/jn.00055.2005. [DOI] [PubMed] [Google Scholar]
- Rosa M, Giannicola G, Servello D, Marceglia S, Pacchetti C, Porta M, Sassi M, Scelzo E, Barbieri S, Priori A. Subthalamic local field beta oscillations during ongoing deep brain stimulation in Parkinson's disease in hyperacute and chronic phases. Neurosignals. 2011;19:151–162. doi: 10.1159/000328508. [DOI] [PubMed] [Google Scholar]
- Sharott A, Gulberti A, Zittel S, Tudor Jones AA, Fickel U, Munchau A, Koppen JA, Gerloff C, Westphal M, Buhmann C, Hamel W, Engel AK, Moll CK. Activity parameters of subthalamic nucleus neurons selectively predict motor symptom severity in Parkinson's disease. J Neurosci. 2014;34:6273–6285. doi: 10.1523/JNEUROSCI.1803-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment. J Neurosci. 2004;24:6417–6426. doi: 10.1523/JNEUROSCI.0836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek JL, Bakay RA, Freeman A, Evatt M, Green J, McDonald W, Haber M, Barnhart H, Wahlay N, Triche S, Mewes K, Chockkan V, Zhang JY, DeLong MR. Randomized trial of pallidotomy versus medical therapy for Parkinson's disease. Ann Neurol. 2003;53:558–569. doi: 10.1002/ana.10517. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Bakay RA, Hashimoto T, Kaneoke Y, Mewes K, Zhang JY, Rye D, Starr P, Baron M, Turner R, DeLong MR. Microelectrode-guided pallidotomy: technical approach and its application in medically intractable Parkinson's disease. J Neurosurg. 1998;88:1027–1043. doi: 10.3171/jns.1998.88.6.1027. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Giroux M. Physiology of hypokinetic and hyperkinetic movement disorders: model for dyskinesia. Ann Neurol. 2000;47:S131–140. [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]