Abstract
Objective
To report our successful outcomes of genital split-thickness skin graft (STSG) in covering major skin loss and providing good functional and cosmetic outcomes.
Materials and Methods
A retrospective chart review was performed for all adult urology patients who underwent STSG at our institution from 1998 to 2014. Patients had a wide range of disease etiologies, including tissue loss (eg post-Fournier's gangrene), lymphedema, buried penis, foreign body injection, and tumors.
Results
A total of 54 patients were identified with the following breakdown of etiology: 13 patients with tissue loss (eg post-Fournier's gangrene), 13 with lymphedema, 12 with buried penis, 8 with foreign body injection, 4 with hidradenitis suppurativa, and 4 with tumors. Fifty-two out of 54 patients had more than 90% graft take, with maintained or improved erection, normal voiding, good cosmetic outcome as judged by the patient and the examining surgeon, and normal mobility. One patient died at 3 months due to cardiovascular cause, and 1 patient had a poor take of the graft.
Conclusion
We show the wide variety of indications for STSG use, the ease of the technique, and its successful outcomes. We believe this procedure should be offered to patients as a first-line treatment and also as a last resort when other more conservative approaches fail.
Several diseases affect the male genitals and may result in tissue loss, severe functional disability, and cosmetic disfigurement. This can have a significant impact on the patient's quality of life, necessitating surgical intervention in many instances. These diseases range from severe tissue loss due to infection (Fournier's gangrene) or trauma and burn injuries, malignancy such as Paget's disease or penile carcinoma in situ (CIS), to severe disfigurements and physical disability as in the case of primary lymphedema.1 Surgical intervention is often necessary to treat these diseases. Typically, surgical intervention entails excision of the genital skin in order to eliminate the disease process and resurface the penis, resulting in major skin loss. Different techniques have been described to cover those defects, including primary closure (whenever feasible), local tissue flaps, full-thickness skin grafts (FTSG), and split-thickness skin grafts (STSG). STSG represents a simple and effective surgical technique that is capable of covering major skin loss and providing good functional and cosmetic outcomes.2
Ollier was the first to experiment with STSG in 1872. The plastic surgeon Earl Pagett and George Hood the mechanical engineer, later on in 1939, invented the electrical dermatome to harvest STSG.3 Tanner et al4 were the first to describe in 1964 the meshed STSG in order to expand the graft, and Davison et al5 in 1986 showed that meshed STSG improves graft take. In this study, we present our experience at a major tertiary reconstructive urology referral center in performing STSG for male genital reconstruction over the last 15 years. We show the diverse indications for this procedure and demonstrate its successful cosmetic and functional outcomes in terms of voiding and sexual performance.
Materials and Methods
Patients
We performed a retrospective analysis of all patients who underwent STSG for genital reconstruction at the University of California San Francisco (UCSF) medical center and San Francisco General Hospital from 1998 to 2014. Approval from University of California San Francisco Institutional Review Board was obtained for the study. Retrospective chart review was performed. Written consents were prospectively obtained from all the patients who had their photographs taken.
Surgical Technique
Preparation
Patients are placed in low lithotomy position. The genitalia, lower abdomen, and both thighs are shaved. We administer broad-spectrum intravenous antibiotics. Then, depending on the extent the disease process, we excise the scrotal and/or penile skin. An effort is made to assess if primary coverage of the defect with local advancement flaps is possible prior to obtaining the graft. For groin and perineal defects, we mobilize local tissue flaps and close primarily, creating the appropriate surface to graft the penis and scrotum.
Graft harvesting
We harvest the STSG by applying mineral oil over the anterior thigh, and using the Padgett dermatome (Integra, Plainsboro, NJ, USA) at 0.015-inch thickness. We may harvest from one or both thighs depending on the defect size, although 2 large grafts can be taken from a single thigh making use of both thighs uncommon in genital-only cases. If a simultaneous abdominoplasty is performed, as in cases of buried penis due to obesity, obtaining the graft from the excised abdominal skin is an option, though we still prefer to take the graft from the thigh even in those cases, based on our subjective, expert opinion. The graft donor site is covered with thrombin-soaked Telfa (Covidien, Minneapolis, MN, USA), which is removed at the end of the case and replaced with Tegaderm dressing (3M, St. Paul, MN, USA).
Scrotal grafting
During the excision of scrotal skin, we always make every effort to preserve the testicle and the external spermatic fascia, however applying the graft directly on the tunica albuginea is possible. We suture the testicles and spermatic cords together with absorbable sutures to prevent the formation of bifid scrotum. We typically mesh the scrotal STSG because this improves take, and makes it more pliable and resembles scrotal rugae. Gravity allows the scrotal graft to expand gradually, giving a natural pendulous neoscrotum as healing occurs.
Penile grafting
When we excise the penile skin, we typically try to preserve as much dartos tissue as possible, since we believe it enhances the extensibility and mobility of the graft. We excise all the skin to the coronal sulcus to avoid lymphedema of any remnant skin. If the glans is involved, we excise the glans skin as well. We always inquire patients preoperatively about their erections and sexual function, and we keep the penile STSG unmeshed in patients who still desire to have erections, as it allows more stretching, and is more cosmetically appealing. However, we generally prefer to mesh the penile skin grafts in patients who do not have erections and do not wish to be sexually active in the future, as meshing improves the take of the graft. While we do not exclusively place one type of graft on irradiated patients, we prefer meshed graft because of general improved graft take. We then place the graft over the defect area and tack it in place using 4-0 chromic sutures. The penile graft is secured at the base and the neo-ventral raphe we create. Silk sutures (4-0) are placed at the coronal sulcus and the base to tie over bolsters.
Dressing
A urethral catheter is placed prior to dressing application. Xeroform dressing (Covidien, Minneapolis, MN, USA) is applied and covered with mineral oil-soaked cotton. Fluffs and bolsters are used to secure the penile and scrotal grafts, and a penile splint ensure immobility as we previously described.6 Figure 1C and Supplementary Figure 2C and 2D show our dressing technique. It is important to note that the penis should be at full stretch when the graft and dressing are applied, as any wrinkling in the graft would prevent proper graft take, and to prevent contracture of the graft.
Figure 1.
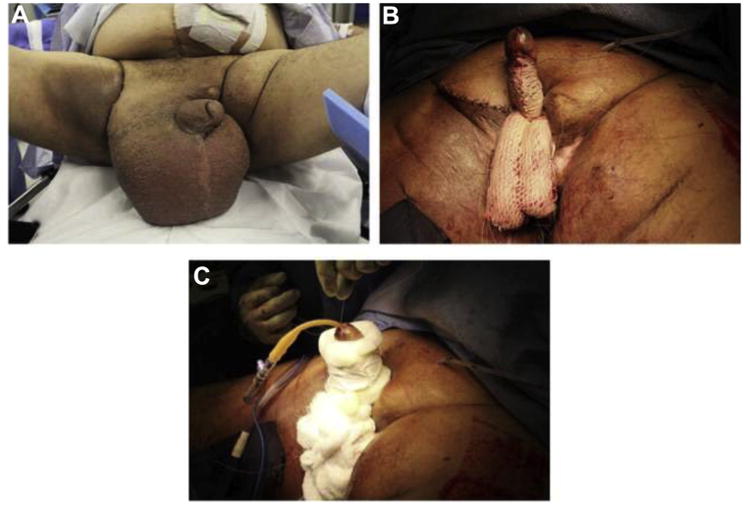
(A) 68-year-old gentleman with genital lymphedema after radiation therapy for prostate cancer and abdominoperineal resection for rectal cancer. (B) The suprapubic skin was tacked to the pubic bone, the penile and scrotal skin were excised, and meshed STSG was applied over the penis and scrotum. (C) Fluffs and penile splint were applied over the graft. (Color version available online.)
Postoperative Care
Patients are kept maintained on bed rest with the dressing in place for 4-5 days postoperatively while being maintained on anticoagulation. Intravenous broad-spectrum antibiotics are given for 24 hours, and then switched to oral cephalexin for 5 days. The graft donor site dressing is removed at day 2 and is replaced with Xeroform, which is left until it falls off spontaneously. Immobility of the graft for 5 days is of utmost importance, to ensure proper take of the graft. The urethral catheter and graft dressing are usually removed on day 5, at which time the patient is allowed to ambulate. The patient is typically discharged home on postoperative day 6. We encourage patients to maintain personal hygiene by showering twice daily and dressing the graft site with Xeroform for 1 month. We instruct the patient to prevent direct contact of the penile and scrotal grafts through the application of Xeroform on both grafts. We encourage the patients to have erections after 5 days postoperatively, in order to expand the length and diameter of the penile graft. Home nursing visits are of great importance in the postoperative period to help ensure the patient's adherence to the care instructions of graft site. We follow the patients closely in the early postoperative through frequent clinic visits, every 2 weeks for 6 weeks, then at 3, 6, and 12 months. During the clinic visit we ask the patients specifically about erections, sexual performance, their satisfaction with the graft cosmetic appearance, and whether they have any difficulties urinating.
Results
Supplementary Table 1 summarizes the patients' outcomes. A total of 54 patients were identified with a wide range of disease etiologies, including 13 patients with tissue loss (eg post-Fournier's gangrene), 13 with lymphedema, 12 with buried penis, 8 with foreign body injection, 4 with hidradenitis suppurativa, and 4 with tumors. Table 1 summarizes the disease etiologies for these patients, and Figures 1 and 2 and Supplementary Figures 1-5 show the photographs of some of these patients. Patients had a follow-up that ranged widely from 6 months up to 10 years. While there was one mortality at 3 months from cardiovascular complications (patient 18) and some patients had significant complications, most patients tolerated the procedure and had excellent outcomes. Nearly all patients (52 of 54 patients) had more than 90% take of the graft, with cosmetically acceptable outcome, normal urination, unchanged or improved baseline erectile and sexual function, and uncompromised ability to ambulate. There were a total of 16 complications, 13 early in the first 1 year and 3 late complications. Patient 21 was found to have invasive squamous cell carcinoma in the pathology specimen, requiring chemotherapy and total penectomy at 1 year postoperative. Patient 19 was the only patient with poor graft take at the scrotum area. He was morbidly obese, with metabolic syndrome and had a history of pelvic irradiation. Patient 5 had left testicular necrosis, requiring left orchiectomy and left scrotal skin debridement at 3 weeks. Patient 6 had pubic wound infection, which was opened (see Fig. 2). Patient 28 had residual silicone material that was resected at 1 year, while the residual silicone material in patient 29 was managed conservatively as per the patient's request. Patient 33 developed a benign fibrotic perineal mass that was excised at 1 year. Patient 38 had hidradenitis suppurativa and he continued to have some drainage from the perineum that resolved spontaneously after 4 years, as he did not want an intervention. Patient 41 had extensive Fournier's gangrene as the etiology for his skin loss, and then developed 2 urethrocutaneous fistulae after STSG placement, which were successfully repaired at 8 months. Patient 46 had bleeding from the wound that had to be controlled in the operating room within the first 24 hours after surgery. Patient 13 had tethered penis requiring penile release and repeat skin grafting at 1 year. In our expert opinion, while the STSG provides good deep touch sensation, enough to maintain adequate erectile function, we noticed that light touch is nearly always affected to varying degrees, resulting in delayed ejaculation in many patients. Nearly all patients experienced pain at the graft donor site for the first few weeks, however we did not encounter any donor site infections or other complications.
Table 1. Etiology for cases undergoing genital STSG.
| Etiology (n) | Percentage (%) |
|---|---|
| Tissue loss7 | |
| Post-Fournier's gangrene8 | 24 |
| Postpenile reimplantation3 | |
| Postprolonged penile ring application1 | |
| Lymphedema7 | |
| Primary lymphedema5 | 24 |
| Postpelvic/penile surgery and/or radiation5 | |
| Posthypospadias repair1 | |
| Klippel-Trenaunay-Weber syndrome1 | |
| Milroy's lymphedema1 | |
| Buried penis9 | |
| Lichen sclerosus4 | 22 |
| Multiple circumcisions3 | |
| Obesity2 | |
| Postpenile radiation1 | |
| Posthypospadias repair1 | |
| Postpartial penectomy1 | |
| Foreign body injection10 | |
| Silicone6 | 15 |
| Steroid1 | |
| Unknown1 | |
| Hidradenitis suppurativa4 | 7 |
| Malignancy2 | |
| Buschkee–Löwenstein tumor1 | 4 |
| Penile CIS1 | |
| Condyloma accuminatum1 | 2 |
| Extramammary Paget's disease1 | 2 |
CIS, carcinoma in situ.
Figure 2.
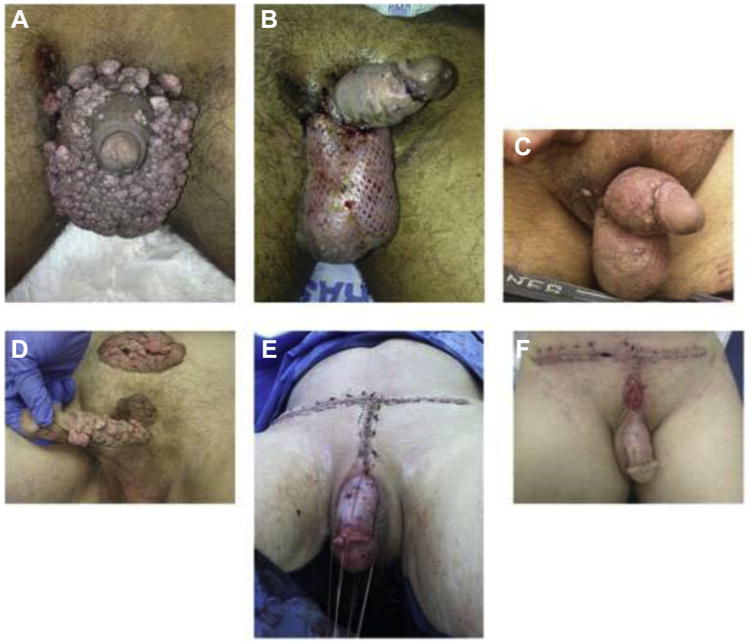
(A) 28-year-old gentleman with Milroy's lymphedema. (B) The involved penile and scrotal skin was excised, meshed STSG applied over the scrotum, and unmeshed STSG applied over the penis. (C) Graft at 3 months. (D) 57-year-old gentleman with condyloma accuminatum involving the penis and the pubic region. (E) All the involved skin was excised. Unmeshed STSG was applied over the penis, and pubic wounds were closed primarily. (F) Picture taken at 1 month. Part of the pubic wound was opened due to wound infection on postoperative day 4. (Color version available online.)
Comment
The main principles of male genital reconstruction are excision of all the diseased skin and coverage of the defect. Other goals of reconstruction include uncompromised physical mobility, normal voiding, normal erectile and sexual function, and satisfactory cosmesis. When primary closure is not possible, other techniques are available and have been described including STSG, and FTSG using remnant foreskin10,11 or scrotal skin,8 which provide excellent cosmesis. However, STSG has several advantages over FTSG, including its hairlessness, excellent take in contaminated fields such as in trauma or hidradenitis, and ease of harvest. In addition, FTSG suffers from increased donor site morbidity and limited donor sites.12
We previously showed the successful utilization of STSG in managing primary lymphedema.13 Several other surgeons reported the same success of STSG in lymphe-dema7,9,14; the largest was a case series of 350 patients with elephantiasis.12 In this study, we report the success of STSG in lymphedema due to a wide range of etiologies (primary, postpelvic radiation/surgery, hypospadias repair, Klippel-Trenaunay-Weber, and Milroy's lymphedema). We also report here the success of STSG in covering the defects from tissue loss due to Fournier's gangrene or any other etiology. We typically apply the graft during the same admission once the wound is stabilized, and no more debridement is needed. We usually mesh penile graft in contaminated wounds. Black et al2 reported the use of meshed penile grafts in all cases of tissue loss. Chen et al15 reported the use of STSG in cases where there is a large surface area of tissue loss in Fournier's gangrene.
Similar to a previous report by Chen et al,16 we show here the successful use of STSG in cases of hydradenitis suppurativa. We also show the effectiveness of STSG in covering genital defects caused by excision of different skin lesions and tumors, namely condyloma accuminatum, Buschke–Löwenstein tumor, penile CIS, and Paget's disease. We, in addition to other surgeons,17 have reported the success of STSG post skin excision in extramammary Paget's disease.18 Ballaro et al19 previously described a technique of penile resurfacing for extensive warts.
Another utility demonstrated here is the use of STSG in buried penis, which could be due to a variety of etiologies, as shown above. This is a common problem, and STSG represents an important option for cases not amenable to less morbid surgical options, such as phalloplasty. Injection of foreign bodies, such as silicone, into the penis is a common procedure performed by nonmedical practitioners or the patient himself for penile augmentation. Just as in previous reports,20,21 we managed foreign body complications through excision of the involved skin and replacing the defect effectively with STSG. In these cases, it is important to remove the involved skin in its entirety, as we did have 2 patients complaining of residual foreign material that required re-excision in one of them. While we do not apply wound VAC therapy to the STSG recipient wound after application of the graft, several authors have reported its successful use in STSG.22,23 We previously reported,1 among other authors,2 that the penis is kept immobilized and on stretch for 5 days we believe that sheering the penile graft off its recipient bed can lead to disruption of the vascular ingrowth process and lead to graft contracture or failure of the graft take. However, some authors reported the use of fibrin glue to improve graft take and reduce the bed rest period.16 We do not employ fibrin glue in our practice.
To our knowledge, this is one of the largest case series on genital STSG, and shows the widest range of indications for its use in the literature. However, there are some limitations in our study. This is a retrospective chart review, and the assessment of surgical outcomes was subjective as no validated instruments were used (such as quality of life and International Index of Erectile Function). To our knowledge, there are no standardized methods for graft take assessment. Therefore, our graft take assessment was subjectively based on surgeon's expertise. Future directions should include prospective examination of STSG outcomes, and applying validated instruments in order to quantify the effect of STSG on sexual function and quality of life.
Conclusions
We report our experience as a tertiary reconstructive urology referral center with male genital STSG. We show the wide variety of indications for its use, the ease of the technique, and its successful outcomes. We believe this procedure should be offered to patients as a first-line therapy as well as a last resort when other more conservative approaches fail.
Supplementary Material
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Appendix: Supplementary Data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.urology.2015.07.005
References
- 1.McAninch J. Management of genital skin loss. Urol Clin North Am. 1989;16:387–397. [PubMed] [Google Scholar]
- 2.Black PC, Friedrich JB, Engrav LH, Wessells H. Meshed unexpanded split-thickness skin grafting for reconstruction of penile skin loss. J Urol. 2004;172:976–979. doi: 10.1097/01.ju.0000133972.65501.44. [DOI] [PubMed] [Google Scholar]
- 3.Letterman GS, Schurter M. How to use the Padgette–Hood dermatome. GP. 1956;14:140. [PubMed] [Google Scholar]
- 4.Tanner JC, Vandeput J, Olley JF. The mesh skin graft. Plast Reconstr Surg. 1964;34:287–292. [PubMed] [Google Scholar]
- 5.Davison PM, Batchelor A, Lewis-Smith PA. The properties and uses of non-expanded machine-meshed skin grafts. Br J Plast Surg. 1986;39:462–468. doi: 10.1016/0007-1226(86)90114-1. [DOI] [PubMed] [Google Scholar]
- 6.Anderson KA, McAninch JW. Penile dressing splint. Urology. 1982;20:188. doi: 10.1016/0090-4295(82)90360-0. [DOI] [PubMed] [Google Scholar]
- 7.Ollapallil JJ, Watters D. Surgical management of elephantiasis of male genitalia. Br J Urol. 1995;76:213–215. doi: 10.1111/j.1464-410x.1995.tb07677.x. [DOI] [PubMed] [Google Scholar]
- 8.Castanãres S, Belt E. Surgical reconstruction of the penis in skin losses, using scrotum skin. Br J Plast Surg. 1968;21:253–257. doi: 10.1016/s0007-1226(68)80032-3. [DOI] [PubMed] [Google Scholar]
- 9.Singh V, Sinha RJ, Sankhwar SN, Kumar V. Reconstructive surgery for penoscrotal filarial lymphedema: a decade of experience and follow-up. Urology. 2011;77:1228–1231. doi: 10.1016/j.urology.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Manchanda RL, Singh R, Keswani RK, Sharma CG. Traumatic avulsion of scrotum and penile skin. Br J Plast Surg. 1967;20:97–103. doi: 10.1016/s0007-1226(67)80012-2. [DOI] [PubMed] [Google Scholar]
- 11.Parkash S, Gajendran V. Surgical reconstruction of the sequelae of penile and scrotal gangrene: a plea for simplicity. Br J Plast Surg. 1984;37:354–357. doi: 10.1016/0007-1226(84)90078-x. [DOI] [PubMed] [Google Scholar]
- 12.Dandapat MC, Mohapatro SK, Patro SK. Elephantiasis of the penis and scrotum. A review of 350 cases. Am J Surg. 1985;149:686–690. doi: 10.1016/s0002-9610(85)80156-2. [DOI] [PubMed] [Google Scholar]
- 13.Morey AF, Meng MV, McAninch JW. Skin graft reconstruction of chronic genital lymphedema. Urology. 1997;50:423–426. doi: 10.1016/S0090-4295(97)00259-8. [DOI] [PubMed] [Google Scholar]
- 14.Garaffa G, Christopher N, Ralph DJ. The management of genital lymphoedema. BJU Int. 2008;102:480–484. doi: 10.1111/j.1464-410X.2008.07559.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen SY, Fu JP, Wang CH, et al. Fournier gangrene: a review of 41 patients and strategies for reconstruction. Ann Plast Surg. 2010;64:765–769. doi: 10.1097/SAP.0b013e3181ba5485. [DOI] [PubMed] [Google Scholar]
- 16.Chen ML, Odom B, Santucci RA. Surgical management of genitoperineal hidradenitis suppurativa in men. Urology. 2014;83:1412–1417. doi: 10.1016/j.urology.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Maruyama S, Ueda K. Scrotal skin replacement for extramammary Paget's disease—a technique. Br J Plast Surg. 2005;58:94–96. doi: 10.1016/j.bjps.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Grossfeld GD, McAninch JW, Santucci R. Extramammary Paget's disease of the penis and scrotum: excision, reconstruction and evaluation of occult malignancy. J Urol. 2001;166:2112–2117. doi: 10.1016/s0022-5347(05)65516-4. [DOI] [PubMed] [Google Scholar]
- 19.Ballaro A, Webster JJ, Ralph D. Penile resurfacing for extensive genital warts. Int J Impot Res. 2001;13:47–48. doi: 10.1038/sj.ijir.3900640. [DOI] [PubMed] [Google Scholar]
- 20.Cavalcanti AG, Hazon A, Favorito LA. Surgical reconstruction after liquid silicone injection for penile augmentation. Plast Reconstr Surg. 2006;117:1660–1661. doi: 10.1097/01.prs.0000208869.97473.8a. [DOI] [PubMed] [Google Scholar]
- 21.Inn FX, Imran F, Ali MF, et al. Penile augmentation with resultant foreign material granuloma and sequelae. Malays J Med Sci. 2012;19:81–83. [PMC free article] [PubMed] [Google Scholar]
- 22.Stokes TH, Follmar KE, Silverstein AD, et al. Use of negative-pressure dressings and split-thickness skin grafts following penile shaft reduction and reduction scrotoplasty in the management of penoscrotal elephantiasis. Ann Plast Surg. 2006;56:649–653. doi: 10.1097/01.sap.0000202826.61782.c9. [DOI] [PubMed] [Google Scholar]
- 23.Weinfeld AB, Kelley P, Yuksel E, et al. Circumferential negative-pressure dressing (VAC) to Bolster skin grafts in the reconstruction of the penile shaft and scrotum. Ann Plast Surg. 2005;54:178–183. doi: 10.1097/01.sap.0000143606.39693.3f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


