Abstract
Background
Cervical disc replacement (CDR) has been widely used to restore and maintain mobility and function of the treated and adjacent motion segments. Posterior longitudinal ligament (PLL) resection has been shown to be efficient in anterior cervical decompression and fusion. However, less is known about the biomechanical effect of PLL removal versus preservation in cervical disc arthroplasty.
Material/Methods
Three motion segments of 24 ovine cervical spines (C2–C5) were evaluated in a robotic spine system with axial compressive loads of 50 N. These cervical spines were divided in three groups according to the following conditions: (1) intact spine, (2) C3/C4 CDR with the Prestige LP prosthesis and PLL preservation, and (3) C3/C4 CDR with the Prestige LP prosthesis and PLL removal. The ranges of motion (ROMs) were recorded and analyzed in each group.
Results
The C3/C4 ROM in group 3 (CDR with PLL removed) increased significantly in flexion-extension and axial rotation compared with group 1 (intact spine). Moreover, in flexion-extension, the mean total ROM was significantly larger in group 3 than in group 1. All the ROM observed in group 2 (CDR with PLL preserved) did not significantly differ from the ROM observed in group 1.
Conclusions
Compared with intact spines, CDR with PLL removal partly increased ROM. Moreover, the ROM in CDR with PLL preservation did not significantly differ from the ROM observed in intact spines. The PLL appears to contribute to the balance and stability of the cervical spine and should thus be preserved in cervical disc replacement provided that the posterior longitudinal ligament is not degenerative and the compression can be removed without PLL takedown.
MeSH Keywords: Biomechanical Phenomena, Longitudinal Ligaments, Total Disc Replacement
Background
Compared with the standard anterior cervical discectomy and fusion, cervical disc replacement (CDR) is an alternative method that can restore and maintain the mobility and function of the treated and adjacent motion segments [1–3]. Clinical results of single-level and multiple-level implantation have demonstrated that CDR is a safe method that shows encouraging clinical and radiological outcomes [4–7]. The benefits of CDR over anterior cervical discectomy and fusion (ACDF), the gold standard technique, have been demonstrated in several prospective randomized controlled trials [4,6,8–10].
Many surgeons recommend the removal of degenerative or hypertrophic posterior longitudinal ligament (PLL) after resection of the proliferative osteophytes and herniated disc in ACDF. It has been shown in the literature that removal of the PLL during anterior decompression procedures for cervical spondylotic myelopathy can provide more decompression and better postoperative clinical results [11–13]. However, the effect of PLL removal in cervical disc arthroplasty remains unclear. According to McAfee et al. [14], PLL plays an important role in postsurgical stability in CDR. Nevertheless, Yang et al. [15] suggested that the PLL resection method could improve the clinical outcomes of CDR; additionally, it does not have a large effect on the motion and balance of the cervical spine. However, the exact role of the PLL in CDR still remains uncertain.
The Prestige LP (Medtronic Sofamor Danek Inc., USA) prosthesis (Figure 1) is an internationally recognized prosthesis model that has provided excellent clinical outcomes and that preserves the overall cervical alignment and the range of motion (ROM) of the treated and adjacent levels [16].
Figure 1.

The Prestige LP prosthesis.
Human spinal specimens are frequently used for in vitro biomechanical analyses. However, the use of human cadaver specimens involves some confounding factors such as age, gender, height, bone quality, and grade of degeneration, which can bias the results of these analyses. Additionally, human cadaver specimens are very difficult to obtain. In contrast, spinal specimens from animals are usually more homogeneous and easier to obtain. Several studies have suggested the use of ovine cervical spine as an accepted model for research on human cervical spine [17–19].
In this study, ovine cervical spines were used to quantify changes in kinematics. The ROMs were compared under the same conditions. We tested the specimens’ physiological motion function using the intact cervical spine, the spine after CDR with the Prestige LP prosthesis and PLL preservation, and the spine after CDR with the Prestige LP prosthesis and PLL removal.
Material and Methods
Specimen preparation
Twenty-four freshly frozen cadaveric cervical spines from two-year-old sheep were utilized in this study. Before starting the biomechanical tests, all specimens were evaluated for bone mineral density (BMD) with dual-energy X-ray absorptiometry scanning to ensure that none of the spines had pathological low BMD (t-score >−2.5). Following the preparation for the biomechanical analysis, the specimens were frozen at −20°C [20,21], then thawed at room temperature for 24 hours before analysis. The ligamentous attachments of each ovine specimen were deliberately preserved. Only muscular and fatty tissues were carefully removed. The C2–C5 vertebrae of the ovine cervical spines were tested in a polysegmental setup. For stabilization purpose, the proximal (C2) and distal (C5) ends of the specimen were embedded in cold curing resin adhesive (HEI-CAST 8012, Heisen Yoko Co. Ltd., Japan). The C2 vertebra was attached to the upper fixture, and the C5 vertebra was mounted to the lower testing platform. Motion capture markers of the optical tracking system were inserted into the vertebral bodies of C2–C4.
Three-dimensional motion testing
The proximal and distal ends of the specimen were mounted on a six-axis spinal robot (Shanghai Sanyou Medical Co. Ltd., Shanghai, China). The moment arm attached to the proximal end of the specimen could apply an axial load and a pure moment, whereas the distal end of the specimen remained fixed to the socket of the robot. The robot was programmed to apply three continuous loading-unloading cycles of applied moment along each primary axis of motion to simulate flexion-extension (FE), lateral bending (LB), and axial rotation (AR). An axial preload of 50 N was given on the C2 vertebra to simulate head weight. All specimens were subjected to three cycles of FE, LB, and AR under nondestructive pure moment of ±2.0 N·m, and the data of the third cycle were used for analysis [22]. The ROM of the C2–C5 polysegment was measured by the optical tracking system. During the biomechanical tests, all specimens were moistened with normal saline to prevent desiccation.
Segmental ROM
An optical tracking system (OptiTrack, NaturalPoint Inc., USA) was used to evaluate the ROM of the C2/C3, C3/C4, and C4/C5 segments. A rigid rod connected to a motion capture marker was inserted into each vertebral body of C2, C3, and C4 (Figure 2). Every motion capture marker was composed of three noncollinear optical balls to ensure it could be detected by the optical tracking system. A marker was placed on the socket to attach the C5 vertebra due to its immovability. The angular ROM value was directly measured by the optical tracking system.
Figure 2.

An intact spine specimen. Each rigid rod connected the motion capture markers to the vertebral bodies for detection by the optical tracking system.
Reconstructive conditions
Twenty-four intact cervical spines were divided into three groups (group 1, group 2, and group 3), with eight specimens per group. A complete discectomy of C3/C4 was performed on all spines of groups 2 and 3. Additionally, PLL resection was performed on the eight cadaveric cervical spines of group 3. Thereafter, the endplates of the ovine spines of groups 2 and 3 were prepared using a high-speed burr to ensure adequate implant positioning, and then CDR was performed. The Prestige LP prostheses were inserted at the C3/C4 level for the two groups (Figure 3). The specimens of the three groups were analyzed.
Figure 3.

The specimens of C3/C4 CDR with the Prestige LP prosthesis.
Radiographic control
Radiographs were taken to ensure that the implants were correctly positioned in the intervertebral space (Figure 4).
Figure 4.
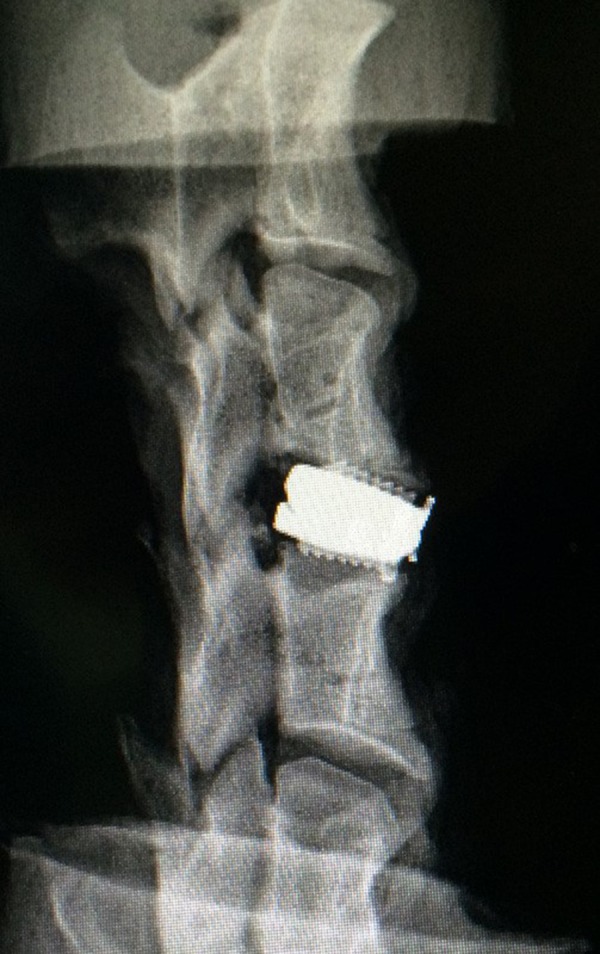
A radiograph showing the correct position of the Prestige LP prosthesis.
Data and statistical analysis
The third loading cycle data for the six spinal motions were used for statistical analysis. The ROM at the C2/C3, C3/C4, and C4/C5 segment levels and the total ROM were quantified at maximum load. The ROM values of the CDR groups and the intact group were compared. The ROMs of group 1 (intact spine) were compared with the ROMs of group 2 (CDR and PLL preservation) and group 3 (CDR and PLL removal) using the unpaired Student’s t-test. The level of significance was set at p<0.05. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 19.0 (IBM Corp., USA).
Results
After biomechanical testing, all ovine spines of groups 2 and 3 were dissected and visually evaluated for damage. No fracture or hardware failure was observed.
As shown in Figure 5, the mean total ROMs of groups 1 and 2 were not significantly different (p>0.05) regardless of the motion direction (FE: mean total ROM=26.07°±2.18° for group 1 vs. 27.24°±3.97° for group 2; LB: mean total ROM=38.54°±4.39° for group 1 vs. 40.10°±4.25° for group 2; AR: mean total ROM=21.02°±4.04° for group 1 vs. 20.89°±3.92° for group 2). For group 3, the mean total ROMs were 29.11°±2.21° (FE), 40.73°±5.05° (LB), and 22.19°±3.27° (AR). Compared with the mean total ROM of group 1, the mean total ROM of group 3 significantly increased only in FE (p<0.05). The mean total ROM in the three motion directions was always recorded at a maximum loading of ±2 N·m.
Figure 5.
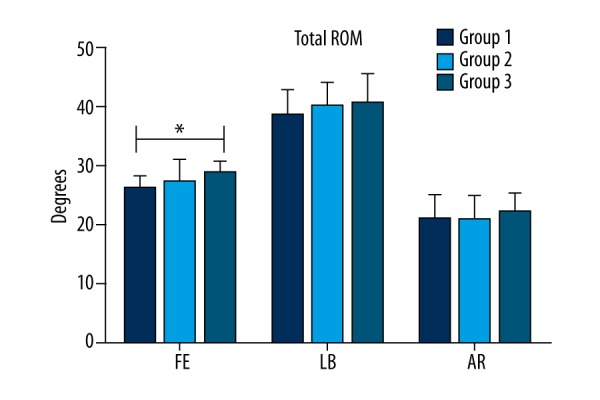
Mean total ROM (±SD) of intact spine (group 1), C3/C4 CDR with PLL preservation (group 2), and C3/C4 CDR with PLL removal (group 3) in the three motion directions. An asterisk (*) indicates a significant difference between the corresponding groups (as indicated by the horizontal bar) at p<0.05.
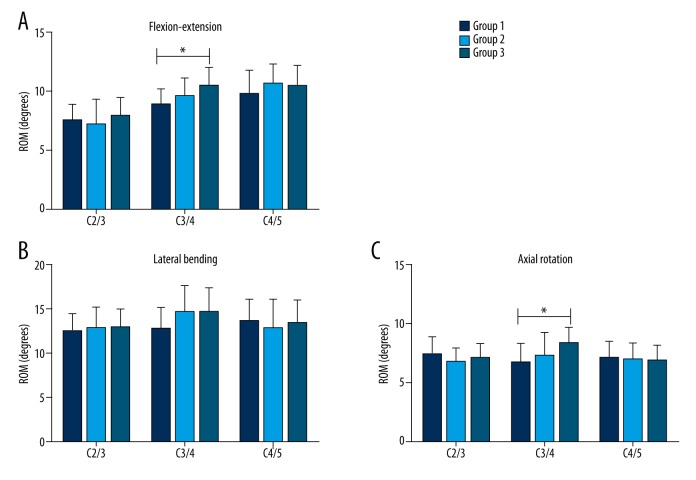
The mean ROMs of C2/C3, C3/C4, and C4/C5 segments in the three motion directions and for each of the three groups are listed in Table 1. Compared with group 1 (intact spine), the C3/C4 ROM in group 3 (CDR with PLL removed) significantly increased (p<0.05) in FE and AR (FE: 10.45°±1.51° for group 3 vs. 8.87°±1.28° for group 1; AR: 8.34°±1.37° for group 3 vs. 6.65°±1.67° for group 1). In addition, the ROM in group 2 (CDR with PLL preserved) was not significantly different from that in group 1 (intact spine) regardless of the motion direction and the vertebral segment (p>0.05) (Figure 6).
Table 1.
ROM of C2/C3, C3/C4 and C4/C5.
| Segment | FE | LB | AR | |
|---|---|---|---|---|
| C2/C3 | Group 1 | 7.49°±1.37° | 12.38°±2.07° | 7.53°±1.54° |
| Group 2 | 7.13°±2.15° | 12.82°±2.40° | 6.72°±1.15° | |
| Group 3 | 7.89°±1.62° | 12.87°±2.14° | 7.04°±1.24° | |
| C3/C4 | Group 1 | 8.87°±1.28° | 12.62°±2.53° | 6.65°±1.67° |
| Group 2 | 9.56°±1.56° | 14.57°±3.08° | 7.23°±1.98° | |
| Group 3 | 10.45°±1.51° | 14.53°±2.82° | 8.34°±1.37° | |
| C4/C5 | Group 1 | 9.71°±2.08° | 13.54°±2.51° | 7.02°±1.44° |
| Group 2 | 10.55°±1.74° | 12.72°±3.32° | 6.94°±1.47° | |
| Group 3 | 10.39°±1.79° | 13.34°±2.70° | 6.80°±1.37° | |
Figure 6.
Mean ROM (±SD) for each of the three groups in flexion-extension (A), lateral bending (B), and axial rotation (C). An asterisk (*) indicates a significant difference between the corresponding groups (as indicated by the horizontal bar) at p<0.05.
Discussion
Cervical fusion sacrifices segmental mobility and increases ROM stress at the adjacent segment. Overloading at the adjacent segments caused by fusion is known to contribute to adjacent segment degeneration [23,24]. Cervical disc replacement is a successful and promising nonfusion technique aimed at restoring normal articular motion and spine kinematics.
Because CDR is of great interest in the operative management of degenerative disc disease in the cervical spine, in vitro studies are of great interest in order to analyze the biomechanics of the different implants. Human cadaveric specimens are very difficult to acquire for such studies. Therefore, animal spines are commonly used to simulate the human spine. ROM testing on many animal spines has confirmed that the ROM of sheep is most similar to that of humans [18]. Moreover, the C2/C3 and C3/C4 segments appear suitable for biomechanical testing. In this study, we tested the kinematics of cadaveric cervical spines from sheep under three conditions (intact, CDR with preserved PLL, and CDR with removed PLL).
Whether to cut off the PLL in cervical disc arthroplasty is a surgeon’s decision. Because of the traditional opinion stating that PLL contributes to the balance and stability of the cervical spine, some authors have suggested preserving the PLL as much as possible if the PLL is not degenerative or hypertrophic [25,26]. However, other authors prefer to remove the PLL in the cervical anterior approach, considering some results that show a better effect of decompression and a similar ROM when PLL is removed compared with when it is preserved [15]. Thus, it appears controversial whether the PLL should be removed during a CDR surgery.
In our study, the CDR with PLL removal partly increased ROM as compared with the intact spines. In addition, the ROM in the CDR with preserved PLL was not significantly different from that in the intact group. Therefore, these findings show that the PLL plays a key role in keeping the balance and stability of the cervical spine.
The PLL lies behind the vertebral bodies, beginning from the occipital bone to the sacrum [27]. The PLL, which is composed of two layers, links up with the intervertebral disc at multiple levels [11,28]. A normal PLL is believed to prevent the disc contents and bone fragments from protruding into the spinal canal [28]. The PLL can also protect the spinal cord if the prosthesis loosens backwards [29]. In addition, the risk of damaging nerve roots, spinal cord, dura, and epidural vascular plexus can increase when the PLL is removed. However, the PLL’s normal biomechanical function of preventing disc protrusion into the spinal canal can be obviously weakened in cases of degeneration or injury, because of the breakdown of the PLL’s elasticity and tensile strength [12]. Moreover, the disc contents may protrude into the degenerative PLL, thus becoming a disc-PLL complex. In that case, the entire disc should be removed to prevent future symptoms. As a result, it is also beneficial to remove the degenerative PLL in CDR. Therefore, we recommend the preservation of the PLL in CDR when the PLL is not degenerative. In cases of degeneration, we recommend that the PLL be routinely removed.
Chen et al. [30] reported that PLL resection can significantly increase the ROM in FE, LB, and AR. McAfee et al. [14] described the importance of the PLL through a biomechanical experiment in CDR. In the present study, the ROMs of the treated levels were significantly increased in FE and AR for group 3 (CDR with removed PLL) compared with group 1 (intact spine), and the mean total ROM of group 3 during FE was significantly larger than that in group 1. In addition, the ROM in group 2 (CDR with preserved PLL) was not significantly different from the ROM observed in group 1 (intact spine), regardless of the motion direction. These results more adequately demonstrate the importance of PLL removal. However, our specimens originated from 2-year-old sheep, which had healthy PLL. Consequently, the present findings only apply to healthy PLL; compared with a healthy PLL, the resection of a degenerative PLL may have a different effect on the cervical spine.
A limitation of our study is that we mainly focused on the extent of motion without considering the stabilizing influence of the paraspinal muscles, the quality of motion, or neural control mechanisms, which is a common limitation in any in vitro cadaveric biomechanical analysis of the cervical spine. Therefore, our results cannot represent the long-term effect of CDR.
Conclusions
Cervical disc replacement with Prestige disc and PLL removal partly increased ROM in a sheep cadaver model, as compared with the intact spines. In addition, the ROM in CDR with PLL preservation did not significantly differ from that in the intact group. Consequently, the PLL appears to contribute to the balance and stability of the cervical spine and should be preserved in CDR if the PLL is not degenerative and the compression can be removed without PLL takedown.
Acknowledgements
The authors declare that they have no competing interests.
Footnotes
Source of support: There is no other support for this article
References
- 1.Coric D, Kim PK, Clemente JD, et al. Prospective randomized study of cervical arthroplasty and anterior cervical discectomy and fusion with long-term follow-up: Results in 74 patients from a single site. J Neurosurg Spine. 2013;18:36–42. doi: 10.3171/2012.9.SPINE12555. [DOI] [PubMed] [Google Scholar]
- 2.Coric D, Nunley PD, Guyer RD, et al. Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2-year follow-up: Clinical article. J Neurosurg Spine. 2011;15:348–58. doi: 10.3171/2011.5.SPINE10769. [DOI] [PubMed] [Google Scholar]
- 3.Sasso RC, Smucker JD, Hacker RJ, Heller JG. Artificial disc versus fusion: A prospective, randomized study with 2-year follow-up on 99 patients. Spine (Phila Pa 1976) 2007;32:2933–40. doi: 10.1097/BRS.0b013e31815d0034. discussion 2941–42. [DOI] [PubMed] [Google Scholar]
- 4.Ren C, Song Y, Xue Y, Yang X. Mid- to long-term outcomes after cervical disc arthroplasty compared with anterior discectomy and fusion: A systematic review and meta-analysis of randomized controlled trials. Eur Spine J. 2014;23:1115–23. doi: 10.1007/s00586-014-3220-3. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y, Zhao YB, Pan SF, et al. Comparison of adjacent segment degeneration five years after single level cervical fusion and cervical arthroplasty: A retrospective controlled study. Chin Med J (Engl) 2012;125:3939–41. [PubMed] [Google Scholar]
- 6.Yin S, Yu X, Zhou S, et al. Is cervical disc arthroplasty superior to fusion for treatment of symptomatic cervical disc disease? A meta-analysis. Clin Orthop Relat Res. 2013;471:1904–19. doi: 10.1007/s11999-013-2830-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S, Wu X, Hu Y, et al. Early and intermediate follow-up results after treatment of degenerative disc disease with the Bryan cervical disc prosthesis: Single- and multiple-level. Spine (Phila Pa 1976) 2008;33:E371–77. doi: 10.1097/BRS.0b013e31817343a6. [DOI] [PubMed] [Google Scholar]
- 8.Wu AM, Xu H, Mullinix KP, et al. Minimum 4-year outcomes of cervical total disc arthroplasty versus fusion: A meta-analysis based on prospective randomized controlled trials. Medicine (Baltimore) 2015;94:e665. doi: 10.1097/MD.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi JS, Lin B, Xue C, et al. Clinical and radiological outcomes following hybrid surgery in the treatment of multi-level cervical spondylosis: Over a 2-year follow-up. J Orthop Surg Res. 2015;10:185. doi: 10.1186/s13018-015-0330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian W, Yan K, Han X, et al. Comparison of the clinical and radiographic results between cervical artificial disc replacement and anterior cervical fusion: A six-year prospective non-randomized comparative study. J Spinal Disord Tech. 2014 [Epub ahead of print] [Google Scholar]
- 11.Avila MJ, Skoch J, Sattarov K, et al. Posterior longitudinal ligament resection or preservation in anterior cervical decompression surgery. J Clin Neurosci. 2015;22:1088–90. doi: 10.1016/j.jocn.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Bai CR, Wang BQ, Li KH, et al. Benefit of degenerative posterior longitudinal ligament removal during anterior decompression in cervical spondylotic myelopathy. Orthopedics. 2015;38:e54–61. doi: 10.3928/01477447-20150105-61. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Chen Y, Chen D, et al. Removal of posterior longitudinal ligament in anterior decompression for cervical spondylotic myelopathy. J Spinal Disord Tech. 2009;22:404–7. doi: 10.1097/BSD.0b013e318187039f. [DOI] [PubMed] [Google Scholar]
- 14.McAfee PC, Cunningham B, Dmitriev A, et al. Cervical disc replacement-porous coated motion prosthesis: a comparative biomechanical analysis showing the key role of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2003;28:S176–85. doi: 10.1097/01.BRS.0000092219.28382.0C. [DOI] [PubMed] [Google Scholar]
- 15.Yang DL, Ding WY, Zhang YZ, et al. Removal versus preservation of the posterior longitudinal ligament in Bryan cervical disc arthroplasty. Chin Med J (Engl) 2013;126:3812–16. [PubMed] [Google Scholar]
- 16.Chen F, Yang J, Ni B, et al. Clinical and radiological follow-up of single-level Prestige LP cervical disc replacement. Arch Orthop Trauma Surg. 2013;133:473–80. doi: 10.1007/s00402-013-1689-6. [DOI] [PubMed] [Google Scholar]
- 17.Sheng SR, Wang XY, Xu HZ, et al. Anatomy of large animal spines and its comparison to the human spine: A systematic review. Eur Spine J. 2010;19:46–56. doi: 10.1007/s00586-009-1192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kandziora F, Pflugmacher R, Scholz M, et al. Comparison between sheep and human cervical spines: An anatomic, radiographic, bone mineral density, and biomechanical study. Spine (Phila Pa 1976) 2001;26:1028–37. doi: 10.1097/00007632-200105010-00008. [DOI] [PubMed] [Google Scholar]
- 19.Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine (Phila Pa 1976) 1997;22:2365–74. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 20.Liao Z, Fogel GR, Wei N, et al. Biomechanics of artificial disc replacements adjacent to a 2-level fusion in 4-level hybrid constructs: An in vitro investigation. Med Sci Monit. 2015;21:4006–14. doi: 10.12659/MSM.896274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao Z, Fogel GR, Pu T, et al. Biomechanics of hybrid anterior cervical fusion and artificial disc replacement in 3-level constructs: An in vitro investigation. Med Sci Monit. 2015;21:3348–55. doi: 10.12659/MSM.896085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilke HJ, Wenger K, Claes L. Testing criteria for spinal implants: Recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998;7:148–54. doi: 10.1007/s005860050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gornet MF, Burkus JK, Shaffrey ME, et al. Cervical disc arthroplasty with PRESTIGE LP disc versus anterior cervical discectomy and fusion: A prospective, multicenter investigational device exemption study. J Neurosurg Spine. 2015 doi: 10.3171/2015.1.SPINE14589. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Virk SS, Niedermeier S, Yu E, Khan SN. Adjacent segment disease. Orthopedics. 2014;37:547–55. doi: 10.3928/01477447-20140728-08. [DOI] [PubMed] [Google Scholar]
- 25.Shim CS, Lee SH, Park HJ, et al. Early clinical and radiologic outcomes of cervical arthroplasty with Bryan Cervical Disc prosthesis. J Spinal Disord Tech. 2006;19:465–70. doi: 10.1097/01.bsd.0000211235.76093.6b. [DOI] [PubMed] [Google Scholar]
- 26.Yoon DH, Yi S, Shin HC, et al. Clinical and radiological results following cervical arthroplasty. Acta Neurochir (Wien) 2006;148:943–50. doi: 10.1007/s00701-006-0805-6. [DOI] [PubMed] [Google Scholar]
- 27.Chin KR, Ghiselli G, Cumming V, et al. Postoperative magnetic resonance imaging assessment for potential compressive effects of retained posterior longitudinal ligament after anterior cervical fusions: A cross-sectional study. Spine (Phila Pa 1976) 2013;38:253–56. doi: 10.1097/BRS.0b013e3182796e9c. [DOI] [PubMed] [Google Scholar]
- 28.Loughenbury PR, Wadhwani S, Soames RW. The posterior longitudinal ligament and peridural (epidural) membrane. Clin Anat. 2006;19:487–92. doi: 10.1002/ca.20200. [DOI] [PubMed] [Google Scholar]
- 29.Misterska E, Jankowski R, Glowacki J, et al. Kinesiophobia in pre-operative patients with cervical discopathy and coexisting degenerative changes in relation to pain-related variables, psychological state and sports activity. Med Sci Monit. 2015;21:181–94. doi: 10.12659/MSM.891045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen TY, Crawford NR, Sonntag VK, Dickman CA. Biomechanical effects of progressive anterior cervical decompression. Spine (Phila Pa 1976) 2001;26:6–13. doi: 10.1097/00007632-200101010-00003. discussion 14. [DOI] [PubMed] [Google Scholar]