Abstract
BACKGROUND
Angiogenesis is critical for the growth of colorectal carcinoma (CRC). Vascular endothelial growth factor is the most important angiogenic growth factor.
METHOD
Fifty cases of CRC operated at INHS Asvini were studied by using immunohistochemical labelling of the tumours by using CD34, p53, and vascular endothelial growth factor (VEGF).
RESULTS
Out of the 50 cases, 31 (62%) were positive for p53; of which 80.7% showed high expression. Significant staining (> 20% tumour cells showing positivity) was seen in 33 patients (66%), while 34% were negative. Of the 33 positive cases, 57.58% showed high-grade VEGF positivity.
CONCLUSION
Vascular endothelial growth factor correlated significantly with the stage and grade of disease. Intra tumours microvessel density as calculated from CD34 expression increased with the stage and grade of the CRC.
Key Words: angiogenesis, colorectal carcinoma, immunohistochemistry
INTRODUCTION
Colorectal carcinoma (CRC), one of the most common cancers causing death worldwide,1 is treated with surgery, and patients with stage III (Dukes' C) and a subset of stage II CRC (Dukes' B2) are treated with adjuvant therapies. Still the locoregional recurrence after curative resection remains a problem. The main prognostic factors in CRC are lymph node involvement, size of the tumour and stage of disease.2 However, these factors do not fully predict individual clinical outcome. Adjuvant treatment is hampered by a lack of reliable prognostic factors in addition to the clinicopathologic staging system.3 Folkman first proposed that tumour angiogenesis could serve as a potential target for anticancer therapy. Normal tissue homeostasis is maintained by balancing cell proliferation with physiologic deletion of senescent cells by apoptosis. Disruption of apoptotic pathway may confer a selective growth advantage, upsetting this homeostatic state.4 In about 50% of CRC, however, mutant p53 aids cancer cell survival.
Angiogenesis is studied by calculating the microvessel density (MVD) i.e. number of vessels per unit area or per field. MVD, as a surrogate marker of tumoural angiogenesis, has been proposed to identify patients at high risk of recurrence. It was first developed by Weidner et al in 1991 using immunohistochemical (IHC) staining of microvessels. Some authors used Chalkley count or computerised image analysis systems, both aimed to minimise subjectivity.5 Tenderenda et al6 carefully counted the microvessels (per 200 × field), and graded (1 to 4 +), in the most active areas of neovascularisation. They concluded that the number of microvessels per 200 × field may be an independent predictor of metastasis. Tomisaki in 1996 studied MVD in CRC and found that microvessel quantification showed a significant correlation with liver metastasis.7
Takahashi et al8 observed a significant correlation between vascular endothelial growth factor (VEGF) expression and metastatic disease. A separate study evaluated factor VIII, VEGF, basic fibroblast growth factor, proliferating cell nuclear antigen, and the presence of vascular, lymphatic, and perineural invasion in CRC node-negative disease.9 Multivariate analysis identified vessel count as a significant correlate with time to recurrence and VEGF expression. VEGF has been reported as an independent prognostic factor by Des et al.10 Kuniyasu et al11 found VEGF levels significantly higher in hyperplastic adjacent mucosa and suggested that it contributes to neoangiogenesis and progression of tumour.
MATERIALS AND METHOD
Fifty resected cases of CRC were studied by Department of Pathology at a tertiary health care centre between January 2000 and May 2007.
Inclusion Criteria
Adenocarcinoma or its variants with at least a six-month follow-up after completion of chemotherapy were selected. Relevant details like site, size, type of growth, whether ulcerative/proliferative, and enlarged nodes were noted. The blocks with maximum depth of tumour invasion were retrieved for performing IHC. The tumours were staged according to TNM classification. Relevant data from oncology files regarding follow up was retrieved to note therapy given, relapse and the survival data.
Immunohistochemistry
Immunohistochemistry (IHC) was performed using standard avidin-biotin-complex (ABC) method on poly-L-lysine coated slides. Sections were brought down to water following which endogenous peroxidase blocking was done with 3% hydrogen peroxide solution in methanol for 20 minutes.
Antigen retrieval for p53 (Monoclonal mouse anti-human p53 antibody, DO-7, Dako) was done by microwaving in TRIS–ethylenediamine tetra acetic acid buffer at pH 9.0 for five cycles, each lasting five minutes. The first cycle was at 100% power, while the remaining were at 60% power. Similarly antigen retrieval for VEGF (Monoclonal mouse anti-human VEGF, Dako) and CD34 (Monoclonal mouse anti-human CD34, QBEnd10, Dako) was done. After the antigen retrieval, the slides were allowed to cool to room temperature and were then flooded with TRIS buffer solution pH 7.3 (TBS) for 10 minutes. Endometrium and placenta were used as positive controls for VEGF and CD34, respectively. For negative control, we omitted the primary antibody each time. The IHC was carried out as per techniques described in the literature.12
Interpretation of Results
p53 is a nuclear stain and VEGF stains cytoplasm and/or membrane. Tumour “hot spots” which often corresponded to the invasive edge were selected, 500 tumour cells counted, and the number of tumour cells positive for p53 and VEGF were noted individually. They were then graded semi-quantitatively as follows:
zero, no expression; 1+, < 20% of tumour cells are positive; 2+, 20–50% positivity; and 3+, > 50% are positive. Only tumours that showed > 20% staining was taken as positive. The expression below 20% positivity was considered negative. CD34 expression in tumour “hot spots” was identified. Branching structures were counted as a single vessel. A single microvessel was defined as any brown immunostained endothelial cell separated from adjacent microvessels, tumour cells and other connective tissue elements. In this way, the number of vessels was noted in ten continuous fields at 400×, i.e. an area of 0.152 mm2.13
Since CD34 expression in the form of intratumoural microvessel density (IMD) is quantitative, and p53, VEGF expressions and grade of the disease are categorical, so to test whether there were significant differences in the IMD value between the different groups of VEGF, p53 and grade of the cancer, ANOVA (analysis of variance) was carried out after testing for homogeneity of variances. Pair-wise comparisons were carried out using LSD method. To find the correlation between the categorical variables χ2-test is carried out using SPSS version 14.0.
RESULTS
In 50 cases of CRC, p53, VEGF, and CD34 immunophenotyping was carried out and their expressions were analysed. Out of the total cases, 34 were males and 16 females with an average age of 58.2 ± 10.0 years. The most common site was rectum (56%). Most patients belonged to stage I, i.e. T1/2 (40%). Sixteen (32%) patients had stage IIA disease, i.e. T3N0. Eleven (22%) patients had locally advanced disease, i.e. T3/4 with lymph node metastasis N1. One patient had stage IIIC disease while two (4%) had distant metastasis.
Twenty-seven (54%) cases showed a moderately differentiated adenocarcinoma. Three cases had a poorly differentiated carcinoma, while 20 cases had a well-differentiated tumour out of which six were mucin secreting. Thirty-seven patients remained free of disease at the endpoint of the study and six patients who died of disease had advanced stage disease, either local or with distant metastasis. Relapses occurred in three patients after 2–5 years of completion of therapy. Out of the three patients that died of unrelated cause, two died of coronary disease while one committed suicide eight months after completion of therapy. One case was lost to follow-up.
Out of the 50 cases, 31 (62%) were positive for p53; of which 19.3% showed low expression while 80.7% showed high expression, i.e. > 50% of the cells showed positive immunoreactivity in the tumour cell nuclei (Figure 1). All cases showed staining with VEGF monoclonal antibody. However, significant staining was seen in 33 patients (66%), while 34% were negative (> 20% stained tumour cells). Of the positive cases, 42.42% had low VEGF staining while 57.58% showed high-grade staining (Figure 2). The IMD ranged from 65 to a maximum of 213/10 high power fields (hpf) (Figure 3 and Table 1).
Figure 1.
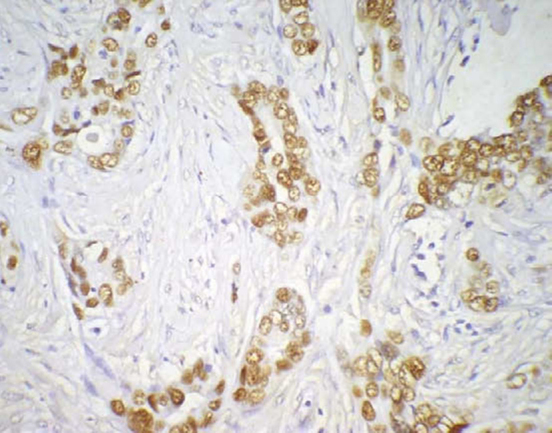
p53 immunoreactivity by the tumour cell nuclei (400×).
Figure 2.
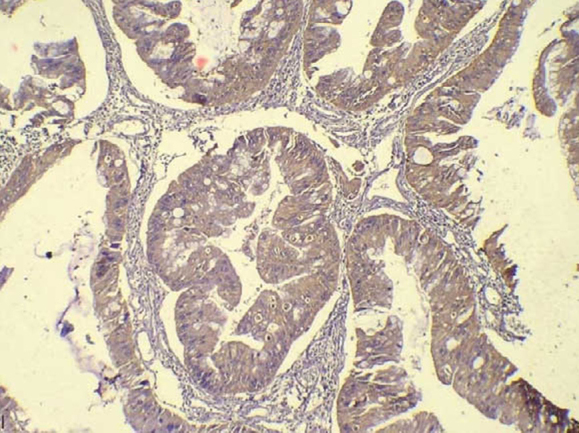
Vascular endothelial growth factor immunopositive high-grade tumour glands of colorectal carcinoma (400×).
Figure 3.

High-grade intratumoural microvessel density highlighted by immunohistochemistry for CD34 on tumour sections (400×).
Table 1.
Average intratumoural microvessel density (standard deviation) as seen in various groups.
| VEGF | p53 | Stage | Grade | ||||
|---|---|---|---|---|---|---|---|
| Expression | Mean IMD | Expression | Mean IMD | Group | Mean IMD | Group | Mean IMD |
| 1+ (negative) | 65 | 0 | 86 | I | 84 | I | 111 |
| 2+ (low) | 129 | 1+ | 104 | II | 153 | II | 178 |
| 3+ (high) | 213 | 2+ | 126.5 | III | 206 | III | 223 |
| − | − | 3+ | 202 | IV | 234 | − | − |
IMD: intratumoural microvessel density, VEGF: vascular endothelial growth factor.
Comparing expression of p53 with VEGF, it was found that 66.7% of cases that were p53 negative, were also VEGF negative; while 84.2% of cases with high-grade immunoreactivity, i.e. 3+ VEGF expression also had grade 3 expression of p53. The correlation in the expression of p53 and VEGF was statistically significant (χ2 = 33.99, P = 0.001) Table 2. Association between p53 and stage using χ2-test shows that there is no association between the p53 expression and stage. Whereas association between VEGF expression by the tumour cells and stage shows that there is significant association between VEGF expression and stage of the disease.
Table 2.
Expression of p53 and vascular endothelial growth factor.
| VEGF/p53 | 1+ | 2+ | 3+ | Total |
|---|---|---|---|---|
| (Negative) | (Low) | (High) | ||
| 0/1+ (negative) | 11 (66.7) | 5 (38.5) | 3 (15.8) | 19 |
| 2+ (low) | 0 (0) | 6 (46.2) | 0 (0) | 6 |
| 3+ (high) | 6 (33.3) | 3 (15.4) | 16 (84.2) | 25 |
| Total | 17 | 14 | 19 | 50 |
VEGF: vascular endothelial growth factor.
P = 0.000.
χ2 = 33.99.
Average IMD also increased from 96.8 and 110.2 (107) in p53 negative tumours to 126.2 in grade 2 to 183.3 in grade 3 tumours. ANOVA reveals that there is a significant difference in the mean CD34 value in the three groups of p53 (P = 0.000). The pair-wise difference in the three groups of p53 was carried out by LSD method. It was found that groups 1 and 2 was not statistically significantly different with respect to CD34 (P = 0.466), whereas there was significant difference in the mean CD34 value in groups 1 and 3 (P = 0.000) and groups 2 and 3 (P = 0.031) (Figure 4). Higher the p53 value, higher was the average IMD value.
Figure 4.
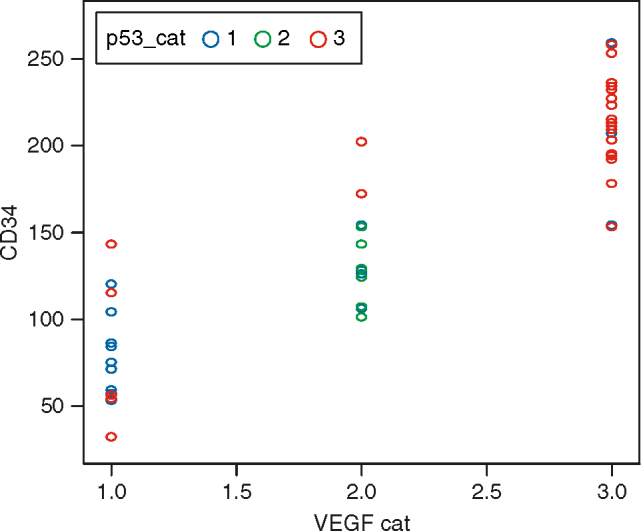
Correlation between p53, vascular endothelial growth factor, and CD34 (intratumoural microvessel density) expression in colorectal carcinomas. VEGF: vascular endothelial growth factor.
The average IMD increased from 65 in VEGF negative tumours to 129 in low-grade and 213 in high-grade expression of VEGF. ANOVA revealed that there is significant difference in the average IMD value in various VEGF grades (P = 0.000). Again pair-wise comparison using LSD shows that there is significant difference in grades 1 and 2 (P = 0.000), grades 1 and 3 (P = 0.000) and also in grades 2 and 3 (P = 0.000). The value of IMD increases with increase in VEGF grade (Table 1).
Intratumoural microvessel density had differed with the stage of the disease (P = 0.00) (Figure 5). Mean IMD increased from 84 in stage I tumours to 206 in stage III, and to 234 in stage IV disease. Pair-wise comparisons revealed that there was no significant difference in the IMD value in stages II and III (P = 0.124) and also in stages III and IV (P = 0.31). All other pairs showed significant difference. Intratumoural microvessel density also differed significantly with the grade of the tumour Table 1, Figure 5). ANOVA shows that there is significant difference in the IMD value in various grades of tumours. However pair-wise comparison shows that there is no significant difference in IMD value in grades 2 and 3 (P = 0.066). Other pairs namely (1 and 2) and (1 and 3) shows significant difference (P = 0.000). Tumours with high IMD were poorly differentiated and had poor prognosis and vice versa. Another important incidental observation was made with respect to mucinous tumours, all of which were well-differentiated. Most mucinous tumours were negative for both p53 and VEGF expression, or they had lower expression. Only one case had grade 3 positivity for p53. Mean IMD (114.0) correlated with that of well-differentiated tumours (mean, 116.0).
Figure 5.
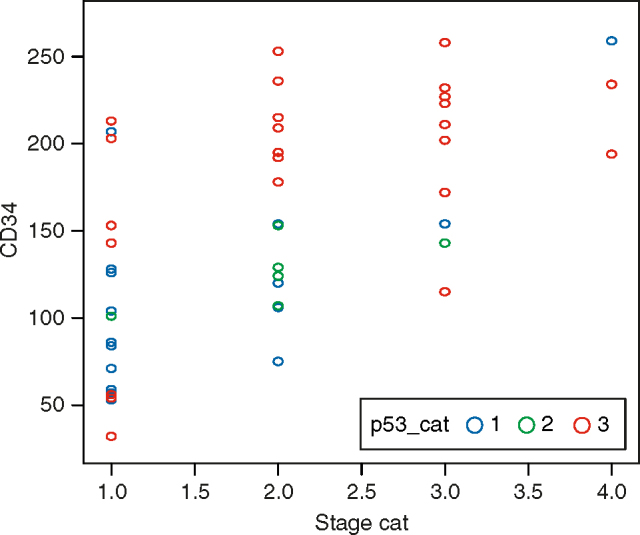
Correlation between CD34 (intratumoural microvessel density), p53, and stage of the colorectal carcinomas.
DISCUSSION
Colorectal carcinoma still remains a major cause of morbidity in the form of recurrence and mortality.1, 2, 3 In this study, p53, VEGF, and IMD by staining for CD34 are considered and correlated with grade, stage, and survival data. Normally p53 prevents propagation of genetically damaged cells. Vascular endothelial growth factor expression is regulated by local tissue hypoxia, cytokines, oncogenes, tumour suppressor genes, and growth factors.14 CD34 is constitutively expressed on endothelial cells.15
p53 was detected in 62% of the cases, of which 19.3% showed low-grade expression while 80.7% had high-grade p53. The incidence of p53 positivity in another study was up to 42%.16 No significant correlation was found with the stage or grade in our study. This corresponded to the findings of Kressner, who stated that p53 is of limited value in CRCs17 unlike Goh et al.18 Similarly Vermuelen et al3 independently found that p53 and VEGF only when considered together are reliable prognostic indicators.
Vascular endothelial growth factor expression correlated significantly with the stage and grade, i.e. grade 3 VEGF was found in higher stage and higher grade of the tumour. This was similar to other studies.9, 19 We found an incidence of 66% positivity for VEGF, but it is not an independent prognostic factor in CRCs. Perrone et al also confirmed a strong correlation of expression of p53 with VEGF expression and microvessel density with increasing stage of tumour.20 Galizia et al21 found a high four-year recurrence in stage pT1–T3 in cases with high-grade p53 and VEGF expression. In contrast, Takahashi found a direct relation of VEGF with metastasis and poor prognosis.22
Intratumoural microvessel density correlated significantly with the stage and grade. Kern found the same correlation.23 Weidner et al5 and Vermuelen et al3 independently found that increasing IMD was an independent risk factor for metastasis. In our study, there was a 66.7% correlation between negative p53 and negative VEGF and a statistically significant relation (P = 0.001) between high-grade expressions of p53 and VEGF (Table 2, Graph 1). Vermuelen3 and Perrone et al20 found a similar direct correlation between p53 and VEGF. Ravi et al24 studied one of the possible mechanisms by which p53 has an effect on VEGF. Pal also found an inverse relation between p53 and VEGF.25 Higher p53 was associated with higher VEGF, which in turn was linked to higher IMD and poor prognosis. Vascular endothelial growth factor and IMD were significantly related (P < 0.00001) with a correlation coefficient of 0.80. Vermuelen,3 Perrone,20 Galizia et al21 have independently found the same.
In our study, p53 and IMD had statistically significant correlation (P = 0.0006) and a correlation coefficient of 0.31. p53 was not as significantly linked to IMD as VEGF, suggesting the role of other proangiogenic factors. Perrone,20 Galizia,21 and Yuan et al26 independently found an association between p53 and IMD. However, Kern23 did not find a significant association between p53 and IMD. He found that vascularity decreased in higher grade and stage tumours and that it did not have any association with metastatic potential.
Assessing parameters related to tumour angiogenesis has provided valuable prognostic information in different tumour types. Alterations in p53 gene have been found to be responsible for changing the local balance of pro- and antiangiogenic factors in favour of the former. Early-stage colon cancer patients (Dukes A or B; pT1-T3 N0 M0) are excluded from adjuvant chemotherapy following potentially curative surgery because they are expected to have good long-term survival. However, some of these patients ultimately succumb from recurrence indicating that conventional staging may be unable to precisely predict.
To summarise, p53 expression did not have an independent statistically significant correlation with the stage or grade. Vascular endothelial growth factor expression correlated significantly with the stage and grade of disease but it was not an independent prognostic factor. Intratumoural microvessel density as calculated from CD34 increased with the stage and grade. p53 and VEGF correlated significantly suggesting a stimulating effect of p53 on VEGF. Vascular endothelial growth factor and IMD had very strong correlation. p53 and IMD also correlated significantly. Although definite trends are seen in this study, a larger study of the target group i.e. stages II and III is needed to substantiate our findings.
Our study did not find p53 and VEGF to be independent prognostic factors in CRCs but suggests that, considered together they are reliable predictors of disease and could help in selection of patients at a risk of progression, metastasis or recurrence and selectively subject them to the newer drugs like the antiVEGF drug, bevacizumab, and look for the response in them in the form of longer disease-free and overall survival.
Intellectual Contributions of Authors
Study concept: Lt Col Ajay Malik, Surg Cmde B Fanthome
Drafting and manuscript revision: Lt Col Ajay Malik
Statistical analysis: SR Patrikar
Study supervision: Surg Cdr Ramesh Rao, Surg Capt RN Mishra (Retd)
ACKNOWLEDGEMENTS
The author wishes to acknowledge the contributions of Dr Kavita Pimpalwar, Resident in Pathology (presently working as Consultant in Civil), for sincerity, hard work and doing the benchwork diligently. We would like to acknowledge the office of the DGAFMS for funding this project (3428/2005).
CONFLICTS OF INTEREST
This study has been financed by the research grants from the office of the DGAFMS.
REFERENCES
- 1.Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. Springer; New York: 2010. [Google Scholar]
- 3.Vermeulen PB, Van den Eynden GG, Huget P. Prospective study of intratumoral microvessel density, p53 expression and survival in colorectal cancer. Br J Cancer. 1999;79:316–322. doi: 10.1038/sj.bjc.6690051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takano Y, Saegusa M, Ikenaga M, Mitomi H, Okayasu Y. Apoptosis of colon cancer: comparison with Ki-67 proliferative activity and expression of p53. J Cancer Res Clin Oncol. 1996;122:166–170. doi: 10.1007/BF01366957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 6.Tenderenda M, Rutkowsi P, Jesionek D, Rubiak R. Expression of CD34 in gastric cancer and its correlation with histology, stage, proliferation activity, p53 expression and apoptotic index. Pathol Oncol Res. 2001;7:129–134. doi: 10.1007/BF03032579. [DOI] [PubMed] [Google Scholar]
- 7.Tomisaki S, Ohno S, Ichiyoshi Y, Kuwano H, Maehara Y, Sugimachi K. Microvessel quantification and its possible relation with liver metastasis in colorectal cancer. Cancer. 1996;77:1722–1728. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1722::AID-CNCR46>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 9.Takahashi Y, Tucker SL, Kitadai Y. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg. 1997;132:541–546. doi: 10.1001/archsurg.1997.01430290087018. [DOI] [PubMed] [Google Scholar]
- 10.Des Guetz G, Uzzan B, Nicolas P. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823–1832. doi: 10.1038/sj.bjc.6603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuniyasu H, Yasui W, Shinohara H. Induction of angiogenesis by hyperplastic colonic mucosa adjacent to colon cancer. Am J Pathol. 2000;157:1523–1535. doi: 10.1016/S0002-9440(10)64790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor CR, Shi SR, Barr NJ. Techniques of immunohistochemistry: principles, pitfalls, and standardization. In: Dabbs DJ, editor. Diagnostic Immunohisto-chemistry. 3rd ed. Saunders, Elsevier; Philadelphia: 2010. pp. 1–41. [Google Scholar]
- 13.Zlobec I, Steele R, Michel RP, Compton CC, Lugli A, Jass JR. Scoring of p53, VEGF, Bcl-2 and APAF-1 immunohistochemistry and interobserver reliability in colorectal cancer. Mod Pathol. 2006;19:1236–1242. doi: 10.1038/modpathol.3800642. [DOI] [PubMed] [Google Scholar]
- 14.Khorana AA, Ryan CK, Cox C, Eberly S, Sahasrabudhe DM. Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma: a role for the host response in prognosis. Cancer. 2003;97:960–968. doi: 10.1002/cncr.11152. [DOI] [PubMed] [Google Scholar]
- 15.Ellis LM, Liu W, Ahmad SA. Overview of angiogenesis: biologic implications for antiangiogenic therapy. Semin Oncol. 2001;28:94–104. doi: 10.1016/s0093-7754(01)90287-8. [DOI] [PubMed] [Google Scholar]
- 16.Tanigawa N, Amaya H, Matsumura M. Tumor angiogenesis and mode of metastasis in patients with colorectal cancer. Cancer Res. 1997;57:1043–1046. [PubMed] [Google Scholar]
- 17.Kressner U, Lindmark G, Gerdin B, Pahlman L, Glimelius B. Immuno-histological p53 staining is of limited value in the staging and prognostic prediction of colorectal cancer. Anticancer Res. 1996;16:951–957. [PubMed] [Google Scholar]
- 18.Goh HS, Yao J, Smith DR. p53 point mutation and survival in colorectal cancer patients. Cancer Res. 1995;55:5217–5221. [PubMed] [Google Scholar]
- 19.Zheng S, Han MY, Xiao ZX, Peng JP, Dong Q. Clinical significance of vascular endothelial growth factor expression and neovascularization in colorectal carcinoma. World J Gastroenterol. 2003;9:1227–1230. doi: 10.3748/wjg.v9.i6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrone G, Vincenzi B, Santini D. Correlation of p53 and bcl-2 expression with VEGF, microvessel density and clinicopathological features in colon cancer. Cancer Lett. 2004;208:227–234. doi: 10.1016/j.canlet.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 21.Galizia G, Ferraraccio F, Lieto E. Prognostic value of p27, p53, and vascular endothelial growth factor in Dukes A and B colon cancer patients undergoing curative surgery. Dis Colon Rectum. 2004;47:1904–1914. doi: 10.1007/s10350-004-0695-8. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi Y, Bucana C, Cleary K, Ellis LM. p53, vessel count and vascular endothelial growth factor expression in human colon cancer. Int J Cancer. 1998;79:34–38. doi: 10.1002/(sici)1097-0215(19980220)79:1<34::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 23.Kern A, Taubert H, Scheele J. Association of p53 mutations, microvessel density and neoangiogenesis in pairs of colorectal cancers and corresponding liver metastases. Int J Oncol. 2002;21:243–249. doi: 10.3892/ijo.21.2.243. [DOI] [PubMed] [Google Scholar]
- 24.Ravi R, Mookerjee B, Bhujwalla ZM. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1a. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 25.Pal S, Datta K, Mukhopadhyay D. Central role of p53 on regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in mammary carcinoma. Cancer Res. 2001;61:6952–6957. [PubMed] [Google Scholar]
- 26.Yuan A, Yu CJ, Luh KT, Kuo SH, Lee YC, Yang PC. Aberrant p53 expression correlates with expression of vascular endothelial growth factor MRNA and interleukin-8 MRNA and neoangiogenesis in non-small-cell lung cancer. J Clin Oncol. 2002;20:900–910. doi: 10.1200/JCO.2002.20.4.900. [DOI] [PubMed] [Google Scholar]


