Abstract
Objective
How advances in the management of ANCA (anti-neutrophil cytoplasmic antibody)-associated vasculitis (AAV) have impacted long-term outcomes is still unclear. We examined temporal changes over 25 years in long-term clinical outcomes, including the impact of renal function at diagnosis (a potential marker of time to disease detection) and duration of cyclophosphamide use in AAV patients with renal involvement.
Methods
ANCA-positive, biopsy-proven patients with AAV diagnosed in 1985–2009 followed in the Glomerular Disease Collaborative Network inception cohort were included. Outcomes included the composite outcome of end-stage renal disease (ESRD) or death as well as relapse. Cox proportional hazard or competing risk regression models were adjusted for potential baseline confounders.
Results
Data from 544 patients were included in the analysis. There was a decreasing 5-year risk of ESRD or death over time (log rank test for trend: p < 0.001). After adjustment for baseline characteristics, the risk of relapse was similar across the time periods (test for trend: p = 0.45). Serum creatinine at baseline was the only significant predictor of an increased risk of ESRD or death (HR 1.11 per 1 mg/dL of serum creatinine [95% CI 1.04–1.18], p = 0.002).
Conclusion
In patients with renal disease secondary to AAV, over 25 years the risk of ESRD or death has decreased but the risk of relapse has not changed. A higher serum creatinine at diagnosis is associated with a higher risk of ESRD or death, suggesting that earlier disease detection is potentially an important measure to improve outcomes in AAV.
Keywords: vasculitis, glomerulonephritis, outcomes, survival, end-stage renal disease, relapse
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of diseases characterized by inflammation of blood vessels often leading to tissue destruction and organ failure. Renal involvement, in the form of glomerulonephritis, is a common complication of AAV and indicates a poor prognosis1–4. Significant morbidities such as end-stage renal disease (ESRD) and frequent relapses lead to poorer survival and quality of life5–7. Over the past several decades patients with AAV have survived longer but whether morbidity has improved in terms of preservation of kidney function is unclear8–10. Examining changes in relapse rates over time was previously hindered by poorly standardized definitions of disease and relapse and lack of appropriate longitudinal data.
Significant progress has been made in the diagnosis and treatment of AAV over the past three decades. ANCA testing was introduced in the late 1980s and by the early 1990s became widely used, leading to a dramatic improvement in detection of AAV11, 12. Clinical trials focused on refining the use of cyclophosphamide, a primary induction agent for severe AAV, to minimize toxicity without losing efficacy13–16. These trials used a shorter duration of cyclophosphamide and found no difference in short-term remission rates; however, extension studies suggest there may be a higher rate of relapse in patients receiving less cumulative cyclophosphamide17–19. Additionally, the effect of a shorter duration of cyclophosphamide on long-term outcomes, such as survival and preservation of kidney function, is not known4, 18, 20.
To better understand the impact of diagnostic and therapeutic changes on long-term outcomes in AAV, this study utilized a large, multi-center inception cohort of patients with AAV and renal involvement to examine changes in long-term outcomes over the past few decades.
PATIENTS AND METHODS
Study Population
Patients enrolled in the Glomerular Disease Collaborative Network (GDCN) AAV registry were eligible for this study. The GDCN began in 1985 and is a collaborative venture between academic and private practice nephrologists that now includes over 600 physicians. Centered at the University of North Carolina (UNC) at Chapel Hill, this registry consists of patients diagnosed with AAV who are primarily located in the Southeastern United States.
Patients were included in this study if they met all of the following 3 criteria: (1) had a native kidney biopsy showing pauci-immune glomerulonephritis or had kidney involvement as determined by the nephrologist based on active urine sediment with or without worsening renal impairment along with a diagnostic biopsy of extra-renal tissue (lung, nerve, or gastrointestinal tract) consistent with small-vessel vasculitis; (2) had a positive test for ANCA, defined as detection of ANCA specific to proteinase 3 (PR3) or myeloperoxidase (MPO) by enzyme-linked immunosorbent assay (ELISA) and/or cytoplasmic ANCA (C-ANCA) or perinuclear ANCA (P-ANCA) staining pattern on indirect immunofluorescence; and (3) were diagnosed between 1985 to 2009. Patients were excluded if they had no renal involvement. Four patients diagnosed with eosinophilic granulomatosis with polyangiitis (EGPA; Churg-Strauss) were excluded from the study since EGPA is considered to have a different clinical outcome than the other AAV diseases21, 22. Four patients who received cyclophosphamide more than 1 year prior to enrollment were also excluded. The 21 patients (4, 4, 8, and 5 in time period 1985–1989, 1990–1994, 1995–1999, and 2000–2004, respectively) who were dialysis dependent at time of diagnosis were also excluded.
Patients were enrolled in the registry at the time of diagnosis and followed prospectively until the occurrence of ESRD, defined as the onset of dialysis or transplantation, or death. Patients who did not reach an endpoint were followed until the date of their most recent office visit or hospital discharge.
Outcomes
The primary outcome was a composite endpoint of either ESRD or death within 5 years of diagnosis (henceforth referred to as ESRD-free survival). Secondary outcomes included ESRD and death separately, occurrence of any relapse, and occurrence of renal relapse (relapse involving the kidneys). The record of death was obtained either through medical records or the Social Security Death Index. Relapse was defined as the occurrence of at least one of the following: (1) a rise in serum creatinine accompanied by an active urine sediment, (2) a renal biopsy demonstrating active necrosis or crescent formation, (3) hemoptysis, pulmonary hemorrhage, or new or expanding pulmonary nodules without evidence for infection, (4) active vasculitis of the respiratory or gastrointestinal (GI) tract as demonstrated by endoscopy with biopsy, (5) inflammatory eye disease, (6) new mononeuritis multiplex, (7) clinical signs or symptoms of upper airway involvement, or (8) necrotizing vasculitis identified by biopsy in any tissue23. The definition of a renal relapse was limited to the first 2 options listed above.
Exposure
The primary exposure of interest was the time period in which the patient was diagnosed, determined by the date of first renal biopsy or a non-renal biopsy demonstrating active vasculitis. Patients were categorized into 5-year time intervals: 1985–1989, 1990–1994, 1995–1999, 2000–2004, and 2005–2009.
Definitions and Clinical Features
The specific AAV subtypes of granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) were defined by the Chapel Hill Consensus Criteria24. Renal-limited vasculitis (RLV) was defined as the presence of renal vasculitis with no other organ involvement including constitutional symptoms. Diagnostic testing to exclude other organ involvement (e.g. pulmonary or upper airway) was up to the discretion of the treating physician and was not required. RLV was categorized as a separate subtype since its onset tends to be more insidious and is associated with a delay in diagnosis25. A small proportion of patients (10%) had a diagnosis of AAV prior to their renal biopsy and were started on an alternative immunosuppressive therapy (e.g. methotrexate) prior to induction therapy with cyclophosphamide; this duration of disease was accounted for in the analysis. Organ involvement of AAV at baseline was determined by biopsy or previously described criteria3, 23, 26.
Peak serum creatinine at diagnosis was determined by selecting the highest serum creatinine during the period spanning the month before to the month after the date of the diagnostic biopsy. The estimated glomerular filtration rate (eGFR) was determined using the Modification of Diet in Renal Disease (MDRD) formula27. Serum creatinine, rather than eGFR, was used as a measure of renal function in the multivariable models in order to examine the influence of age, sex, and race separately (as these are incorporated in the MDRD formula). Duration of cyclophosphamide was examined as a proxy for cumulative cyclophosphamide dose since dose adjustments are frequently made in the setting of renal impairment.
Statistical Analysis
Kaplan-Meier estimators were used to plot the univariable probability of outcomes. Cox proportional hazards models estimated the effect of time period on composite ESRD or death as well as death alone with hazards ratios (HRs) and 95% confidence intervals (CI) presented. The assumption of proportionality was tested using Schoenfeld residuals. A secondary analysis was performed evaluating changes in ESRD-free survival among patients with severe renal disease at diagnosis (a priori chosen as an eGFR ≤ 45 ml/min/1.73 m2) to determine if length-biased sampling, a form of selection bias, was occurring due to increasing detection of more indolent, slowly-progressing disease over time.
Competing risks regression was used to estimate the effect of time period on relapse with ESRD and death considered competing events. An analysis accounting for competing risks was used because the competing events (ESRD and death) are likely not independent from relapse and may informatively censor patients; for example, patients censored for death may have developed a higher rate of relapses if they had lived. Similarly, for the outcome of ESRD, death was treated as a competing event. Competing risks models were presented with subdistribution hazards ratios (SHRs) and 95% CI. Cumulative incidence graphs censoring ESRD and death were compared to those not censoring for ESRD and death to determine if improving survival could account for increasing relapse rates.
To evaluate the effect of duration of cyclophosphamide use on long-term outcomes, the subgroup of patients who received cyclophosphamide and had dates of cyclophosphamide use available was examined (N = 340). Because there were only 5 such patients in the first time period (1985–1989), these patients were combined with the next time period (1990–1994). Univariate models were performed using age, sex, race, ANCA type (C/PR3 or P/MPO), AAV disease type (GPA, MPA, or RLV), duration of disease prior to biopsy (if previously diagnosed), organ involvement (pulmonary, upper respiratory, joint, muscle, skin, GI, or neurological), use of plasma exchange for induction, and site where patient was primarily treated (tertiary care vs community practice). The proportion of patients managed at a tertiary care center likely increased over time stemming from changes in enrollment by the GDCN registry which began placing more emphasis on enrolling patients from UNC during the later time periods. Predictors of interest were peak serum creatinine at diagnosis and duration of cyclophosphamide use. Select interactions between duration of cyclophosphamide and ANCA type as well as disease subtype were evaluated to determine if the effect of the duration of cyclophosphamide differed between ANCA or disease groups. All variables associated with the outcome with a p-value < 0.10 were included in a final multivariable model, except for age, ANCA type, serum creatinine, and duration of cyclophosphamide which were forced into all final models due to clinical relevance. Since inclusion of the duration of cyclophosphamide limited the analysis to only patients who received and had available data on cyclophosphamide use, we performed a sensitivity analysis excluding duration of cyclophosphamide from the multivariable analysis, therefore utilizing the entire cohort. Additional sensitivity analysis was performed including route of administration of cyclophosphamide (oral vs intravenous) in the multivariable analysis.
Duration of cyclophosphamide use was analyzed as a time-varying covariate since this variable was not constant throughout follow-up. In the analysis of ESRD-free survival, relapse was also treated as a time-varying covariate as determined by the start and end date of each relapse (if end date of relapse was not available, then the duration of relapse was assumed to be 3 months). All other variables were kept constant.
A two-tailed P value < 0.05 was considered significant for all analyses. All analyses were conducted using Stata (Version 12.1, StataCorp, College Station, TX).
The study was approved by the institutional review boards of the University of North Carolina at Chapel Hill and the University of Pennsylvania.
RESULTS
Patient characteristics
A total of 554 patients were included in the study with a median follow-up of 31 months (interquartile range [IQR] 11–67). Baseline characteristics by time period are presented in Table 1. The median age of this population was 60 years and 47% of the patients were female; mean age and sex ratios were similar across the time periods. Sixty percent of patients were anti-MPO-positive and more than 80% had MPA or RLV. ANCA type and disease type were similar across the time periods. Approximately half of the patients had lung involvement. 542 (98%) patients had a diagnostic renal biopsy while the remaining 12 (2%) patients were diagnosed by their nephrologist based on active urine sediment (with or without rising serum creatinine). Renal function at diagnosis significantly improved over time with a median eGFR of 11 ml/min/1.73 m2 in the earliest time period and 23 ml/min/1.73 m2 in the latest period. There was also a significant increase over time in the proportion of patients who used cyclophosphamide and a significant decline in the duration of cyclophosphamide used per patient.
Table 1.
Baseline characteristics of cohort by time period
| Characteristic | All | Time Period
|
P-value for trend | ||||
|---|---|---|---|---|---|---|---|
| 85–89 | 90–94 | 95–99 | 00–04 | 05–09 | |||
| N | 554 | 84 | 76 | 131 | 172 | 91 | -- |
|
| |||||||
| Median age, years (IQR) | 60 (47–71) | 62 (45–70) | 61 (43–68) | 64 (49–72) | 58 (47–71) | 58 (46–68) | 0.98 |
|
| |||||||
| Female, % | 47% | 46% | 54% | 45% | 49% | 42% | 0.42 |
|
| |||||||
| Race, % | |||||||
| White | 85% | 87% | 86% | 91% | 83% | 79% | 0.15 |
| Black | 9% | 12% | 8% | 8% | 9% | 9% | 0.61 |
| Other | 6% | 1% | 7% | 2% | 8% | 12% | 0.02 |
|
| |||||||
| Diagnosis, % | |||||||
| GPA | 19% | 15% | 18% | 18% | 20% | 24% | 0.15 |
| MPA | 56% | 58% | 51% | 56% | 55% | 58% | 0.82 |
| RLV | 25% | 26% | 30% | 26% | 24% | 18% | 0.10 |
|
| |||||||
| ANCA ELISA, % | |||||||
| PR3/C | 40% | 44% | 33% | 43% | 41% | 37% | 0.78 |
| MPO/P | 60% | 56% | 67% | 57% | 59% | 63% | |
|
| |||||||
| Organ Involvement, % | |||||||
| Lung | 49% | 50% | 38% | 49% | 52% | 53% | 0.23 |
| Joint | 41% | 36% | 36% | 40% | 44% | 48% | 0.05 |
| Upper respiratory | 35% | 36% | 29% | 34% | 33% | 44% | 0.28 |
| Skin | 23% | 21% | 26% | 25% | 19% | 19% | 0.37 |
| Gastrointestinal | 11% | 15% | 16% | 12% | 8% | 4% | 0.005 |
| Neurologic | 10% | 14% | 8% | 15% | 8% | 7% | 0.13 |
| Muscle | 3% | 6% | 7% | 2% | 3% | 0% | 0.06 |
|
| |||||||
| Pre-existing AAV prior to diagnostic biopsy, % | 10% | 7% | 11% | 10% | 10% | 11% | 0.45 |
|
| |||||||
| Median duration of follow-up, months (IQR) | 31 (11–67) | 35 (11–68) | 32 (7–72) | 23 (7–49) | 29 (14–91) | 38 (11–59) | 0.98 |
|
| |||||||
| Tertiary care, % (vs community practice) | 48% | 30% | 26% | 40% | 55% | 82% | < 0.001 |
|
| |||||||
| Median serum creatinine, mg/dL (IQR) | 3.6 (2–5.9) | 4.8 (3–8.6) | 3.7 (2.1–6.4) | 4.1 (2.3–6.1) | 3.2 (1.8–5.1) | 2.8 (1.6–4.8) | < 0.001 |
|
| |||||||
| Median Glomerular Filtration Rate, ml/min/1.73 m2 (IQR)* | 16 (9–32) | 11 (7–20) | 16 (8–30) | 14 (8–29) | 17 (11–37) | 23 (12–37) | < 0.001 |
|
| |||||||
| Used plasma exchange, % | 16% | 2% | 4% | 10% | 22% | 40% | < 0.001 |
|
| |||||||
| Cyclophosphamide | |||||||
| Ever used, % | 89% | 73% | 80% | 90% | 95% | 99% | < 0.001 |
| Dates of use available, % | 79% | 8% | 72% | 88% | 91% | 96% | < 0.001 |
|
| |||||||
| Median duration of cyclophosphamide, months (IQR) | 7 (4–13) | 17 (14–20) | 8 (5–21) | 7 (5–15) | 7 (6–13) | 6 (4–8) | 0.009 |
Glomerular filtration rate was calculated using the Modification of Diet in Renal Disease (MDRD) formula. AAV, ANCA-associated vasculitis. ANCA, anti-neutrophil cytoplasmic antibody. GPA, granulomatosis with polyangiitis. IQR, interquartile range. MPA, microscopic polyangiitis. MPO/P, myeloperoxidase antibody and/or perinuclear pattern. PR3/C, proteinase 3 antibody and/or cytoplasmic pattern. RLV, renal-limited vasculitis.
Primary outcome: ESRD-free survival
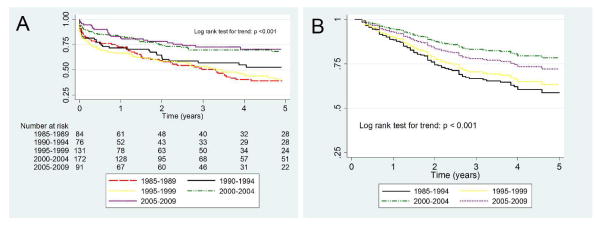
Within the entire cohort, there were 260 (47%) patients who developed ESRD and/or died during the follow-up period and by 5 years from diagnosis, 180 patients (32%) were lost to follow-up. The incidence of first occurrence of ESRD and/or death was 12.9 events per 100 person-years. Overall, the 1-year ESRD-free survival rate was 75% and the 5-year rate was 54%. Comparing the 5 time periods, the ESRD-free survival rates improved over time both in the Kaplan-Meier curve (log rank test for trend: p < 0.001; Figure 1A) and the adjusted curve (log rank test for trend: p < 0.001; Figure 1B). Secondary analysis of patients presenting with severe renal disease defined as eGFR ≤ 45 ml/min/1.73 m2 also demonstrated that ESRD-free survival significantly improved over time (log rank test for trend: p < 0.001).
Figure 1.
Risk of developing ESRD or death in 5 years stratified by year of diagnosis, shown as Kaplan-Meier curve (1A) and multivariable Cox proportional hazards curve (1B). Patients diagnosed in earlier time periods have poorer ESRD-free survival. Multivariable analysis adjusted for age, race, ANCA type, site, baseline serum creatinine, duration of cyclophosphamide use, and occurrence of relapse. Time period 1985–1989 combined with time period 1990–1994 due to missing cyclophosphamide data in the 1985–1989 group.
Of the 494 patients who received cyclophosphamide, 388 (79%) had available data on duration of cyclophosphamide use; among these patients, 48 (12%) were missing additional baseline data (e.g. serum creatinine or extra-renal manifestations). Thus, 340 patients were included in this subgroup analysis. There were no major differences between patients with or without available data on cyclophosphamide use (data not shown). In the multivariable Cox regression analysis, after adjustment for potential confounders, an elevated serum creatinine at diagnosis was the only significant factor associated with a higher risk of ESRD or death (HR 1.11 per 1 mg/dL of serum creatinine [95% CI 1.04–1.18], p = 0.002) while ANCA type, age, and duration of cyclophosphamide use were not significantly associated with the outcome (Table 3). Adjustment for route of administration of cyclophosphamide (oral vs intravenous) led to similar results (data not shown). Furthermore, the ANCA-by-cyclophosphamide duration and disease subtype-by-cyclophosphamide duration interactions were not significant (p for interaction 0.72 and 0.49, respectively), indicating that the effect of cyclophosphamide did not differ between ANCA and disease groups.
Table 3.
Risk factors for the occurrence of ESRD or death in 5 years
| Variable | Univariate model | Multivariable model* | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Time Period | ||||
| 85–94† | 1 (reference) | -- | 1 (reference) | -- |
| 95–99 | 0.94 (0.51–1.73) | 0.84 | 1.03 (0.53–2.01) | 0.94 |
| 00–04 | 0.51 (0.27–0.95) | 0.03 | 0.58 (0.29–1.16) | 0.13 |
| 05–09 | 0.66 (34–1.28) | 0.22 | 0.80 (0.38–1.71) | 0.57 |
|
| ||||
| Age, years | 1.00 (0.99–1.01) | 0.60 | 1.00 (0.99–1.01) | 0.99 |
|
| ||||
| Sex (female vs male) | 0.96 (0.62–1.46) | 0.84 | ||
|
| ||||
| Race | ||||
| White | 1 (reference) | -- | 1 (reference) | -- |
| Black | 1.06 (0.51–2.20) | 0.88 | 0.97 (0.45–2.08) | 0.95 |
| Other | 0.29 (0.07–1.20) | 0.09 | 0.15 (0.02–1.07) | 0.07 |
|
| ||||
| Diagnosis | ||||
| GPA | 1 (reference) | -- | ||
| MPA | 1.43 (0.84–2.45) | 0.20 | ||
| RLV | 1.21 (0.61–2.41) | 0.58 | ||
|
| ||||
| ANCA (MPO/P vs PR3/C) | 0.95 (0.62–1.45) | 0.80 | 0.92 (0.57–1.47) | 0.71 |
|
| ||||
| Organ Involvement | ||||
| Lung | 1.23 (0.80–1.89) | 0.34 | ||
| Joint | 0.83 (0.54–1.27) | 0.39 | ||
| Upper respiratory | 0.81 (0.52–1.26) | 0.34 | ||
| Skin | 1.06 (0.65–1.72) | 0.81 | ||
| Gastrointestinal | 0.91 (0.44–1.89) | 0.81 | ||
| Neurologic | 1.10 (0.55–2.19) | 0.79 | ||
| Muscle | 0.70 (0.17–2.83) | 0.61 | ||
|
| ||||
| Duration of disease prior to biopsy, months | 1.00 (0.99–1.00) | 0.47 | ||
|
| ||||
| Serum creatinine, mg/dL | 1.12 (1.06–1.19) | < 0.001 | 1.11 (1.04–1.18) | 0.002 |
|
| ||||
| Site (community vs tertiary) | 1.46 (0.96–2.24) | 0.087 | 1.01 (0.60–1.70) | 0.96 |
|
| ||||
| Used plasma exchange | 1.17 (0.71–1.92) | 0.54 | ||
|
| ||||
| Duration of cyclophosphamide, months | 0.98 (0.93–1.02) | 0.27 | 0.96 (0.92–1.01) | 0.14 |
|
| ||||
| Occurrence of relapse | 0.93 (0.44–1.94) | 0.84 | 0.92 (0.42–2.05) | 0.84 |
The effects are expressed as hazards ratio (HR) for ESRD or death in 5 years.
Multivariable model included variables with p < 0.10 in univariate analysis along with pre-specified variables of interest (time period, age, ANCA type, serum creatinine, cyclophosphamide, and relapse).
There were only 5 patients in 85–89 group so these patients were combined with 90–94 group.
ANCA, anti-neutrophil cytoplasmic antibody. GPA, granulomatosis with polyangiitis. MPA, microscopic polyangiitis. MPO/P, myeloperoxidase antibody and/or perinuclear pattern. PR3/C, proteinase 3 antibody and/or cytoplasmic pattern. RLV, renal-limited vasculitis.
After removing duration of cyclophosphamide from the model and utilizing the entire cohort for a sensitivity analysis, a significant trend in improvement in outcome across the time periods was still observed (test for trend: p < 0.001).
Patient survival and renal survival
Among the 554 patients, 160 (29%) deaths were observed during the follow-up period with an incidence rate of 7.0 deaths per 100 patient-years. The 1-year patient survival for the entire cohort was 91% and 5-year survival was 72%. Survival significantly improved over time even after adjusting for age, ANCA type, baseline serum creatinine, and duration of cyclophosphamide use (log rank test for trend: p = 0.03).
There were 181 (33%) patients who developed ESRD during the observational period. At 1 year from diagnosis, 20% developed ESRD while 5% had died without developing ESRD; and at 5 years, 65% had developed ESRD while 23% had died without developing ESRD. Renal survival significantly improved across the 5 time periods even after adjusting for age, ANCA type, baseline serum creatinine, and duration of cyclophosphamide use (test for trend: p = 0.03) and the highest yearly incidence of ESRD occurred within the first year.
Relapse
Of the 554 patients, 185 (33%) patients experienced a relapse after diagnosis for an incidence rate of relapse of 13.9 events per 100 patient-years. The cumulative occurrence of relapse at 1 year, 3 years, and 5 years was 11%, 29%, and 35%, respectively. Among the 185 patients who experienced a relapse, 136 (74%) had kidney involvement at the time of relapse and 112 (61%) used cyclophosphamide to treat the relapse.
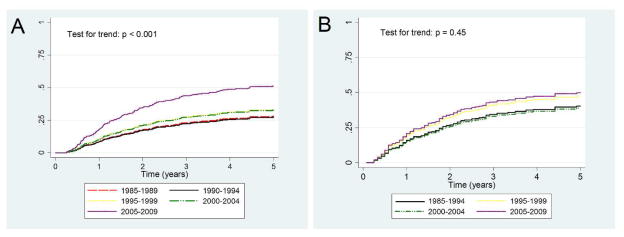
The unadjusted occurrence of relapse increased over time as shown in the cumulative incidence curves in Figure 2A (test for trend: p < 0.001). However, in the subgroup of patients with data on cyclophosphamide use (n = 340), after adjusting for potential confounders in a multivariable competing risk regression, this trend was no longer significant (p for trend = 0.45; Figure 2B). When examining predictors of relapse, there were no studied factors associated with relapse. Notably, ANCA type and duration of cyclophosphamide use were not associated with the occurrence of relapse (Table 4). Adjustment for route of administration of cyclophosphamide (oral vs intravenous) led to similar results (data not shown). The ANCA-by-cyclophosphamide interaction and the disease subtype-by-cyclophosphamide interaction were not significant (p for both interactions = 0.41) indicating that the effect of the duration of cyclophosphamide is not modified by ANCA or disease type.
Figure 2.
Cumulative incidence of relapse stratified by year of diagnosis. Unadjusted incidence (2A) shows more relapses occur in patients diagnosed in later time periods, but no difference is seen after adjusting for baseline variables (2B). Adjusted for age, diagnosis, ANCA type, site, baseline serum creatinine, joint/upper respiratory/skin involvement, and duration of cyclophosphamide use. Time period 1985–1989 combined with time period 1990–1994 due to missing cyclophosphamide data in the 1985–1989 group.
Table 4.
Risk factors for disease relapse
| Univariate model | Multivariable model* | |||
|---|---|---|---|---|
|
|
||||
| Variable | SHR (95% CI) | P-value | SHR (95% CI) | P-value |
| Time Period | ||||
| 85–94† | 1 (reference) | -- | 1 (reference) | -- |
| 95–99 | 1.47 (0.83–2.61) | 0.20 | 1.26 (0.68–2.33) | 0.47 |
| 00–04 | 1.21 (0.69–2.12) | 0.50 | 0.96 (0.53–1.77) | 0.91 |
| 05–09 | 1.72 (0.99–3.01) | 0.06 | 1.35 (0.71–2.55) | 0.35 |
|
| ||||
| Age, years | 0.99 (0.99–1.00) | 0.17 | 1.00 (0.99–1.01) | 0.92 |
|
| ||||
| Sex (female vs male) | 0.99 (0.70–1.39) | 0.94 | ||
|
| ||||
| Race | ||||
| White | 1 (reference) | -- | ||
| Black | 0.63 (0.30–1.30) | 0.21 | ||
| Other | 0.99 (0.48–2.04) | 0.98 | ||
|
| ||||
| Diagnosis | ||||
| GPA | 1 (reference) | -- | 1 (reference) | -- |
| MPA | 0.65 (0.45–0.94) | 0.02 | 0.75 (0.48–1.18) | 0.21 |
| RLV | 0.39 (0.22–0.68) | 0.001 | 0.59 (0.29–1.18) | 0.14 |
|
| ||||
| ANCA (MPO/P vs PR3/C) | 0.74 (0.52–1.04) | 0.08 | 0.91 (0.62–1.34) | 0.63 |
|
| ||||
| Organ Involvement | ||||
| Lung | 1.14 (0.81–1.61) | 0.45 | ||
| Joint | 1.58 (1.12–2.22) | 0.008 | 1.21 (0.82–1.79) | 0.33 |
| Upper respiratory | 1.53 (1.09–2.15) | 0.015 | 1.08 (0.70–1.65) | 0.74 |
| Skin | 1.46 (1.00–2.11) | 0.46 | 1.25 (0.82–1.90) | 0.30 |
| Gastrointestinal | 0.97 (0.54–1.74) | 0.92 | ||
| Neurologic | 1.34 (0.79–2.29) | 0.28 | ||
| Muscle | 1.00 (0.34–2.91) | 0.99 | ||
|
| ||||
| Duration of disease prior to biopsy, months | 1.00 (1.00–1.00) | 0.92 | ||
|
| ||||
| Serum creatinine, mg/dL | 0.89 (0.81–0.97) | 0.011 | 0.92 (0.84–1.01) | 0.07 |
|
| ||||
| Site (community vs tertiary) | 0.71 (0.50–1.00) | 0.055 | 0.81 (0.52–1.25) | 0.34 |
|
| ||||
| Used plasma exchange | 1.30 (0.87–1.94) | 0.20 | ||
|
| ||||
| Duration of cyclophosphamide, months | 1.02 (0.99–1.05) | 0.176 | 1.01 (0.98–1.04) | 0.55 |
The effects are expressed as hazards ratio (HR) for relapse in 5 years.
Multivariable model included variables with p < 0.10 in univariate analysis along with pre-specified variables of interest (time period, age, ANCA type, serum creatinine, and cyclophosphamide).
There were only 5 patients in 85–89 group so these patients were combined with 90–94 group.
ANCA, anti-neutrophil cytoplasmic antibody. GPA, granulomatosis with polyangiitis. MPA, microscopic polyangiitis. MPO/P, myeloperoxidase antibody and/or perinuclear pattern. PR3/C, proteinase 3 antibody and/or cytoplasmic pattern. RLV, renal-limited vasculitis. SHR, subdistribution hazard ratio.
In a sensitivity analysis, the duration of cyclophosphamide was removed from the model (to allow for analysis of entire cohort) along with variables that correlate with ANCA type (disease subtype and upper respiratory involvement); there continued to be no significant change in occurrence of relapse over the time periods (test for trend: p = 0.24).
To determine whether the increasing hazard rates for relapse were the result of better ESRD-free survival (i.e. more patients developed relapse because patients were living longer), cumulative incidence graphs with and without censoring ESRD and death were compared (data not shown). The unadjusted cumulative incidence of relapse increases over time whether or not ESRD and death were censored (test for trend: p < 0.001) or not censored (i.e. treated as competing event; test for trend: p < 0.001), suggesting that the higher rate of relapses in the unadjusted analysis is not simply due to longer ESRD-free survival.
A total of 136 (25%) patients experienced a renal relapse and 74% of patients who experienced at least one relapse had relapse of their kidney disease. There was no significant difference in the occurrence of renal relapses over time (proportion of patients with renal relapse was 23%, 26%, 18%, 24%, 32% in 1985–1989, 1990–1994, 1995–1999, 2000–2004, 2005–2009 respectively, test for trend: p = 0.13).
DISCUSSION
Both patient and renal survival significantly improved between 1985 and 2009 in this cohort of patients with AAV and renal disease. Serum creatinine at diagnosis was the only predictor associated with this better outcome, suggesting that early detection is fundamental to improving ESRD-free survival in AAV. After adjusting for potential confounders, the risk of relapse did not significantly change over time.
While many studies have examined predictors of outcome in AAV, to our knowledge only 5 studies have addressed how outcomes have temporally changed over the time span of several decades8–10, 28, 29. This study was the largest study examining long-term changes over time and the largest study to evaluate the impact of the duration of cyclophosphamide on long-term outcomes. Hilhorst et. al. examined 181 patients in The Netherlands who were diagnosed with ANCA-associated glomerulonephritis between 1979 and 2009 and found significant improvement in patient and renal survival over time9. Holle et. al. studied 290 patients diagnosed with AAV (specifically GPA) in Germany between 1994 and 2002 and compared them to a historical cohort of 155 patients diagnosed between 1966 and1993. They found a significant reduction in mortality but, unlike the results found in this study, a lower rate of relapses was seen in their study over time28. Differences in the population studied may explain the contradictory results since only about half the patients in the German cohort had renal disease and most patients were PR3-ANCA positive. In addition, competing risks analysis was not used in their study and, therefore, the rate of relapse may have appeared higher in the earlier time periods as more patients were being censored for death.
Compared to prior studies determining predictors of outcomes, our study showed consistent results indicating that better renal function at diagnosis is associated with improved survival and renal outcomes30–32. Lung involvement, age, and upper respiratory involvement have also been shown to be associated with outcome, although these factors were not associated with events in this study32, 33. Similar to previous studies, better renal function at diagnosis was associated with a higher risk of relapse34, 35.
Neither changes in the definition of relapse nor severity of relapse likely explains the lack of improvement in the risk of relapse seen in the current study. A standard definition of relapse was implemented throughout the entire cohort. A large majority of patients experienced a relapse involving the kidneys and this rate did not change over time. It is notable that ANCA type was not associated with the occurrence of relapse in this study. A prior study by Hogan et. al. using this cohort did show that patients with PR3-positive ANCA are more likely to relapse compared to patients with MPO-positive ANCA and similar findings have been shown in other cohorts26, 36, 37. When the same inclusion and exclusion criteria and analytic approach as described in Hogan et. al. was used, similar results were obtained including a significant association between ANCA type and relapse. Therefore, it is likely this relationship was attenuated in the current analysis due to the enrichment of the cohort with patients with renal disease (excluding patients with primarily upper airway disease who are more prone to relapse) and censoring all patients after 5 years of follow-up. Additionally, since one of the aims of this study was to examine the effect of duration of cyclophosphamide use on relapse, only patients who received cyclophosphamide were included in the multivariable models; this subgroup of patients were more likely to be on longer periods of maintenance therapy after cyclophosphamide resulting in lower occurrence of relapse.
The current study also found no association between duration of cyclophosphamide use and ESRD-free survival or relapse. The lack of effect of cyclophosphamide use on relapse seen in this study contradicts prior studies, including several long-term follow-up studies of clinical trials examining maintenance therapies in AAV17–19. Several possible reasons may explain why no association between duration of cyclophosphamide and long-term outcomes was seen in this study. First, the extension studies of the clinical trials compared results based on initial randomization but most did not account for repeated uses of cyclophosphamide in their analysis. It is possible that if overall use of cyclophosphamide was included in the prior studies no difference in outcomes would be seen. Second, the cohort used in the current study had a different overall clinical phenotype compared to the patients in the trials, including ANCA and disease type. This is further reflected by the large proportion of patients who had a renal relapse. It is possible that a longer duration of cyclophosphamide use does not affect renal relapses; this contention is further supported by findings in the CYCAZAREM extension study which found no difference in renal relapses between patients on shorter versus longer durations of cyclophosphamide.
The current study has several strengths in the data source and methodology that enhanced its ability to address research questions of interest. The GDCN inception cohort is an optimal source to study renal disease in this rare disease. This cohort is unique in the U.S. and one of the largest cohorts of patients with ANCA-associated renal disease. All patients in this cohort had kidney involvement, a biopsy-proven diagnosis, and were closely followed by a nephrologist. Although the patients were confined to a particular geographic region (the Southeastern US), the GDCN provides uniformity and standardization, both of which are difficult to achieve in a multi-centered cohort. Patients were enrolled at diagnosis and followed prospectively, allowing examination of time-to-event without being prone to recall bias. Lastly, disease severity at diagnosis, an important confounder when examining long-term outcomes, was accounted for and the examination of cyclophosphamide as a time-varying covariate enabled a more real-world depiction of cyclophosphamide use in clinical practice.
There were some limitations to this study. There are several unmeasured confounders that may influence the interpretation of the findings. The dose and duration of use of glucocorticoids and other immunosuppressive therapies were not accounted for in the analysis. However, the results of this study are still noteworthy because they demonstrate continued improvement in outcomes in the setting of routine clinical care. Furthermore, a prior study using the same cohort found no association with duration of glucocorticoid therapy and risk of relapse38. Improvements in healthcare were not captured in the analysis and were likely affecting the long-term outcomes, particularly patient and renal survival. Frequency in follow-up was determined by individual clinicians and, therefore, was not standardized; this may have affected the ability to detect relapses. Lead-time bias is unlikely to be an issue due to the rapidly progressive nature of the disease that becomes clinically apparent in weeks to months. Therefore, improvements in long-term outcomes are expected to be a much higher magnitude (e.g. years) than any lead time in diagnosis based on our understanding of the disease (e.g. weeks to months). Similarly, length-biased sampling may have potentially occurred (e.g. ANCA testing could have led to greater detection of indolent disease, thereby giving the false appearance of improved outcomes). However, when the sample was restricted to patients with an eGFR ≤ 45 ml/min/1.73 m2, a significant improvement in ESRD-free survival was still seen. Therefore, length-biased sampling was not likely an issue in this study. Additionally, there were 32% lost to follow-up by 5 years from diagnosis which, as in any observational cohort, may have affected results. Lastly, generalizability may be limited to other populations of AAV.
In conclusion, this analysis of a multi-centered inception cohort demonstrates that for patients with AAV and renal disease, ESRD-free survival has improved over the past several decades. The finding that a lower serum creatinine at diagnosis was the only significant predictor associated with a lower risk of ESRD or death underscores the importance of early disease detection and the need for diagnostic tools to identify patients with AAV before the onset of irreversible renal damage. Nonetheless, despite advances in disease detection and therapeutic management, relapses continue to be an important clinical problem in AAV. Further studies are needed to address the problem of relapse, including better identification of at-risk populations and refining the use of current therapies to maintain better disease control without losing what has been gained in patient and renal survival.
Table 2.
Cumulative incidence of study outcomes by time period
| Outcome | All | Time Period
|
P-value for trend | |||||
|---|---|---|---|---|---|---|---|---|
| 85–89 | 90–94 | 95–99 | 00–04 | 05–09 | ||||
| ESRD-free survival | 1-year | 75% | 73% | 72% | 66% | 82% | 82% | 0.02 |
| 3-year | 61% | 51% | 57% | 53% | 69% | 72% | < 0.001 | |
| 5-year | 54% | 39% | 53% | 40% | 68% | 70% | < 0.001 | |
|
| ||||||||
| Patient survival | 1-year | 91% | 89% | 89% | 89% | 93% | 90% | 0.36 |
| 3-year | 81% | 74% | 84% | 73% | 87% | 83% | 0.15 | |
| 5-year | 72% | 64% | 71% | 58% | 84% | 83% | < 0.001 | |
|
| ||||||||
| Renal survival | 1-year | 80% | 77% | 77% | 70% | 86% | 89% | 0.005 |
| 3-year | 69% | 60% | 61% | 60% | 76% | 84% | < 0.001 | |
| 5-year | 65% | 51% | 61% | 53% | 76% | 82% | < 0.001 | |
|
| ||||||||
| Relapse | 1-year | 11% | 5% | 7% | 12% | 14% | 15% | 0.008 |
| 3-year | 29% | 22% | 25% | 28% | 28% | 40% | 0.01 | |
| 5-year | 35% | 28% | 27% | 33% | 30% | 57% | < 0.001 | |
ESRD, end-stage renal disease
Acknowledgments
Funding: Dr. Rhee receives support from the Rheumatology Research Foundation and the Vasculitis Foundation. This work was supported by Program Project Grant P01-DK5-30834 from the National Institute for Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Footnotes
Disclosures:
Dr. Merkel has received consulting fees from Actelion, Alexion, ChemoCentryx, and Sanofi, and research funding from Actelion, Bristol-Myers Squibb, Celgene, ChemoCentryx, Genentech/Roche, and GlaxoSmithKline.
References
- 1.Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003;41(4):776–84. doi: 10.1016/s0272-6386(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 2.Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70(3):488–94. doi: 10.1136/ard.2010.137778. [DOI] [PubMed] [Google Scholar]
- 3.Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7(1):23–32. doi: 10.1681/ASN.V7123. [DOI] [PubMed] [Google Scholar]
- 4.Neumann I, Kain R, Regele H, Soleiman A, Kandutsch S, Meisl FT. Histological and clinical predictors of early and late renal outcome in ANCA-associated vasculitis. Nephrol Dial Transplant. 2005;20(1):96–104. doi: 10.1093/ndt/gfh563. [DOI] [PubMed] [Google Scholar]
- 5.Tomasson G, Boers M, Walsh M, LaValley M, Cuthbertson D, Carette S, et al. Assessment of health-related quality of life as an outcome measure in granulomatosis with polyangiitis (Wegener’s) Arthritis Care Res (Hoboken) 2012;64(2):273–9. doi: 10.1002/acr.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basu N, McClean A, Harper L, Amft EN, Dhaun N, Luqmani RA, et al. The characterisation and determinants of quality of life in ANCA associated vasculitis. Ann Rheum Dis. 2014;73(1):207–11. doi: 10.1136/annrheumdis-2012-202750. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe K, Tani Y, Kimura H, Tanaka K, Hayashi Y, Asahi K, et al. Clinical outcomes of Japanese MPO-ANCA-related nephritis: significance of initial renal death for survival. Intern Med. 2012;51(15):1969–76. doi: 10.2169/internalmedicine.51.7727. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson P, Jacobsson L, Lindell A, Nilsson JA, Skogh T. Improved outcome in Wegener’s granulomatosis and microscopic polyangiitis? A retrospective analysis of 95 cases in two cohorts. J Intern Med. 2009;265(4):496–506. doi: 10.1111/j.1365-2796.2008.02060.x. [DOI] [PubMed] [Google Scholar]
- 9.Hilhorst M, Wilde B, van Paassen P, Winkens B, van Breda Vriesman P, Cohen Tervaert JW. Improved outcome in anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis: a 30-year follow-up study. Nephrol Dial Transplant. 2013;28(2):373–9. doi: 10.1093/ndt/gfs428. [DOI] [PubMed] [Google Scholar]
- 10.Takala JH, Kautiainen H, Leirisalo-Repo M. Survival of patients with Wegener’s granulomatosis diagnosed in Finland in 1981–2000. Scand J Rheumatol. 2010;39(1):71–6. doi: 10.3109/03009740903140701. [DOI] [PubMed] [Google Scholar]
- 11.Andrews M, Edmunds M, Campbell A, Walls J, Feehally J. Systemic vasculitis in the 1980s--is there an increasing incidence of Wegener’s granulomatosis and microscopic polyarteritis? J R Coll Physicians Lond. 1990;24(4):284–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Gross WL, Hauschild S, Mistry N. The clinical relevance of ANCA in vasculitis. Clin Exp Immunol. 1993;93(Suppl 1):7–11. doi: 10.1111/j.1365-2249.1993.tb06215.x. 0009-9104 (Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Groot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52(8):2461–9. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 14.Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniene J, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349(1):36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 15.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363(3):211–20. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 17.Faurschou M, Westman K, Rasmussen N, de Groot K, Flossmann O, Hoglund P, et al. Brief Report: long-term outcome of a randomized clinical trial comparing methotrexate to cyclophosphamide for remission induction in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64(10):3472–7. doi: 10.1002/art.34547. [DOI] [PubMed] [Google Scholar]
- 18.Walsh M, Faurschou M, Berden A, Flossmann O, Bajema I, Hoglund P, et al. Long-Term Follow-Up of Cyclophosphamide Compared with Azathioprine for Initial Maintenance Therapy in ANCA-Associated Vasculitis. Clin J Am Soc Nephrol. 2014 doi: 10.2215/CJN.00100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper L, Morgan MD, Walsh M, Hoglund P, Westman K, Flossmann O, et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis. 2012;71(6):955–60. doi: 10.1136/annrheumdis-2011-200477. [DOI] [PubMed] [Google Scholar]
- 20.Guillevin L, Cohen P, Mahr A, Arene JP, Mouthon L, Puechal X, et al. Treatment of polyarteritis nodosa and microscopic polyangiitis with poor prognosis factors: a prospective trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in sixty-five patients. Arthritis Rheum. 2003;49(1):93–100. doi: 10.1002/art.10922. [DOI] [PubMed] [Google Scholar]
- 21.Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, Viallard JF, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013;65(1):270–81. doi: 10.1002/art.37721. [DOI] [PubMed] [Google Scholar]
- 22.Gibelin A, Maldini C, Mahr A. Epidemiology and etiology of wegener granulomatosis, microscopic polyangiitis, churg-strauss syndrome and goodpasture syndrome: vasculitides with frequent lung involvement. Semin Respir Crit Care Med. 2011;32(3):264–73. doi: 10.1055/s-0031-1279824. [DOI] [PubMed] [Google Scholar]
- 23.Nachman PH, Hogan SL, Jennette JC, Falk RJ. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7(1):33–9. doi: 10.1681/ASN.V7133. [DOI] [PubMed] [Google Scholar]
- 24.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 25.Rupcic V, Jakic M, Rupcic V, Vizjak V, Fijacko M. Clinical course and outcome predictors in pauci-immune ANCA-positive renal-limited vasculitis. Acta Clin Croat. 2011;50(4):475–83. [PubMed] [Google Scholar]
- 26.Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143(9):621–31. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 28.Holle JU, Gross WL, Latza U, Nolle B, Ambrosch P, Heller M, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63(1):257–66. doi: 10.1002/art.27763. [DOI] [PubMed] [Google Scholar]
- 29.Stratta P, Marcuccio C, Campo A, Sandri L, Messuerott A, Colla L, et al. Improvement in relative survival of patients with vasculitis: study of 101 cases compared to the general population. Int J Immunopathol Pharmacol. 2008;21(3):631–42. doi: 10.1177/039463200802100317. [DOI] [PubMed] [Google Scholar]
- 30.de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, Jayne DR, Gaskin G, Rasmussen N, et al. Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: A prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol. 2006;17(8):2264–74. doi: 10.1681/ASN.2005080870. [DOI] [PubMed] [Google Scholar]
- 31.Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003;41(4):776–84. doi: 10.1016/s0272-6386(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 32.Mukhtyar C, Flossmann O, Hellmich B, Bacon P, Cid M, Cohen-Tervaert JW, et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann Rheum Dis. 2008;67(7):1004–10. doi: 10.1136/ard.2007.071936. [DOI] [PubMed] [Google Scholar]
- 33.Bligny D, Mahr A, Toumelin PL, Mouthon L, Guillevin L. Predicting mortality in systemic Wegener’s granulomatosis: a survival analysis based on 93 patients. Arthritis Rheum. 2004;51(1):83–91. doi: 10.1002/art.20082. [DOI] [PubMed] [Google Scholar]
- 34.Walsh M, Flossmann O, Berden A, Westman K, Hoglund P, Stegeman C, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64(2):542–8. doi: 10.1002/art.33361. [DOI] [PubMed] [Google Scholar]
- 35.Pierrot-Deseilligny Despujol C, Pouchot J, Pagnoux C, Coste J, Guillevin L. Predictors at diagnosis of a first Wegener’s granulomatosis relapse after obtaining complete remission. Rheumatology (Oxford) 2010;49(11):2181–90. doi: 10.1093/rheumatology/keq244. [DOI] [PubMed] [Google Scholar]
- 36.Miloslavsky EM, Specks U, Merkel PA, Seo P, Spiera R, Langford CA, et al. Clinical outcomes of remission induction therapy for severe antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2013;65(9):2441–9. doi: 10.1002/art.38044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahr A, Katsahian S, Varet H, Guillevin L, Hagen EC, Hoglund P, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72(6):1003–10. doi: 10.1136/annrheumdis-2012-201750. [DOI] [PubMed] [Google Scholar]
- 38.McGregor JG, Hogan SL, Hu Y, Jennette CE, Falk RJ, Nachman PH. Glucocorticoids and relapse and infection rates in anti-neutrophil cytoplasmic antibody disease. Clin J Am Soc Nephrol. 2012;7(2):240–7. doi: 10.2215/CJN.05610611. [DOI] [PMC free article] [PubMed] [Google Scholar]