Abstract
Background and Aim
Individuals with type 2 diabetes are at heightened risk for nonalcoholic fatty liver disease, which gives rise to nonalcoholic steatohepatitis (NASH) and cirrhosis. Yet, current guidelines do not recommend screening for NASH among these high-risk patients. Using a simulation model, we assessed the effectiveness and cost-effectiveness of screening diabetic patients for NASH.
Methods
A Markov model was constructed to compare two management strategies for 50-year-olds with diabetes. In the No Screening strategy, patients do not undergo screening, although NASH may be diagnosed incidentally over their lifetime. In the NASH Screening strategy, all patients receive a one-time screening ultrasound. Individuals with fatty infiltration on ultrasound then have a liver biopsy, and those found to have NASH receive medical therapy, which decreases progression to cirrhosis. End-points evaluated included quality-adjusted life years (QALYs) gained, costs, and incremental cost-effectiveness ratios (ICERs).
Results
Screening for NASH decreased the number of individuals who developed cirrhosis by 12.9 % and resulted in an 11.9 % decrease in liver-related deaths. However, screening resulted in 0.02 fewer QALYs, due to the disutility associated with treatment, and was therefore dominated by the No Screening strategy. When the model excluded this quality-of-life decrement, screening became cost-effective, at an ICER of $42,134 per QALY.
Conclusions
Screening for NASH may improve liver-related outcomes, but is not cost-effective at present, due to side effects of therapy. As better tolerated treatments for NASH become available, even with modest efficacy, screening for NASH will become cost-effective.
Keywords: Nonalcoholic steatohepatitis, Fibrosis, Cirrhosis, Screening
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease in the USA, with a prevalence over 30 % in the general population [1]. The progressive form of NAFLD, nonalcoholic steatohepatitis (NASH), can give rise to cirrhosis and hepatocellular carcinoma. NASH is the third most common indication for liver transplantation in the USA and is predicted to be the leading indication by 2025 [2].
NAFLD is strongly associated with type 2 diabetes, with the prevalence of NAFLD among diabetics ranging up to 75 % [3, 4]. Among individuals with both NAFLD and diabetes, NASH is unusually common, with a prevalence of 63–87 % [5–8]. Advanced fibrosis is also especially frequent in these individuals, affecting 34–60 % of patients with NAFLD and diabetes [6]. In addition, diabetes may increase the risk of developing hepatocellular carcinoma by as much as threefold [9, 10].
Diabetic individuals are generally evaluated for NAFLD when routine laboratory testing reveals elevated amino-transferase levels or when fatty infiltration of the liver is incidentally noted on imaging studies such as ultrasound or abdominal computed tomography (CT) scan. Despite the high prevalence of NASH and advanced fibrosis among individuals with diabetes, and their high risk of progression to end-stage liver disease, there is no established screening protocol for NASH in this population. Guidelines from the American Association for the Study of Liver Diseases (AASLD) do not recommend screening for NASH in high-risk groups because of a lack of evidence demonstrating benefit from screening, concerns surrounding cost of screening examinations, and a lack of available NASH therapeutics [11]. Under these circumstances, where long-term clinical data are limited, modeling can help to integrate the best available short-term data and project potential future outcomes of hypothetical screening and treatment strategies. Modeling also allows for cost-effectiveness analysis, which can enable interrogation of concerns about screening costs that contributed in part to current guidelines.
The aim of our study was to determine the clinical outcomes and cost-effectiveness of a hypothetical screening strategy for NASH in patients with diabetes, with the goal of providing data to guide clinical and policy decision making. To that end, a Markov model was constructed to compare outcomes in 50-year-old subjects with diabetes, using two approaches: (1) no screening (“No Screening strategy”) and (2) ultrasound screening (“NASH Screening strategy”) followed by liver biopsy for patients found to have fatty liver infiltration, with medical intervention for patients with NASH.
Methods
Model Structure
We developed a Markov model using TreeAge Pro 2014 (TreeAge, Williamstown, MA). The model assessed two different strategies for the screening and treatment of NASH in hypothetical patients with diabetes: the No Screening strategy and the NASH Screening strategy.
The model included six health states: (1) no liver disease, (2) simple steatosis or NAFLD, (3) NASH with fibrosis stage 0–3, (4) compensated NASH cirrhosis, (5) decompensated NASH cirrhosis, and 6) death. Possible causes of death included complications due to liver biopsy, liver-related mortality, and age-related mortality. The Markov cycle length, or time between state transitions, was 1 year. The model began with a hypothetical cohort of 50-year-old men and women who were followed until death. In each 1-year cycle, patients could either remain in the same health state, progress to another health state, or die (Fig. 1).
Fig. 1.

Schematic of Markov model. The ovals within the schematic depict the health states of patients in the model. The star indicates the health state in which patients may receive treatment to slow progression toward cirrhosis
Competing Strategies in the Model
In the No Screening strategy, NASH is incidentally detected due to abnormal aminotransferase levels in 21 % of patients [1]. In the NASH Screening strategy, all patients initially receive an abdominal ultrasound to assess for fatty infiltration of the liver regardless of aminotransferase levels. Abdominal ultrasound can result in a positive finding for fatty infiltration, a finding of no fatty infiltration, a false positive test for fatty infiltration, or a false negative normal ultrasound. Individuals found to have fatty infiltration (both true and false positive results) and then undergo a percutaneous ultrasound-guided liver biopsy. Complications of biopsy include hospitalization and death. Death was predicted to occur in 0.01 % of patients receiving liver biopsy (Table 1) [12]. Biopsy was selected as the screening procedure in the model rather than transient elastography, which is less invasive but cannot reliably identify early fibrosis and has a high failure rate in individuals with high BMI—a major limitation for diagnosis in the diabetic population [11, 13].
Table 1. Model inputs.
| Parameters | Base case | References |
|---|---|---|
| Natural history parameters | ||
| Annual probability of decompensating | 6 % | [23] |
| Compensated cirrhosis—annual all-cause mortality | 2 % | [23] |
| Decompensated cirrhosis—annual all-cause mortality | 13 % | [24, 26] |
| Screening and pioglitazone parameters | ||
| Probability of mortality from biopsy | 0.01 % | [12] |
| Probability of major complication from biopsy | 0.5 % | [38] |
| Risk ratio of histological improvement (pioglitazone) | 1.38 | [15] |
| Annual probability of stopping pioglitazone treatment | 28 % | [39] |
| Costs (2013 US$) | ||
| Annual costs by health state | ||
| NASH without cirrhosis | $1149.08 | [40] |
| Compensated cirrhosis | $1291.90 | [40] |
| Decompensated cirrhosis | $17,296.79 | [40] |
| Annual costs of screening and treatment | ||
| Abdominal ultrasound | $127.56 | [41, 42] |
| Ultrasound-guided liver biopsy | $992.44 | [41, 42] |
| Pioglitazone 30 mg daily | $188.31 | [43] |
| Major complication of biopsy | $6126.99 | [41, 44, 45] |
| Quality-of-life values | ||
| NASH without cirrhosis | 0.92 | [26, 46] |
| Compensated cirrhosis | 0.89 | [26, 46] |
| Decompensated cirrhosis | 0.81 | [26, 46] |
| Pioglitazone 30 mg daily | −0.01 | [47, 48] |
Patients found to have NASH on liver biopsy were treated with pioglitazone 30 mg daily, until either they died or stopped adhering to treatment. Pioglitazone was selected as the treatment for NASH based on guidelines from the AASLD [11]. It has been shown to lead to histological improvements in patients with NASH and diabetes [14]. Efficacy of treatment was derived from a meta-analysis of seven randomized trials on thiazolidinediones that assessed histological endpoints [15]. The model incorporated the effect of treatment by reducing the rate of progression toward cirrhosis. Vitamin E was not included in our model because guidelines do not recommended it for diabetic patients [11], meta-analysis results showed no histological benefits of vitamin E [16], and its use has been associated with increased all-cause mortality [17].
Clinical Parameters for Model Input
The model included a wide range of estimates that were derived from the literature (Table 1). Based on prevalence studies of NAFLD in diabetics, we assumed 65.4 % of patients in the model have steatosis [18]. Of these patients, the proportion with NASH was assumed to be 78 % [6]. The distribution of NASH patients into different stages of disease progression was based on a study by Leite et al. [6]. No patients in the model initially have decompensated cirrhosis, as patients with decompensated liver disease come to clinical attention without screening.
The rate of disease progression in patients with NASH was calibrated so that 20 % of them ultimately develop cirrhosis in their lifetime [19–22]. The annual probability of decompensation for patients with cirrhosis was based on a prospective study of NASH cirrhosis [23]. The model also included different estimates for annual mortality rates for patients with compensated and decompensated cirrhosis: 2 and 13 %, respectively (Table 1) [23, 24].
Costs and Quality-of-Life Adjustments
Base-case costs are summarized in Table 1. Medicare reimbursement rates for physicians and facilities were used to estimate direct costs of procedures, including ultrasound and ultrasound-guided percutaneous liver biopsy. Medication costs were obtained from the 2013 Red Book average wholesale prices. Costs from prior years were converted to 2013-year dollars using the Consumer Price Index [25].
Because there are no quality-of-life studies focused on patients with NASH cirrhosis, utility estimates were derived from studies on patients with cirrhosis from other causes; this approach was used in a previous cost-effectiveness analysis of NASH [26]. In addition to the utility values for each health state, the model incorporated a disutility for medical intervention because pioglitazone causes weight gain. Other side effects of pioglitazone, such as increased risk of bladder cancer and bone fracture, are exceedingly rare and were therefore not incorporated into the model [27, 28]. All utilities and costs were discounted at an annual rate of 3 % to adjust for the relative value of present dollars or a present year of life [29].
Outcomes
The primary outcome of the analysis was the incremental cost-effectiveness ratio (ICER) per quality-adjusted life year (QALY) gained between competing strategies. A willingness-to-pay (WTP) of $100,000/QALY was used as a threshold to determine cost-effectiveness [30]. Other outcomes included QALYs gained, life years (LY) gained, and cost. We also assessed clinical outcomes, such as the percentage of patients who progressed to cirrhosis and the percentage who died from liver-related mortality.
Analyses Performed
A base-case analysis using the best estimates of all parameters was performed, in order to compare the effectiveness and cost-effectiveness of the No Screening strategy and NASH Screening strategy. One-way sensitivity analyses assessed the impact of model input uncertainty on outcomes across a wide range of values (Table 3). A sensitivity analysis was also conducted to examine the outcomes of a NASH Screening strategy given the availability of improved medication, with twice the efficacy of pioglitazone, half the non-adherence rate, and no quality-of-life decrement (Table 4). Lastly, we performed probabilistic sensitivity analysis (Fig. 2). Distributions for model parameters were assigned based on the literature, and 100,000 iterations were performed to gain insight into the optimal strategy under uncertain conditions within our defined WTP threshold.
Table 3. Sensitivity analyses: ICERs for NASH Screening strategy versus No Screening strategy.
| Parameter | Quality-adjusted ICER ($/QALY) | Non-quality-adjusted ICER ($/LY) |
|---|---|---|
| Base case | Dominated | $17,985 |
| Probability of false negative on ultrasound (10 %) | ||
| 0 % (low) | Dominated | $17,809 |
| 30 % (high) | Dominated | $18,487 |
| Probability of false positive on ultrasound (3.7 %) | ||
| 0 % (low) | Dominated | $17,825 |
| 10 % (high) | Dominated | $18,259 |
| Probability of abnormal liver function tests in patients with NASH (21 %) | ||
| 0 % (low) | Dominated | $20,198 |
| 50 % (high) | Dominated | $9870 |
| Relative risk of histological improvement—pioglitazone (1.38) | ||
| 1.01 (low) | Dominated | $1,787,148 |
| 1.89 (high) | $329,756 | $9606 |
| Annual treatment non-adherence rate (27.5 %) | ||
| 0 % (low) | Dominated | $13,405 |
| 60 % (high) | Dominated | $39,544 |
| Adherence to biopsy (100 %) | ||
| 25 % (low) | Dominated | $43,936 |
| 50 % (low) | Dominated | $26,522 |
| Probability of mortality due to liver biopsy (0.01 %) | ||
| 0 % (low) | Dominated | $17,637 |
| 0.1 % (high) | Dominated | $21,900 |
| 0.3 % (high) | Dominated | $43,113 |
| Probability of major complications due to biopsy (0.5 %) | ||
| 0 % (low) | Dominated | $17,776 |
| 2.5 % (high) | Dominated | $18,821 |
| 5 % (high) | Dominated | $19,867 |
| Probability of developing cirrhosis in lifetime for NASH patients (20 %) | ||
| 10 % (low) | Dominated | $49,033 |
| 30 % (high) | $345,378 | $9891 |
| Annual probability of decompensating (5.8 %) | ||
| 1 % (low) | Dominated | $31,068 |
| 10 % (high) | Dominated | $14,187 |
| Annual mortality of individuals with compensated cirrhosis (2 %) | ||
| 0 % (low) | Dominated | $25,113 |
| 5 % (high) | Dominated | $14,116 |
| 10 % (high) | $380,619 | $11,486 |
| Annual mortality of individuals with decompensated cirrhosis (13 %) | ||
| 5 % (low) | Dominated | $21,907 |
| 10 % (low) | Dominated | $18,849 |
| 40 % (high) | Dominated | $15,878 |
| Cost of decompensated cirrhosis | ||
| 50 % of baseline | Dominated | $19,813 |
| 200 % of baseline | Dominated | $14,328 |
| Cost of screening (ultrasound and biopsy) | ||
| 50 % of baseline | Dominated | $13,802 |
| 200 % of baseline | Dominated | $26,351 |
| Cost of medical intervention | ||
| 50 % of baseline | Dominated | $11,758 |
| 300 % of baseline | Dominated | $42,895 |
| Disutility due to pioglitazone | ||
| 0 % of baseline | $42,134 | – |
| 50 % of baseline | $187,054 | – |
Base-case values are in parentheses
Table 4. Analysis of NASH Screening with improved medical intervention.
| Outcome | No Screening strategy | NASH Screening strategy | NASH Screening with improved intervention |
|---|---|---|---|
| Life years | 30.02 | 30.09 | 30.18 |
| QALYs | 17.47 | 17.45 | 17.54 |
| Costs ($) | $13,477 | $14,693 | $14,741 |
| ICER ($/QALY) | – | Dominated | $18,579 |
| ICER ($/LY) | – | $17,985 | $7954 |
| Proportion with cirrhosis (%) | 10.22 | 8.90 | 7.13 |
| Proportion liver-related deaths (%) | 5.12 | 4.51 | 3.74 |
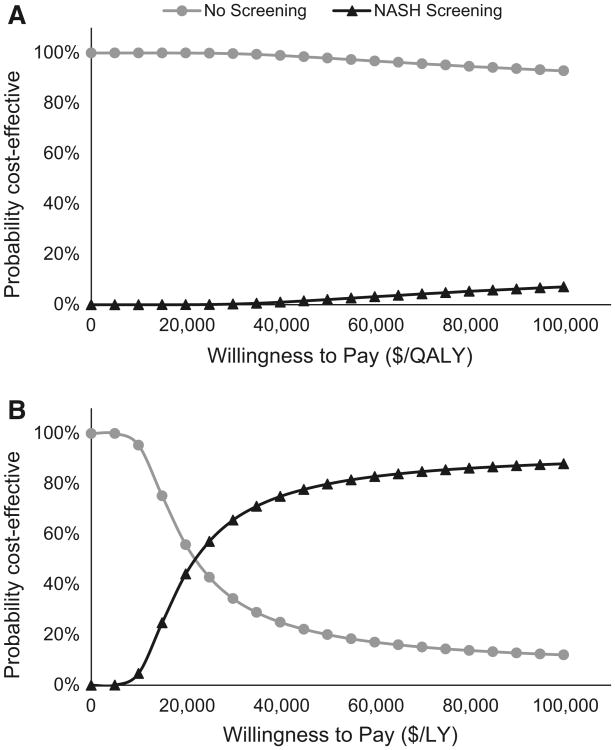
Fig. 2.
Probabilistic sensitivity analysis. a The results of probabilistic sensitivity analysis in our quality of life-adjusted model. b The results of probabilistic sensitivity in our non-quality of life-adjusted model
Results
Base-Case Results
In the base-case, or primary analysis, the NASH Screening strategy reduced the number of individuals who developed cirrhosis by 12.9 % and led to an 11.9 % decrease in liver-related deaths (Table 2). However, compared with No Screening, the NASH Screening strategy also resulted in 0.02 fewer QALYs, mainly because of the disutility associated with the treatment, and was therefore dominated by the No Screening strategy.
Table 2. Base-case results.
| Outcome | No Screening strategy | NASH Screening strategy |
|---|---|---|
| Life years | 30.02 | 30.09 |
| QALYs | 17.47 | 17.45 |
| Costs ($) | $13,477 | $14,693 |
| ICER ($/QALY) | – | Dominated |
| ICER ($/LY) | – | $17,985 |
| Percent with cirrhosis (%) | 10.22 | 8.90 |
| Percent liver-related deaths (%) | 5.12 | 4.51 |
When the analysis was run without incorporating quality-of-life utilities, the NASH Screening Strategy resulted in an additional 0.07 LYs at an incremental cost of $1216, yielding a favorable ICER of $17,985 per LY, well below a WTP threshold of $100,000 per LY (Table 2). The key driver of this difference between the quality of life-adjusted life expectancy and unadjusted life expectancy was the weight gain associated with medical intervention.
Sensitivity Analysis
Results of one-way sensitivity analyses are summarized in Table 3. The quality of life-adjusted results suggest that the NASH Screening strategy is unlikely to be cost-effective even if treatment efficacy is set at the upper bound of the 95 % confidence interval from meta-analysis [15]. Based on sensitivity analysis, screening for NASH will be cost-effective only if medical intervention involves negligible quality-of-life decrement. When the quality of life-adjusted model excluded any disutility associated with medication, the NASH Screening strategy became cost-effective, with an ICER of $42,134 per QALY (Table 3).
One-way sensitivity analyses were also performed on a version of the model that did not account for quality of life (Table 3). These analyses indicate that uncertainty associated with ultrasound does not have a major effect on model output. The screening strategy also remained cost-effective, even if >50 % of patients refused to undergo a screening biopsy. Additionally, raising the mortality of liver biopsy had little effect on the cost-effectiveness of the NASH Screening strategy. When the probability of death due to liver biopsy was raised to 30-times its base-case value, screening remained cost-effective. In contrast, non-adherence to medication and efficacy of treatment had a large impact on results. The NASH Screening strategy became increasingly cost-effective at low non-adherence rates, as expected. This strategy ceased to be cost-effective when non-adherence rates exceeded approximately 77 % per year. The NASH Screening Strategy also stopped being cost-effective when treatment efficacy was set at the minimum value reported in the literature.
Results from the version of the model that did not adjust for quality of life were sensitive to changes in the costs of screening. As these costs decrease, the ICER associated with the NASH Screening strategy also decreases, or becomes more cost-effective. In addition, increasing the cost of treating decompensated cirrhosis also improved the cost-effectiveness of the NASH Screening strategy, because treatment prevents patients from progressing to cirrhosis, a costly health state.
As part of our sensitivity analysis, we examined the effectiveness and cost-effectiveness of the NASH Screening strategy given the availability of a hypothetical treatment for NASH with the same cost as pioglitazone, but with superior adherence, efficacy, and side effect profile (Table 4). This analysis showed that the NASH Screening strategy is likely to be cost-effective given the availability of a superior treatment, in both the quality of life-adjusted and unadjusted versions of the model.
Probabilistic sensitivity analysis in the quality-adjusted model showed that the NASH Screening strategy was not cost-effective at a WTP of $100,000 per QALY in 93 % of trials. In the version of the model that was not quality of life-adjusted, PSA showed that screening was the optimal strategy above a WTP of ~$22,000 per LY. Below this threshold, the No Screening strategy becomes the optimal strategy, in terms of cost-effectiveness.
Discussion
Our analysis demonstrates the potential value of ultrasound screening for NASH among adults with diabetes and provides an evaluation of the key impediments to screening in high-risk individuals. Ultrasound screening with subsequent liver biopsy and medical intervention, when indicated, improved liver-related outcomes. This strategy resulted in an additional 0.07 LY, decreased the prevalence of cirrhosis by 12.9 %, and reduced liver-related deaths by 11.9 % (Table 2). However, in the quality of life-adjusted cost-effectiveness analysis, the No Screening strategy dominated the NASH Screening strategy, due to the decrement in quality of life associated with medical treatment.
This analysis suggests that the main obstacle to cost-effective screening for NASH is the poor tolerability of pioglitazone, the recommended treatment for NASH according to AASLD guidelines [11]. Screening for NASH, accompanied by treatment for affected individuals, is likely to be cost-effective only if there is no quality-of-life decrement associated with medical therapy. When the model excluded the disutility of side effects due to pioglitazone in our sensitivity analyses, the NASH Screening strategy became cost-effective in the quality of life-adjusted model, with an ICER of $42,134 per QALY, below the WTP threshold of $100,000 per QALY (Table 3). Similarly, in the version of the model that did not incorporate quality-of-life utilities, the NASH Screening Strategy was cost-effective, with a base-case ICER of $17,985 per LY gained (Table 2). The main factors contributing to these favorable ICERs—in addition to the exclusion of a quality-of-life decrement for pioglitazone—include the high prevalence of NASH among individuals with diabetes, the reduced progression toward cirrhosis for patients receiving treatment, and the costs avoided by preventing or delaying cirrhosis. While it is possible that a less invasive screening procedure, such as transient elastography, might improve the cost-effectiveness of screening, our results suggest that this is not the case: in sensitivity analyses, No Screening remains the optimal strategy even when the screening procedure involves lower costs, decreased risk of mortality, and decreased risk of complications (Table 3).
If the model includes quality-of-life utilities for pioglitazone, the NASH Screening strategy is not cost-effective, even if the efficacy of treatment is raised to the maximum value from the literature (Table 3). Additionally, even if medication adherence is raised to 100 %, the No Screening strategy still dominates the NASH Screening strategy, due to the side effects of pioglitazone. The quality of life-adjusted model's insensitivity to changing adherence rates and efficacy underscores that pioglitazone's poor tolerability is the key impediment to cost-effective screening. To examine the impact of improved therapy for NASH, the NASH Screening strategy was altered to incorporate a hypothetical treatment for NASH with twice the efficacy of pioglitazone, half the non-adherence rate, and no quality-of-life decrement (Table 4). This analysis generated a favorable ICER of $18,579 per QALY, which falls well below the WTP threshold of $100,000 per QALY. This result highlights the need for and promise of improved therapeutics for NASH.
As with any model, our model has several limitations. First, the quality-adjusted version of the model included a quality-of-life decrement only for the weight gain associated with pioglitazone. Other side effects, such as increased risk of bladder cancer and bone fracture, are exceedingly rare and were therefore not included [27, 28]. Thus, it is possible that our model underestimates the quality-of-life decrease due to these risks and other possible side effects, such as fluid retention and heart failure, which we did not include for the sake of simplicity and because thiazolidinediones have not been shown to increase mortality due to heart failure [31]. However, our model also excludes any diabetes-related benefits of pioglitazone, which may counterbalance the omission of these minor risks [32]. In addition, our model does not account for diabetic patients who are already taking pioglitazone before screening. We do not view this as a major limitation because a small portion of diabetic patients take thiazolidinediones, and the use of these medications is falling out of favor due to their risk profile [33, 34]. The model is also limited by the lack of long-term data supporting pioglitazone treatment for NASH. Trials on pioglitazone for NASH have a short duration of less than 2 years [15]. In the absence of long-term data, our model may provide a useful way of forecasting the potential outcomes of treatment over time, while accounting for the uncertainty inherent in such a projection. Lastly, given the lack of economic data for the health states in our model, we were forced to utilize cost data for similar liver-related conditions. As a stand-in for the costs associated with NASH, our model used cost data for chronic hepatitis. Likewise, our model assumed that NASH-associated cirrhosis and hepatitis-associated cirrhosis involve similar treatment costs.
Despite these caveats, the present model has several strengths. A comparison of the model's clinical outcomes to endpoints derived from the literature, which were not used to build our model, helps affirm the validity of our results. First, in the model, ~10 % of patients with NASH ultimately die from liver-related causes, compared to 17.5 % in a study of 173 NAFLD patients with a median follow-up of 10.5 years [35]. In addition, in the model approximately half of all hypothetical patients with NASH cirrhosis ultimately die from liver-related mortality. For comparison, 40 % of NASH cirrhosis patients die from liver-related causes over a 10-year period in Matteoni et al. [20]. Lastly, 5.1 % of hypothetical patients with diabetes in the model die from liver-related causes, which falls within the range of 4.4–12.5 % in the literature [36].
Though other studies have explored the cost-effectiveness of medications for NASH, to the best of our knowledge, our model is the first to assess the value of screening for NASH in individuals with diabetes [26]. While our model includes several assumptions raising concerns about the findings, as with any model, it provides a useful examination of current guidelines, which do not support NASH screening in high-risk groups because of concerns surrounding cost and treatment efficacy. Our analysis confirms this view—screening is not effective or cost-effective at present—and it also provides a better understanding of the key impediments to cost-effective screening. The model results suggest that screening for NASH, given the availability of medication with mild side effects, even with modest efficacy, is likely to be cost-effective. Although no such medication is currently available, there has been rapid development in this field, with over 200 studies listed on ClinicalTrials.gov and encouraging results from a recent trial on obeticholic acid [37]. As our model shows, even relatively minor advances in this arena hold great promise to enhance the feasibility of NASH screening in high-risk patients.
Acknowledgments
Kathleen E. Corey and Raymond T. Chung received grant support from NIH K23 DK099422 and K24 DK078772, respectively.
Footnotes
Author contribution Kathleen E. Corey, Matthew J. Klebanoff and Chin Hur were involved in study concept and design, and acquisition of data; Kathleen E. Corey, Matthew J. Klebanoff, Raymond T. Chung, Chin Hur were involved in analysis and interpretation of data; Kathleen E. Corey, Matthew J. Klebanoff, Chin Hur drafted the manuscript; Kathleen E. Corey, Matthew J. Klebanoff, Raymond T. Chung, Chin Hur were involved in critical revision of the manuscript for important intellectual content; Kathleen E. Corey, Matthew J. Klebanoff, Angela C. Tramontano were involved in administration; Kathleen E. Corey, Matthew J. Klebanoff, Angela C. Tramontano provided technical or material support; and Kathleen E. Corey, Chin Hur were involved in study supervision.
Compliance with ethical standards: Conflict of interest Dr. Chung received funding from Gilead Sciences (not for NAFLD research). The other authors declare that they have no conflict of interest.
Contributor Information
Kathleen E. Corey, Email: kcorey@partners.org, kcorey@mgh.harvard.edu.
Matthew J. Klebanoff, Email: mklebanoff@mgh.harvard.edu.
Angela C. Tramontano, Email: angela@mgh-ita.org.
Raymond T. Chung, Email: rtchung@mgh.harvard.edu.
Chin Hur, Email: chur@mgh.harvard.edu.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 3.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 4.Prashanth M, Ganesh HK, Vima MV, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2009;57:205–210. [PubMed] [Google Scholar]
- 5.Abdelmalek MF, Diehl AM. Nonalcoholic fatty liver disease as a complication of insulin resistance. Med Clin North Am. 2007;91:1125–1149. doi: 10.1016/j.mcna.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Leite NC, Villela-Nogueira CA, Pannain VL, et al. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: prevalences and correlated factors. Liver Int. 2011;31:700–706. doi: 10.1111/j.1478-3231.2011.02482.x. [DOI] [PubMed] [Google Scholar]
- 7.Gupte P, Amarapurkar D, Agal S, et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19:854–858. doi: 10.1111/j.1440-1746.2004.03312.x. [DOI] [PubMed] [Google Scholar]
- 8.Doycheva I, Patel N, Peterson M, Loomba R. Prognostic implication of liver histology in patients with nonalcoholic fatty liver disease in diabetes. J Diabetes Complicat. 2013;27:293–300. doi: 10.1016/j.jdiacomp.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 11.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 12.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;15(344):495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 13.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 14.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;30:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 15.Mahady SE, Webster AC, Walker S, Sanyal A, George J. The role of thiazolidinediones in non-alcoholic steatohepatitis—a systematic review and meta analysis. J Hepatol. 2011;55:1383–1390. doi: 10.1016/j.jhep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 17.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 18.Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 19.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 20.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 21.Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 22.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 23.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682–689. doi: 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 24.Ratziu V, Bonyhay L, Di Martino V, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485–1493. doi: 10.1053/jhep.2002.33324. [DOI] [PubMed] [Google Scholar]
- 25.United States Bureau of Labor Statistics. CPI Inflation Calculator. [Accessed March 13, 2015];2015 Available at: http://www.bls.gov/data/inflation_calculator.htm.
- 26.Mahady SE, Wong G, Craig JC, George J. Pioglitazone and vitamin E for nonalcoholic steatohepatitis: a cost utility analysis. Hepatology. 2012;56:2172–2179. doi: 10.1002/hep.25887. [DOI] [PubMed] [Google Scholar]
- 27.Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR PROactive investigators. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf. 2009;32:187–202. doi: 10.2165/00002018-200932030-00002. [DOI] [PubMed] [Google Scholar]
- 28.Grey A. Skeletal consequences of thiazolidinedione therapy. Osteoporos Int. 2008;19:129–137. doi: 10.1007/s00198-007-0477-y. [DOI] [PubMed] [Google Scholar]
- 29.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996:23–30. 1339–1341. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 30.Winkelmayer WC, Weinstein MC, Mittleman MA, Glynn RJ, Pliskin JS. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Making. 2002;22:417–430. doi: 10.1177/027298902236927. [DOI] [PubMed] [Google Scholar]
- 31.Rinella ME, Loomba R, Caldwell SH, et al. Controversies in the diagnosis and management of NAFLD and NASH. Gastroenterol Hepatol (NY) 2014;10:219–227. [PMC free article] [PubMed] [Google Scholar]
- 32.Valentine WJ, Tucker D, Palmer AJ, et al. Long-term cost-effectiveness of pioglitazone versus placebo in addition to existing diabetes treatment: a US analysis based on PROactive. Value Health. 2009;12:1–9. doi: 10.1111/j.1524-4733.2008.00403.x. [DOI] [PubMed] [Google Scholar]
- 33.Desai NR, Shrank WH, Fischer MA, et al. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med. 2012;125:302.e1–302.e7. doi: 10.1016/j.amjmed.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the U.S., 2003–2012. Diabetes Care. 2014;37:1367–1374. doi: 10.2337/dc13-2289. [DOI] [PubMed] [Google Scholar]
- 35.Rafiq N, Bai C, Fang Y, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734–743. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 37.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, nonalcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindor KD, Bru C, Jorgensen RA, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology. 1996;23:1079–1083. doi: 10.1002/hep.510230522. [DOI] [PubMed] [Google Scholar]
- 39.Hansen RA, Farley JF, Droege M, Maciejewski ML. A retrospective cohort study of economic outcomes and adherence to monotherapy with metformin, pioglitazone, or a sulfonylurea among patients with type 2 diabetes mellitus in the United States from 2003 to 2005. Clin Ther. 2010;32:1308–1319. doi: 10.1016/j.clinthera.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Eckman MH, Kaiser TE, Sherman KE. The cost-effectiveness of screening for chronic hepatitis B infection in the United States. Clin Infect Dis. 2011;52:1294–1306. doi: 10.1093/cid/cir199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Medicare & Medicaid Services. Physician fee schedule look-up. [Accessed January 7, 2015];2015 http://www.cms.gov/apps/physician-fee-schedule/
- 42.Centers for Medicare & Medicaid Services. Addendum B.-Final OPPS Payment by HCPCS Code for CY 2013. [Accessed October 23, 2014]; goo.gl/M2cX3F.
- 43.Red Book Online. [Accessed January 7, 2015];2015 www.micromedexsolutions.com.
- 44.Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology. 1998;27:1220–1226. doi: 10.1002/hep.510270506. [DOI] [PubMed] [Google Scholar]
- 45.Zauber AG, Lansdorp-Vogelaar I, Wilschut J, Knudsen AB, Ballegooijen M, Kuntz KM. Cost-Effectiveness of DNA Stool Testing to Screen for Colorectal Cancer. Rockville: Agency for Healthcare Research and Quality; 2007. [PubMed] [Google Scholar]
- 46.McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making. 2008;28:582–592. doi: 10.1177/0272989X08315240. [DOI] [PubMed] [Google Scholar]
- 47.Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17:462–470. doi: 10.1016/j.jval.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;6:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]