Abstract
Background
Patients with sickle cell disease are at risk for developing chronic kidney disease. Acute kidney injury has been linked to progression to chronic kidney disease but limited data exists to determine its role in acute complications of sickle cell disease. We hypothesized that acute kidney injury occurs in pediatric patients admitted for acute chest syndrome and prolongs hospitalization.
Methods
We conducted a six year retrospective review of cases of acute chest syndrome admitted at a single institution.
Results
We identified that 12 of 149 (8%) patients developed acute kidney injury. A larger drop in hemoglobin from baseline (2.7 vs 1.4g/dL, p=0.003), lower admission hemoglobin (6.4 vs. 7.5g/dL, p=0.03), and increased admission white blood cell count (33.1 vs. 19.8 × 109/L, p<0.0001) were significantly associated with acute kidney injury as compared to no acute kidney injury. Acute kidney injury (p<0.0001) along with need for advanced respiratory support (Bipap or mechanical ventilation) (p<0.0001), and need for exchange transfusion (<0.0001) were associated with prolonged hospitalization.
Conclusions
Clinicians should monitor for the development of acute kidney injury during pediatric hospitalizations for acute chest syndrome as a potentially modifiable risk factor for prolonged hospitalizations.
Keywords: Sickle Cell Disease, Acute Kidney Injury, Nephropathy, Acute Chest Syndrome, Outcomes
Introduction
Forty percent of adults with sickle cell disease (SCD) develop chronic kidney disease (CKD) and 15–30% of deaths in SCD are related to kidney disease.[1–5] Children with SCD develop hyposthenuria, hyperfiltration, and microalbuminuria; early identification of risk factors and therapies to mitigate disease progression could have significant long term impact.[6–10] Growing evidence shows that acute kidney injury (AKI) contributes to CKD in other populations.[11] Understanding the prevalence of AKI during SCD events is paramount.
While the role of AKI in the development of CKD has become illuminated in other diseases, little research exists to determine the role which AKI has on SCD nephropathy.[12–15] We are aware of only one prior study to evaluate the incidence of AKI during SCD crisis, which suggests that 7–13% of adult SCD patients hospitalized with acute chest syndrome (ACS) develop AKI.[16] However, limitations in this retrospective study include a lack of appropriate evaluation of serum creatinine (SCr) values, as they only looked at day 1 SCr (compared to prior baseline) to diagnose AKI. In addition, patients in this adult cohort, as compared to pediatrics, may have underlying CKD which could influence development of AKI during the acute setting. There are several potential risk factors for developing AKI during ACS. SCD is characterized by acute hemolytic/anemic events, ischemia/reperfusion episodes, hypoxia, and inflammation that may worsen during ACS. The risk of AKI may also be exacerbated by use of nephrotoxic agents (vancomycin and ketorolac) and concern for dehydration (poor PO intake during crisis and hyposthenuria).
To better understand the link between admission for ACS and AKI, we conducted a 6 year retrospective cohort of pediatric patients admitted with ACS to determine the prevalence of AKI. We hypothesized that AKI is prevalent during ACS events and leads to prolonged hospital length of stay.
Methods
We conducted an IRB-approved six year retrospective cohort study of all pediatric SCD patients admitted to Children’s of Alabama with an ICD-9 code of ACS (517.3) as a primary or secondary diagnosis to determine the prevalence of AKI, identify potential risk factors for AKI at admission, and to evaluate the association between AKI and hospital length of stay. We excluded patients coded as ACS (517.3) who did not have an identifiable pulmonary infiltrate on chest X-ray during their hospitalization or who were hospitalized for less than 24 hours. AKI was defined by Kidney Disease Improving Global Outcomes (KDIGO) definition of either a ≥ 0.3 mg/dL or 50% increase in serum creatinine (SCr) from baseline. [17] Per institutional standard of care, patients with fever and pulmonary infiltrates (ACS) are treated with vancomycin, azithromycin, and ceftriaxone in the emergency room and can receive non-steroidal inflammatory medications (ibuprofen or ketorolac) as needed for pain or fevers.[18] After admission for ACS, individual attending physicians can determine whether they will discontinue vancomycin based on their initial clinical evaluation, wait for 48 hour cultures to result negative, or continue vancomycin until clinically stable. The type (simple or exchange) and timing of transfusion is determined by SCD provider preferences rather than an institutional protocol.
We conducted a chart review to determine the sickle cell genotype, age, current SCD modifying therapy, most recent outpatient (within one year of admission) complete blood count (Baseline: Hemoglobin (Hb), white blood cell count (WBC), and platelet (plt)) and SCr of patients diagnosed with ACS. During their admission, we collected their emergency room and daily inpatient complete blood counts and SCr values. We calculated the difference in hemoglobin and SCr from baseline (last Hb or SCr prior to admission and within one year) to their admission for ACS Hb and SCr values. We identified any transfusion intervention during their hospitalization, transfer to pediatric intensive care unit, need for enhanced respiratory support (Bipap or mechanical ventilation) and length of hospital stay. We created two categorical variables to reflect the clinical severity for patients based on SCD genotype: severe (HbSS, SB0 thalassemia, and SD) and mild SCD (HbSC and SB+ thalassemia). Among patients with multiple ACS admissions, we included patients’ data only from their most recent hospital admission.
Characteristics of patients by AKI status were summarized using descriptive statistics. Comparisons of these characteristics were performed using t-test for continuous variables and Fisher’s exact test for categorical variables. To determine variables associated with AKI, nominal logistic regression was performed with baseline, admission, and change in hemoglobin (Hb), white blood cells (WBC) and platelets (as explanatory variables. Similarly, we performed nominal logistic regression for type of transfusion support using laboratory variables Hb (baseline, admission, and change in Hb), WBC, and platelets. Kaplan-Meier curves and log rank tests were used to separately investigate the association of length of hospital stay with development of AKI, need for exchange transfusion, and need for increase in respiratory support. As intubation by itself can increase LOS, we stratified the association of LOS and AKI by need for increased respiratory support (Bipap or Intubation). All analyses were conducted using JMP Pro 10 (Cary, NC).
Results
We identified 236 episodes of ACS coded admissions in 163 patients during a six-year period. As we analyzed only the most recent episode of ACS, we excluded a total of 73 episodes of ACS as they were repeat episodes of ACS in the same patient. Of the 163 unique patients with ACS, we excluded 14 patients who did not have baseline SCr collected in the preceding year and therefore we could not determine AKI during the admission. Among the 149 eligible patients, the mean SCr at baseline was 0.4 mg/dL (s.d.0.1) and the mean maximum serum creatinine during hospitalization was 0.5 mg/dL (s.d. 0.1). The mean age of patients admitted with ACS was 9.5 years (s.d. 4.8) and 64% were male. Genotypes included 119 patients (80%) with HbSS, 7 patients (5%) with HbSB0 thalassemia, 8 patients (5%) with HbSB+ thalassemia), 14 patients (9%) with HbSC and 1 patient (1%) with HbSD. Four patients (2%) were on chronic transfusion at the time of ACS, 50 patients (32%) were on hydroxyurea and 95 patients (66%) were on no disease modifying therapy. Twenty eight patients were admitted or transferred to the ICU of which 22 required more than one day in the ICU. Eleven patients (7%) required mechanical ventilation and two were escalated to Bipap (1%) but not intubated. Thirty three patients (22%) required red cell exchange therapy, 102 patients (69%) required simple transfusion, and 14 patients (9%) were not transfused.
Table I summarizes the characteristics of the patients by AKI status as defined by KDIGO. AKI was identified in 12 of 149 (8%) episodes of ACS over a six year period. Compared to children without AKI, those patients with AKI had a larger drop in hemoglobin (2.7 vs. 1.4 g/dL, p=0.003), lower admission Hb (6.4 vs. 7.5 g/dL, p=0.03), and higher admission WBC (33.1 vs 19.8 × 103/L, p<0.0001). The groups were similar for age, gender, and platelet count. We identified 10 episodes of AKI in 127 cases of ACS for patients with severe SCD phenotypes and 2 episodes of AKI in 22 cases of ACS for patients with milder SCD phenotypes. No significant differences in the development of AKI were identified by genotype (p=0.8). Among the 149 defined cases, 2 of 50 patient cases (4%) on hydroxyurea developed AKI, 0 of 4 (0%) on transfusion developed AKI, and 10 of 95(11%) on no disease modifying therapy developed AKI. No differences in the development of AKI were identified by current SCD therapy (p=0.3).
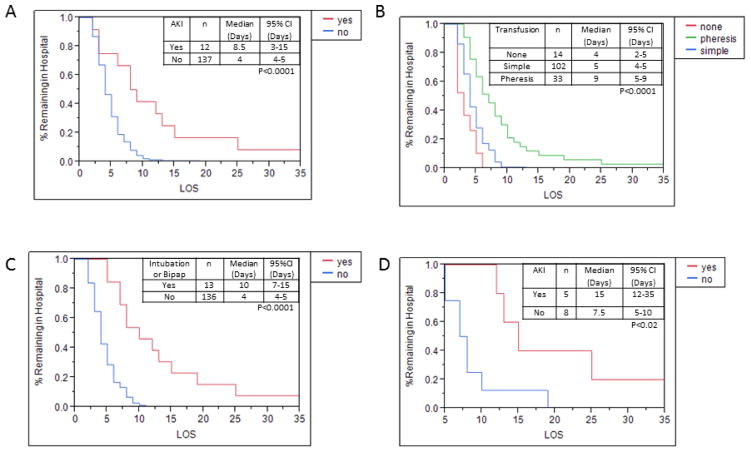
Using nominal logistic regression, drop in admission Hb from baseline and admission (g/dL) WBC (× 103/L) were associated with AKI (p-values=0.01 and 0.0007, respectively). The estimated odds ratios are 1.81 (95% CI: 1.17, 2.92) for change in HB (g/dL) and 1.10 (95% CI: 1.05, 1.18) for WBC at admission (× 103/L). The majority of cases developed AKI within two days of admission. AKI resolved at a median of 2 days (range 1–7 days). The median length of hospitalization stay (LOS) for all patients was 4 days (range 2–35 days). The median and mean length of stay was significantly longer among patients that developed AKI (p<0.0001). (Figure 1a) In addition, the median and mean length of stay was significantly longer among patients that required exchange transfusion (p<0.0001) (Figure 1b) and in patients who required increased respiratory support either intubation or Bipap (p<0.0001) (Figure 1c). As increased respiratory support (intubation) was an expected strong predictor of length of stay, additional analysis on AKI and length of stay was performed with stratification by need for increased respiratory support. Among those who were intubated or required Bipap, the development of AKI was still significantly associated with length of stay (p=0.02). (Figure 1d)
Figure 1.
Length of hospital stay for patients admitted with acute chest syndrome (ACS). a. Total length of hospital stay by acute kidney injury (AKI) b. Total length of hospital stay by type of transfusion (no transfusion, simple RBC transfusion, Red cell pheresis) c. Total length of hospital stay by type of respiratory support (Intubation or Bipap vs no increase in respiratory support) d. Total length of hospital stay by AKI among patients that required Intubation or Bipap
Discussion
This research shows that a subset of pediatric patients with SCD who were admitted for ACS develop AKI during their hospitalizations. Clinicians should monitor for this complication with daily SCr during admission for ACS as it could alter management strategies. At our institution, nephrotoxic agents are often administered to these patients with severe ACS and fever (vancomycin and ibuprofen) or pain (ketorolac) that could potentiate kidney injury.(Add Miller Blood 2011) As protection against pneumococcus from vaccination improves and resistance to third generation cephalosporin stays limited institutional policies and individual clinicians should consider withholding or early discontinuation of vancomycin to treat presumed resistant pneumococcus as a rare pathogen for ACS and consider less aggressive management of fever with ibuprofen or ketorolac in patients that develop AKI.[19]
One variable identified as a strong risk factor for developing AKI was an acute drop in hemoglobin. For every one unit drop in Hb rom baseline to admission, the odds of AKI is estimated to increase by about 81% While prior research has identified severe anemia at baseline as a risk factor for overall disease severity in pediatric SCD, clinicians should also consider an acute drop in hemoglobin from baseline as a risk factor for kidney injury during hospitalization.[20, 21] Patients with acute and sustained drop in hemoglobin during hospitalization are at risk for complicated ICU admissions, stroke and ischemic brain injury, and now AKI.[22–26] Potential mechanisms linking an acute anemic event and AKI include renal ischemia from reduced oxygen carriage or acute increases in plasma free heme/hemoglobin from hemolysis producing direct kidney injury.[27, 28] Future prospective studies should evaluate the relationship between anemia and kidney injury in patients with sickle cell disease. Prior studies of hydroxyurea in pediatrics have suggested a protective effect on progressive sickle cell nephropathy in children with SCD, yet the role of hydroxyurea in preventing AKI has not been explored.[6, 29] While hydroxyurea can prevent admissions for ACS, we were unable to demonstrate that patients prescribed hydroxyurea had statistically lower rates of AKI (2 of 50 cases of AKI) as compared to patients not on disease modifying therapy (10 of 95 cases of AKI).[8] One prior study evaluating the role of hydroxyurea in preventing kidney injury suggests that at steady state, hydroxyurea reduced biomarkers of inflammation and oxidative stress in the kidney.[30] The lack of a statistical advantage for hydroxyurea against AKI may reflect an underpowered retrospective study, poor adherence to hydroxyurea among those patients admitted with ACS, issues inherent in defining AKI by SCr in SCD, or lack of efficacy of hydroxyurea to prevent AKI.
An additional finding suggests that the development of AKI may contribute to possible prolonged hospitalization. The length of hospitalization for ACS frequently results from time to being able to discontinue supplemental oxygenation. Recent research in bi-directional organ cross talk during critical illness offers insight into the association between AKI and Acute Lung Injury. Hypoxemia and acute lung injury due to respiratory failure leads to a decrease in renal blood flow and is a risk factor for AKI.[31, 32] Understanding the pathophysiology of ACS in context of AKI could explain part of the severity of this disease and prolonged need for respiratory support.
The strengths of this study are the use of the most contemporary definition of AKI, clear SCD phenotypes, including standard therapy, and that most kids had known baseline serum creatinine values. Despite these strengths, we acknowledge several limitations to this study by its nature of being a retrospective study. As some patients in this study are seen at satellite sickle cell clinics, a baseline SCr was not performed on all patients admitted to our hospital. Similarly, all laboratory data and clinical records were reviewed from Children’s of Alabama electronic medical records; therefore patients admitted and transferred from an outside emergency room or hospital may not have additional SCr values that we did not evaluate. As some patients in this study did not have daily serum creatinine, it is possible that a few cases of AKI may not have been appropriately captured. Strict urine output was not calculated in most patients, and our definition only uses SCr-based criteria. An inherent concern in AKI reporting in SCD is that serum creatinine may not accurately reflect eGFR. Despite the concern that SCD patients may have a lower serum creatinine, this study utilized the contemporary definition of AKI which incorporates a change in serum creatinine from baseline. Large multi-center studies will be needed to determine if this current definition of AKI predicts short and long term outcomes. A limitation affecting the generalizability of these findings to other institutions is the inclusion of vancomycin at diagnosis of acute chest syndrome. The only adult study identified a similar incidence of AKI (7% in moderate ACS and 14% in severe ACS) but that manuscript did not include their antibiotic regimen for ACS. Finally as we evaluated the most recent admission for patients with ACS in a single center, we identified 12 cases of AKI among 149 unique patients admitted over a six year period. As not all patients with HbSS and SB0 thalassemia develop ACS, this study is limited by a small sample size of patients that developed AKI. Future studies that could better delineate risk factors and outcomes of AKI require a larger, multicenter study.
In conclusion, AKI occurs during hospitalization for ACS and the role of AKI in progression to SCD nephropathy needs further evaluation. We suggest that clinicians monitor for the risk factors of AKI that we identified, acute decline in hemoglobin concentration and leukocytosis, and consider modifying empiric therapy for ACS by limiting nephrotoxic agents and optimizing fluid balance. We are currently conducting a long term prospective study for all SCD patients admitted with ACS to better define AKI and evaluate a potential mechanistic links between ACS, AKI and progression to CKD. With early detection of AKI, better methods for identifying AKI, and modification to therapy for patients with AKI, we hope to improve outcomes in sickle cell nephropathy.
Table 1.
Baseline Characteristics of patients admitted for ACS
| AKI (n=12) | No AKI (n=137) | p-value | |
|---|---|---|---|
| Continuous variables Mean (SD) | |||
| Age (yrs) | 8.7 (5.3) | 9.8 (4.8) | 0.5 |
| Baseline Hb (g/dL) | 8.8 (0.9) | 8.9 (1.6) | 0.96 |
| Admission Hb (g/dL) | 6.4 (2.0) | 7.5 (1.7) | 0.03 |
| Drop in Hb (g/dL) | 2.7(1.9) | 1.4 (1.4) | 0.003 |
| Admission WBC ×109/L | 33.1 (16.2) | 19.8 (9.7) | <0.0001 |
| Admission Platelet count ×109/L | 297 (145) | 332(184) | 0.5 |
| Nominal Variables | |||
| Phenotype | AKI pts | No AKI pts | |
| Severe SCD (SS, SB0 thalassemia, SD) (n=127) | 10 (8%) | 117 (92%) | 0.8 |
| Mild SCD (n=22) | 2 (9%) | 20 (91%) | |
| Disease Modifying Therapy | |||
| No therapy (n=95) | 10 (11%) | 85 (89%) | 0.3 |
| Hydroxyurea (n=50) | 2 (4%) | 48 (96%) | |
| Transfusions (n=4) | 0 (0%) | 4 (100%) | |
ACS=acute chest syndrome, AKI=acute kidney injury, SCD=sickle cell disease
Acknowledgments
J.L would like to acknowledge the NIH (1K23HL127100-01) and American Society of Hematology Scholar Award for funding an ongoing cohort to study progression to CKD. The authors would like to thank the participants living with sickle cell disease as we strive to improve their care. The authors would like to thank the additional members of the UAB Pediatric Sickle Cell team (Lee Hilliard, MD, Christina Bemrich-Stolz, MD, MSPH, Kristen Osborn CRNP, Susan Dobbins, CRNP, Heather Carlton, CRNP, Michelle McCall CRNP, and the SCD clinic nurses) for providing excellent care. The authors also thank the UAB Pediatric Research office for their helpful resources and the Pediatric and Infant Center for Acute Nephrology.
Footnotes
Conflicts of Interest: The authors have no conflict of interest to disclose
References
- 1.Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999–2009) Pediatr Blood Cancer. 2013;60:1482–1486. doi: 10.1002/pbc.24557. [DOI] [PubMed] [Google Scholar]
- 2.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 3.Gosmanova EO, Zaidi S, Wan JY, Adams-Graves PE. Prevalence and progression of chronic kidney disease in adult patients with sickle cell disease. J Investig Med. 2014;62:804–807. doi: 10.1097/01.JIM.0000446836.75352.72. [DOI] [PubMed] [Google Scholar]
- 4.McClellan AC, Luthi JC, Lynch JR, Soucie JM, Kulkarni R, Guasch A, Huff ED, Gilbertson D, McClellan WM, DeBaun MR. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol. 2012;159:360–367. doi: 10.1111/bjh.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM, Johnson C. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med. 1991;115:614–620. doi: 10.7326/0003-4819-115-8-614. [DOI] [PubMed] [Google Scholar]
- 6.Lebensburger J, Johnson SM, Askenazi DJ, Rozario NL, Howard TH, Hilliard LM. Protective role of hemoglobin and fetal hemoglobin in early kidney disease for children with sickle cell anemia. Am J Hematol. 2011;86:430–432. doi: 10.1002/ajh.21994. [DOI] [PubMed] [Google Scholar]
- 7.Miller ST, Wang WC, Iyer R, Rana S, Lane P, Ware RE, Li D, Rees RC, Investigators B-H. Urine concentrating ability in infants with sickle cell disease: baseline data from the phase III trial of hydroxyurea (BABY HUG) Pediatr Blood Cancer. 2010;54:265–268. doi: 10.1002/pbc.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, Rana S, Thornburg CD, Rogers ZR, Kalpatthi RV, Barredo JC, Brown RC, Sarnaik SA, Howard TH, Wynn LW, Kutlar A, Armstrong FD, Files BA, Goldsmith JC, Waclawiw MA, Huang X, Thompson BW investigators B-H. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377:1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Statius van Eps LW, Pinedo-Veels C, de Vries GH, de Koning J. Nature of concentrating defect in sickle-cell nephropathy. Microradioangiographic studies. Lancet. 1970;1:450–452. doi: 10.1016/s0140-6736(70)90836-6. [DOI] [PubMed] [Google Scholar]
- 10.Aygun B, Mortier NA, Smeltzer MP, Hankins JS, Ware RE. Glomerular hyperfiltration and albuminuria in children with sickle cell anemia. Pediatr Nephrol. 2011;26:1285–1290. doi: 10.1007/s00467-011-1857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C Beginning Ending Supportive Therapy for the Kidney I. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 13.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 14.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38:933–939. doi: 10.1097/CCM.0b013e3181cd12e1. [DOI] [PubMed] [Google Scholar]
- 16.Audard V, Homs S, Habibi A, Galacteros F, Bartolucci P, Godeau B, Renaud B, Levy Y, Grimbert P, Lang P, Brun-Buisson C, Brochard L, Schortgen F, Maitre B, Mekontso Dessap A. Acute kidney injury in sickle patients with painful crisis or acute chest syndrome and its relation to pulmonary hypertension. Nephrol Dial Transplant. 2010;25:2524–2529. doi: 10.1093/ndt/gfq083. [DOI] [PubMed] [Google Scholar]
- 17.Kidney Diseases: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guidelines for Acute kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 18.Miller ST. How I treat acute chest syndrome in children with sickle cell disease. Blood. 2011;117:5297–5305. doi: 10.1182/blood-2010-11-261834. [DOI] [PubMed] [Google Scholar]
- 19.De Montalembert M, Abboud MR, Fiquet A, Inati A, Lebensburger JD, Kaddah N, Mokhtar G, Piga A, Halasa N, Inusa B, Rees DC, Heath PT, Telfer P, Driscoll C, Al Hajjar S, Tozzi A, Jiang Q, Emini EA, Gruber WC, Gurtman A, Scott DA. 13-valent pneumococcal conjugate vaccine (PCV13) is immunogenic and safe in children 6–17 years of age with sickle cell disease previously vaccinated with 23-valent pneumococcal polysaccharide vaccine (PPSV23): Results of a phase 3 study. Pediatr Blood Cancer. 2015;62:1427–1436. doi: 10.1002/pbc.25502. [DOI] [PubMed] [Google Scholar]
- 20.Meier ER, Wright EC, Miller JL. Reticulocytosis and anemia are associated with an increased risk of death and stroke in the newborn cohort of the Cooperative Study of Sickle Cell Disease. Am J Hematol. 2014;89:904–906. doi: 10.1002/ajh.23777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebensburger JD, Miller ST, Howard TH, Casella JF, Brown RC, Lu M, Iyer RV, Sarnaik S, Rogers ZR, Wang WC, Investigators BH. Influence of severity of anemia on clinical findings in infants with sickle cell anemia: analyses from the BABY HUG study. Pediatr Blood Cancer. 2012;59:675–678. doi: 10.1002/pbc.24037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowling MM, Quinn CT, Plumb P, Rogers ZR, Rollins NK, Koral K, Buchanan GR. Acute silent cerebral ischemia and infarction during acute anemia in children with and without sickle cell disease. Blood. 2012;120:3891–3897. doi: 10.1182/blood-2012-01-406314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowling MM, Quinn CT, Rogers ZR, Buchanan GR. Acute silent cerebral infarction in children with sickle cell anemia. Pediatr Blood Cancer. 2010;54:461–464. doi: 10.1002/pbc.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cecchini J, Lionnet F, Djibre M, Parrot A, Stojanovic KS, Girot R, Fartoukh M. Outcomes of adult patients with sickle cell disease admitted to the ICU: a case series*. Crit Care Med. 2014;42:1629–1639. doi: 10.1097/CCM.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 25.Bernaudin F, Verlhac S, Arnaud C, Kamdem A, Vasile M, Kasbi F, Hau I, Madhi F, Fourmaux C, Biscardi S, Epaud R, Pondarre C. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood. 2015;125:1653–1661. doi: 10.1182/blood-2014-09-599852. [DOI] [PubMed] [Google Scholar]
- 26.Wierenga KJ, Serjeant BE, Serjeant GR. Cerebrovascular complications and parvovirus infection in homozygous sickle cell disease. J Pediatr. 2001;139:438–442. doi: 10.1067/mpd.2001.117070. [DOI] [PubMed] [Google Scholar]
- 27.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol. 2007;18:414–420. doi: 10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- 28.Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol. 2013;88:116–119. doi: 10.1002/ajh.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.dos Santos TE, Goncalves RP, Barbosa MC, da Silva GB, Jr, de Daher EF. Monocyte chemoatractant protein-1: a potential biomarker of renal lesion and its relation with oxidative status in sickle cell disease. Blood Cells Mol Dis. 2015;54:297–301. doi: 10.1016/j.bcmd.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Kilburn KH, Dowell AR. Renal function in respiratory failure. Effects of hypoxia, hyperoxia, and hypercapnia. Arch Intern Med. 1971;127:754–762. [PubMed] [Google Scholar]
- 32.Basu RK, Wheeler DS. Kidney-lung cross-talk and acute kidney injury. Pediatr Nephrol. 2013;28:2239–2248. doi: 10.1007/s00467-012-2386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]