Abstract
Objective
To determine the impact of MRSA/VRE designations, or flags, on selected hospital operational outcomes.
Design
Retrospective cohort study of inpatients admitted to the Massachusetts General Hospital during 2010–2011.
Methods
Operational outcomes were time to bed arrival, acuity-unrelated within-hospital transfers, and length of stay. Demographic and clinical characteristics – including age, gender, severity of illness on admission, admit day of week, residence prior to admission, hospitalization within the prior 30 days, clinical service, and discharge destination – were used as covariates.
Results
A total of 81,288 admissions were included. After adjusting for covariates, patients with a MRSA/VRE flag at the time of admission experienced a mean delay in time to bed arrival of 1.03 (9.63 [95% CI 9.39–9.88] hours vs. 8.60 [95% CI 8.47–8.73] hours); had 1.19 times the odds [95% CI, 1.13–1.26] of experiencing an acuity-unrelated within-hospital transfer, and experienced a mean length of stay 1.76 days longer (7.03 [95% CI 6.82–7.24] days vs. 5.27 [95% CI 5.15–5.38] days) compared to patients with no MRSA/VRE flag.
Conclusions
MRSA/VRE designation was associated with delays in time to bed arrival, increased likelihood of acuity-unrelated within-hospital transfers, and extended length of stay. Efforts to identify patients who have cleared MRSA/VRE colonization are critically important to mitigate inefficient use of resources and improve inpatient flow.
Keywords: VRE, MRSA, length of stay, infection control
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) are endemic in hospital settings and long-term care facilities, and the prevalence of colonization is increasing.1,2 When admitted to hospitals, it is recommended that patients with a MRSA/VRE designation be placed in either a single-occupancy room or cohorted with another patient with the same designation in a double-occupancy room.3,4 Few studies estimate the operational impact of MRSA/VRE designation, though limited studies based on either survey data or small retrospective studies suggest that the MRSA/VRE label may affect patient movement in the hospital through delays in bed assignments5,6 and within-hospital transfers,7 as well as disposition, through delayed discharge to post-acute care facilities.8,9 In hospitals with double-occupancy accommodations, the additional requirement to match patients on MRSA/VRE designation can introduce inefficiencies when ready matches are not available and patients must queue. In institutions with uniformly single-occupancy accommodations, the impact of MRSA/VRE designation remains relevant through discharge disposition and costs of implementation of contact precautions. We assembled a large data repository to examine the association between MRSA/VRE designation and time to bed arrival, acuity-unrelated within-hospital transfers, and length of stay. We hypothesized that these measures of operational efficiency would be adversely affected by the MRSA/VRE designation.
METHODS
Data Sources and Variables
The study utilized a novel data warehouse created through merging several clinical and administrative databases generating complete records for inpatient admissions to the Massachusetts General Hospital (MGH) during 2010–2011, including: MRSA/VRE flag status on admission, age, gender, residence, recent hospitalizations, admitting clinical service, discharge destination, and length of stay in each patient location.
Hospital Structure
Between January 2010 and September 2011, the MGH had 782 adult licensed beds (excluding obstetrics and psychiatry). There were six adult intensive care units (ICUs) accounting for 98 single-occupancy beds (64 surgical, 34 medical), two step-down units accounting for 57 beds, 23 general care units accounting for 613 beds (294 surgical, 319 medical), and an observation unit with 14 beds. All ICUs feature only single-occupancy rooms. Outside of ICUs, 31% of beds were single-occupancy with the remainder double-occupancy. In September 2011, a new inpatient building opened, increasing the number of adult licensed beds by 26 to 808 (excluding obstetrics and psychiatry); the overall proportion of double-occupancy rooms decreased from 60% to 50% for the final 4 months of the study; overall hospital occupancy remained stable. The MGH operates at occupancy levels well above national esimates10 and in comparison to other academic teaching hospitals.11
Study Sample
The study sample was restricted to adult medical, surgical, and observation inpatient encounters completed during the 2010–2011 study period (N=81,288, Figure 1).
Figure 1.
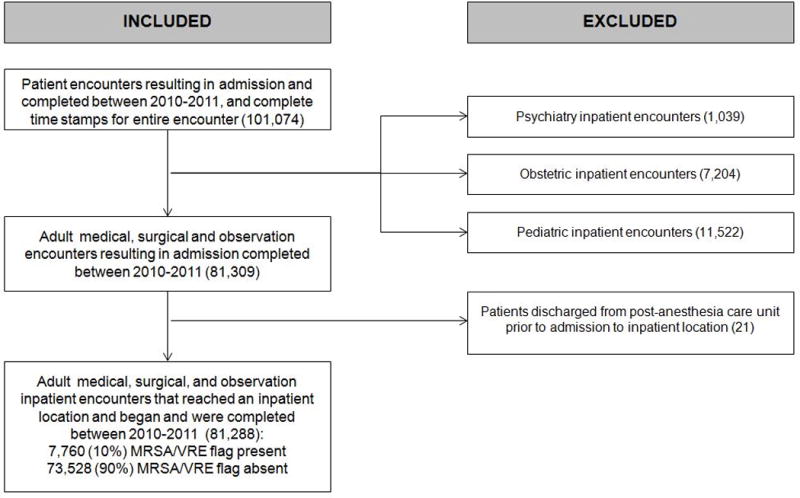
Flow diagram of patient inclusion for analyses. The data warehouse includes all patient encounters at the Massachusetts General Hospital between 2010–2011. A subset of the cohort, inclusive of all patient encounters resulting in admissions completed between January 1, 2010 and December 31, 2011 for which complete time stamps were available documenting patient movement, were included in the analyses. The final sample excluded patients admitted to inpatient psychiatry, obstetric, and pediatric services as well as patients who were discharged to home directly from a post-anesthesia care unit.
MRSA/VRE Flags
Patients with a MRSA/VRE flag on admission were identified, or “flagged”, within the hospital’s electronic health record (EHR). Flag status was defined by an absent or present flag within 48 hours of admission. Patients flagged after 48 hours were considered to be in the no flag category. Institutional policy for active surveillance included culture-based surveillance on admission for MRSA and VRE for ICU patients and those admitted to specific high-risk units. Although a hospital protocol was in place for screening and deflagging of both MRSA- and VRE-flagged patients, both were implemented infrequently.12
Study Outcomes
Three statistical outcomes of interest were assessed: mean time to bed arrival, the likelihood of experiencing acuity-unrelated within-hospital transfers, and mean length of stay. The adjusted geometric means were reported to reflect the non-normal distribution for time to bed arrival and length of stay.
Time to Bed Arrival
Time to bed arrival was defined as the time in hours until a patient reached their first inpatient bed. The first stamp recorded for patients entering the bed queue was considered to be the beginning of this process. For Emergency Department (ED) patients, post-operative patients, direct admission, and transfer patients, these times corresponded to: registration in the ED, time of admission to the post-anesthesia care unit, arrival in the admissions office, and registration and initiation of bed placement prior to physical transfer, respectively. The statistical summary outcome for this variable was mean time to bed arrival in hours.
Within-Hospital Transfers
Within-hospital transfers were defined as a physical move from one inpatient hospital location to another. Acuity-unrelated transfers were identified as two consecutive inpatient beds matching in acuity level (i.e. a transfer not resulting from a change in acuity level). Transfers were defined as a binary variable, categorizing each patient encounter as having experienced, or not experienced any acuity-unrelated transfer. Acuity-related transfers were not included in this analysis, as the odds of experiencing such moves are dominated by acuity on admission (data not shown). This analysis was focused on acuity-unrelated transfers as this phenomenon encompasses efforts to optimize use of single- and double-occupancy accommodations. The statistical summary outcome presented was likelihood of experiencing any acuity-unrelated transfer.
Patient Length of Stay
Length of stay is number of days a patient remains in the hospital from arrival in their initial bed to discharge. The statistical summary outcome for this variable was mean length of stay in days.
Study Predictors
The primary predictor of interest was MRSA/VRE flag status. Patients were categorized having a MRSA/VRE flag on admission or no flag for MRSA/VRE on admission. A flag for MRSA, VRE, or both, were grouped together as having a MRSA/VRE flag. Covariates, many of which were included in multivariate models to account for patient severity of illness during the hospitalization, were: age, gender, severity of illness (acuity) on admission, admit day of week, residence, hospitalization at the same institution within previous 30 days, admitting clinical service, and discharge destination. Acuity on admission was inferred from the patient’s initial admission location—either observation unit, general care unit, step-down unit, or intensive care unit (ICU). This proxy measure of patient severity of illness was utilized because it corresponded most readily to staffing levels and available support services considered indicators of patient acuity. Residence was noted as either home or facility. Prior hospitalization in the preceding 30 days was included as well as a proxy for patient severity of illness. Admitting clinical service was defined as either surgical or medical. Discharge destination was categorized as either to home, a facility, or deceased and was considered an additional proxy measure of patient severity of illness.
Statistical Analysis
Baseline characteristics of the cohort were summarized using counts and proportions, mean ± standard deviation, or median with lower and upper quartiles as appropriate. Univariate models were initially fit to describe the unadjusted associations between MRSA/VRE flag status and each of the study outcomes, and these associations were adjusted, by multivariate models, for the impacts of the covariates. For the adjusted analyses, associations of MRSA/VRE status with the likelihoods of transfer was modeled using multivariate logistic models, and those with length of stay and time to bed arrival were modeled by using exponential models for time-to-event outcomes, and Least-Squares means (LSMEANS) were reported.13
RESULTS
Patient and Admission Characteristics
Of 81,288 patient admissions included in the analysis, 7,760 (10%) were admitted with a flag and 73,528 (90%) were admitted with no flag (Table 1). The majority of admissions were via the ED (65%), followed by PACU (25%), direct admissions (6%) and transfers (4%). The route of admission did not influence the study outcomes (data not shown). Patients with a flag at admission were less often female (43% vs. 49%) and older (64 vs. 60 years) compared to patients without a flag. A larger proportion of flagged patients were admitted to an ICU (12% vs. 8%), admitted from a facility (19% vs. 10%), hospitalized at the same institution within the previous 30 days (39% vs. 19%), admitted to a medical rather than surgical service (65% vs. 53%), and were discharged to a facility (19% vs. 15%) or died during their hospitalization (5% vs. 2%) compared to patients without flags. Patients with flags had a longer mean time to bed arrival (10 ± 7 vs 9 ± 6 hours) compared to patients without flags. A larger proportion of flagged patients experienced any within-hospital transfer (39% vs. 30%). Flagged patients had more of both acuity-related within-hospital transfers (19% vs. 16%) and acuity-unrelated transfers (27% vs. 20%) compared to those without a flag. Patients with a flag had a longer total mean length of stay (7 ± 8 days vs. 5 ± 6 days) and length of stay spent in mixed-occupancy units (6 ± 7 days vs. 4 ± 5 days).
Table 1.
Patient cohort characteristics (N=81,288)
| Overall | MRSA/VRE Flag Status | ||
|---|---|---|---|
| MRSA/VRE Flag1 | No flag for MRSA/VRE | ||
| N | 81,288 | 7,760 (10%) | 73,528 (90%) |
| Gender (N, % female) | 39,596 (49%) | 3,352 (43%) | 36,244 (49%) |
| Age (mean ± SD) in years | 60 ± 18 | 64 ± 18 | 60 ± 18 |
| Severity of illness (acuity) on admission (N, %) | |||
| Observation Unit | 12,395 (15%) | 599 (8%) | 11,796 (16%) |
| General Care Unit | 55,582 (68%) | 5,780 (74%) | 49,802 (68%) |
| Step-Down Unit | 6,196 (8%) | 457 (6%) | 5,739 (8%) |
| Intensive Care Unit | 7,115 (9%) | 924 (12%) | 6,191 (8%) |
| Residence prior to admission (N, %) | |||
| Home | 72,514 (89%) | 6,272 (81%) | 66,242 (90%) |
| Facility | 8,774 (11%) | 1,488 (19%) | 7,286 (10%) |
| Hospitalization within previous 30 days (N, %) | 16,921 (21%) | 3,033 (39%) | 13,888 (19%) |
| Clinical service (N, %) | |||
| Surgical | 36,966 (45%) | 2,688 (35%) | 34,278 (47%) |
| Medical | 44,322 (55%) | 5,072 (65%) | 39,250 (53%) |
| Discharge destination (N, %) | |||
| Home | 66,479 (82%) | 4,852 (63%) | 61,627 (84%) |
| Facility | 13,242 (16%) | 2,541 (19%) | 10,701 (15%) |
| Death | 1,567 (2%) | 367 (5%) | 1,200 (2%) |
| Time to bed arrival in hours (mean ± SD; geometric mean; median [25th–75th percentiles])2 | 9 ± 7 8 8 [5–11] |
10 ± 7 8 8 [5–12] |
9 ± 6 8 8 [5–11] |
| Occurrence of within-hospital transfers (N, %)2 | |||
| No transfers | 56,036 (69%) | 4,756 (61%) | 51,280 (70%) |
| Any transfers1 | 25,252 (31%) | 3,004 (39%) | 22,248 (30%) |
| Acuity-related transfers | 13,050 (16%) | 1,475 (19%) | 11,575 (16%) |
| Acuity-unrelated transfers | 16,566 (20%) | 2,126 (27%) | 14,440 (20%) |
| Length of stay in days (mean ± SD; geometric mean; median [25th 75th percentiles])2 | |||
| Total | 5 ± 6 3 3 [1–6] |
7 ± 8 5 5 [3–9] |
5 ± 6 3 3 [1–5] |
| Spent in double-occupancy units | 4 ± 5 2 3 [1–5] |
6 ± 7 4 4 [2–8] |
4 ± 5 2 2 [1–5] |
Of the 10% of patients with the MRSA/VRE flag (N=7,760), 38% (N= 2,949) had a history of MRSA, 41% (N= 3,181) had a history of VRE, and 21% (N= 1,630) had a history of both MRSA and VRE.
There was no significant difference in time to bed arrival, occurrence of within-hospital transfers or length of stay during the study period prior to the new inpatient building opening (1/1/2010–9/7/2011) and afterwards (9/8/2011–12/31/2011).
Patients contributing to the frequency of “Any transfers” may contribute to either OR both of the “Acuity-related transfers” or “Acuity-unrelated transfers” categories.
Factors Influencing Time to Bed Arrival
In the unadjusted model, patients with a flag on admission experienced an excess mean time to bed arrival of 47 minutes (10.14 [95% CI 9.92–10.37] vs. 9.36 [95% CI 9.29–9.43] hours). In the multivariate model, flagged patients had an excess mean time to bed arrival of 62 minutes (9.63 [95% CI 9.39–9.88] vs. 8.60 [95% CI 8.47–8.73] hours) compared to patients with no flag for MRSA/VRE (Table 2). This effect exceeded the estimated impact of gender, age, day of week of admission, residence prior to admission, and recent hospitalization. Patient severity of illness on admission, and admitting clinic service were associated with significant and substantial effects on time to bed arrival. Among acuity levels, the time to bed arrival for step-down unit beds was the longest, at 14.71 hours. Patients requiring surgical beds experienced close to a 2 hour delay in bed arrival compared to patients awaiting medical beds.
Table 2.
Factors influencing time to bed arrival
| Factors | LS mean (95% CI, hrs) | Adjusted increase in time to bed arrival, compared to referent group* | p value |
|---|---|---|---|
| MRSA/VRE Flag Status | |||
| MRSA/VRE Flag1 | 9.63 (9.39–9.88) | 62 Minutes | <.0001 |
| No flag for MRSA/VRE | 8.60 (8.47–8.73) | Ref | |
| Gender | |||
| Female | 9.29 (9.12–9.47) | 23 Minutes | <.0001 |
| Male | 8.92 (8.76–9.08) | Ref | |
| Age | |||
| < 65 years | 9.29 (9.12–9.46) | 22 Minutes | <.0001 |
| ≥ 65 years | 8.92 (8.76–9.09) | Ref | |
| Acuity on admission | |||
| Observation Unit | 6.25 (6.10–6.41) | Ref | <.0001 |
| General Care Unit | 10.20 (10.04–10.37) | 4 Hours | |
| Step-Down Unit | 14.71 (14.29–15.15) | 8 Hours 30 Minutes | |
| Intensive Care Unit | 7.31 (7.12–7.51) | 1 Hour | |
| Admit day of week | |||
| Weekday (M–F) | 9.37 (9.21–9.53) | 32 Minutes | <.0001 |
| Saturday/Sunday | 8.84 (8.66–9.03) | Ref | |
| Residence prior to admission, home | |||
| No | 9.12 (8.90–9.34) | 2 Minutes | 0.7666 |
| Yes | 9.09 (8.94–9.23) | Ref | |
| Hospitalization within previous 30 days | |||
| No | 9.03 (8.88–9.19) | Ref | 0.0864 |
| Yes | 9.17 (8.98–9.36) | 8 Minutes | |
| Admitting inpatient service | |||
| Surgical | 10.14 (9.94–10.34) | 2 Hours | <.0001 |
| Medical | 8.17 (8.03–8.32) | Ref |
LS Mean: multivariate model based predicted mean, the average of predicted marginal mean over the classes of the simultaneously controlled covariates. Unadjusted observed means for MRSA/VRE flag status were 10.14 [95% CI 9.92–10.37] for patients with a flag and 9.36 [95% CI 9.29–9.43] for patients with no flag, which is an adjusted increase of 47 minutes for patients with a flag on admission (data not shown).
Length of delay was rounded to the nearest integer
MRSA flag, VRE flag, and both MRSA and VRE flag were associated with an excess mean time to bed arrival of 55 minutes, 51 minutes, and 95 minutes, compared to patients with no flag, respectively.
Factors Influencing Within-Hospital Transfers
Patients with a MRSA/VRE flag on admission had 1.55 the odds [95% CI: 1.47–1.36] of experiencing an acuity-unrelated transfer compared to patients without the flag in the unadjusted model. In the multivariate model, flagged patients had 1.19 times the odds [95% CI: 1.13–1.26] of experiencing an acuity-unrelated transfer compared to patients with no flag for MRSA/VRE (Table 3). Considering patients admitted to general care units as the referent population, the odds of experiencing such transfers was similar to that of patients admitted to ICUs (1.24 [95% CI 1.16–1.31]), although less than that attributable to admission to a step-down unit (1.41 [95%CI 1.32–1.5]). Clinical service had minimal influence on acuity-unrelated transfers. Considering patients discharged to home as the referent population, the odds of experiencing an acuity-unrelated transfer were 2.23 [95% CI 2.13–2.33] for patients ultimately discharged to a facility. As patients with longer lengths of stay would be expected to have a greater likelihood of ever experiencing an acuity-unrelated transfer, we stratified the analysis by encounters with length of stay in double-occupancy units (the time during which patients are at risk for experiencing acuity-unrelated transfers). For encounters with less than 24 hours, flagged patients had 0.754 times the odds [95% CI 0.587–0.970] of experiencing acuity-unrelated within-hospital transfers compared to patients with no flag for MRSA/VRE, however, for encounters with 24 or more hours in double-occupancy units, flagged patients had 1.19 times the odds [95% CI 1.12–1.26] of experiencing acuity-unrelated transfers compared to patients with no flag for MRSA/VRE.
Table 3.
Factors influencing acuity-unrelated within-hospital transfers
| Factors | Adjusted OR | 95% CI | p value | |
|---|---|---|---|---|
| MRSA/VRE Flag Status | ||||
| MRSA/VRE Flag1 | 1.19 | 1.13 | 1.26 | <.0001 |
| No flag for MRSA/VRE | Ref | |||
| Severity of illness (acuity) on admission | ||||
| Observation Unit | 0.68 | 0.64 | 0.72 | <.0001 |
| General Care Unit | Ref | |||
| Step-Down Unit | 1.41 | 1.32 | 1.50 | <.0001 |
| Intensive Care Unit | 1.24 | 1.16 | 1.31 | <.0001 |
| Clinical service | ||||
| Surgical | Ref | <.0001 | ||
| Medical | 1.08 | 1.04 | 1.12 | |
| Discharge destination | ||||
| Home | Ref | |||
| Facility | 2.23 | 2.13 | 2.33 | <.0001 |
| Death | 1.62 | 1.45 | 1.82 | 0.1422 |
Adjusted ORs were also controlled for gender, age, admit day of week, residence prior to admission and hospitalization within the previous 30 days (data not shown). Unadjusted OR for MRSA/VRE flag status of flag vs. no flag was 1.55 [95% CI: 1.47 – 1.63] (data not shown).
The odds of experiencing acuity-unrelated transfers for patients with a MRSA flag, VRE flag, and both MRSA and VRE flag were 1.24, 1.27, and 0.98, compared to patients with no flag, respectively.
Factors Influencing Length of Stay
In the unadjusted model, patients with a flag on admission experienced an excess length of stay of 2 days and 22 hours (2.86 days, 6.99 [95% CI 6.84–7.15] vs. 4.13 [95% CI 4.10–4.16] days). In the multivariate model, flagged patients had an excess mean length of stay 1 day and 18 hours longer (1.76 days, 7.03 [95% CI 6.82–7.24] vs. 5.27 [95% CI 5.15–5.38] days) compared to patients with no flag for MRSA/VRE after (Table 4). This excess attributable length of stay was greater than that attributable to age, residence, prior hospitalization, and clinical service. The greatest impacts were patient severity of illness on admission and discharge destination. Considering observation unit patients as the referent population, patients requiring general care unit, step-down unit, or ICU level care on admission had extended hospitalizations of 5 days 4 hours, 5 days 7 hours, and 10 days 17 hours, respectively. Similarly, considering discharge to home as the referent category, patients discharged to facilities or who died during the admission had excess length of stay of 4 days 7 hours and 3 days 16 hours, respectively.
Table 4.
Factors influencing length of stay
| Factors | LS mean (95% CI, days) | Adjusted increase in length of stay, as compared to referent group* | p value |
|---|---|---|---|
| MRSA/VRE Flag Status | |||
| MRSA/VRE Flag1 | 7.03 (6.83–7.24) | 1 day 18 hours | <.0001 |
| No flag for MRSA/VRE | 5.27 (5.15–5.38) | Ref | |
| Age | |||
| < 65 years | 6.00 (5.85–6.14) | Ref | <.0001 |
| ≥ 65 years | 6.17 (6.03–6.32) | 4 hours | |
| Severity of illness (acuity) on admission | |||
| Observation Unit | 2.04 (1.98–2.10) | Ref | <.0001 |
| General Care Unit | 7.22 (7.06–7.39) | 5 days 4 hours | |
| Step-Down Unit | 7.31 (7.08–7.55) | 5 days 7 hours | |
| Intensive Care Unit | 12.73 (12.35–13.11) | 10 days 17 hours | |
| Residence prior to admission, home | |||
| No | 6.31 (6.13–6.49) | 11 hours | <.0001 |
| Yes | 5.86 (5.73–6.00) | Ref | |
| Hospitalization within previous 30 days | |||
| No | 5.72 (5.59–5.85) | Ref | <.0001 |
| Yes | 6.47 (6.30–6.64) | 18 hours | |
| Clinical service | |||
| Surgical | 5.46 (5.33–5.60) | Ref | <.0001 |
| Medical | 6.77 (6.62–6.93) | 1 day 7 hours | |
| Discharge destination | |||
| Home | 3.77 (3.70–3.84) | Ref | <.0001 |
| Facility | 8.05 (7.87–8.23) | 4 days 7 hours | |
| Death | 7.43 (7.06–7.82) | 3 days 16 hours |
LS Mean: multivariate model based predicted mean, the average of predicted marginal mean over the classes of the simultaneously controlled covariates. The multivariate model also controlled for gender and admit day of week. Unadjusted observed means for MRSA/VRE flag status were 6.99 [95% CI 6.84–7.15] for patients with a flag and 4.13 [95% CI 4.10–4.16] for patients with no flag, which is an adjusted increase of 2 days and 21 hours for patients with a flag on admission (data not shown).
Length of increase in length of stay was rounded to the nearest integer
MRSA flag, VRE flag, and both MRSA and VRE flag were associated with an excess length of stay of 17 hours, 2 days and 3 hours, and 1 day and 14 hours, respectively, compared to patients with no flag.
DISCUSSION
We used a large retrospective cohort of admissions to examine the relationship between MRSA/VRE designation and selected operational outcomes and found that patients admitted with MRSA/VRE flag compared to those without a MRSA/VRE flag experienced a longer time to bed arrival, increased likelihood of acuity-unrelated within-hospital transfers, and extended length of stay. These analyses quantify what clinicians and hospital administrators have understood intuitively: that the MRSA/VRE designation affects operational efficiency.
The excess time to bed arrival of 1 hour associated with the MRSA/VRE flag is operationally notable, and potentially clinically significant. This delay may be explained by the additional time required to match such patients based on colonization status5 and is consistent with at least one other study.6 Some studies have demonstrated an association between length of emergency department boarding of patients and mortality, increased overall length of stay,14 medication delays, and adverse events.15,16
The nearly 20% increase in odds for MRSA/VRE flagged patients experiencing an acuity-unrelated within-hospital transfer may be the result of the practice of “bed moves,” or transfers of patients to optimize use of available beds, particularly for double-occupancy accommodations. Because such transfers are attributed only to the patient who experienced the event and not to the patient triggering the transfer or series of transfers, it is possible that this finding underestimates the impact of the flag designation. Within-hospital transfers are burdensome to both patients and staff and may represent an inefficient use of resources and potentially may contribute to patient harm17 and excess costs.18 At times during which hospitals are operating at very high occupancy, such potentially avoidable transfers may further affect the flow of patients. The frequency and operational impact of acuity-unrelated transfers, however, will depend on the specific combination of bedding arrangements across varying levels of acuity and services, an analysis which is beyond the scope of this study, and which is better suited to simulation approaches.
Our findings for length of stay highlight the need for mechanisms to mitigate the impact of the MRSA/VRE designation to improve patient flow in the hospital. The factors that result in this extended length of stay are not known with certainty, but it is possible that the flag, through delays in delivery of care, adverse events, or other sequellae, results in overall less efficient care. Over the past several decades, length of stay for large nonfederal community hospitals has declined from 9.1 to 5.7 days.10 To the extent that a substantial portion of a patient’s length of stay is associated with MRSA/VRE flag status, this represents a need for focused efforts to limit the operational impact of the flag, such as programs to document clearance of colonization, and removal of the MRSA/VRE designation. We have previously demonstrated the efficacy12 and effectiveness19 of this approach for MRSA. Assuming half of the cohort had cleared colonization at the time of admission20 and the excess length of stay predicted by the model, a substantial increase in available patient days could be realized. Further, it is possible that administrative delays due to lack of single-occupancy accomodations at post-acute care facilities contribute to observed length of stay among flagged patients.
A growing body of evidence demonstrates that the duration of colonization with MRSA and VRE is not life-long,21–26 and possibly much shorter than previously believed, even in the setting of recent infection.20,27–29 There are no consensus guidelines to inform the appropriate time interval to wait prior to screening, the anatomical sites to screen, specific screening assay, number of screens, and interpretation of the results in the presence of antibiotics with activity against MRSA or VRE.30 In the absence of clear guidance we have previously demonstrated widespread variation in contact precautions discontinuation protocols, although the majority rely on passive surveillance, effectively resulting in a persistent MRSA/VRE designation for patients previously identified as infected or colonized with MRSA or VRE.5 Thus, the persistence of the MRSA/VRE flag represents a potential target to reduce barriers to patient flow throughout the hospital. In fact, de-flagged patients have fewer associated idle beds.19
The study was conducted at a large tertiary care medical center with long-standing use of the EHR to document MRSA/VRE flag status. Thus, in settings in which MRSA/VRE flag status is not as prominently displayed, or not displayed at all, our findings may not be as compelling. Our institution additionally includes flags for multidrug-resistant gram-negative organisms and Clostridium difficile infection, not evaluated in the current study. Patients with these flags were grouped in the no flag group, which would be expected to bias findings towards the null. The outcomes addressed—time to bed arrival, acuity-unrelated within hospital transfers, and length of stay—are influenced by hospital structure, including the number, acuity levels, and types of beds, and the proportions of beds with specific characteristics. Despite a lack of consensus in the literature regarding the economic, operational, and clinical tradeoffs between single- and double-occupancy accommodations,17,31,32 there is no doubt that double-occupancy accommodations introduce inefficiencies through matching requirements—inefficiencies that may manifest in delays to bed assignment, patient transfers and prolonged hospital stays. The findings reported here are not immediately transferrable to any one individual hospital. In addition to hospital structure, the patient population analyzed likely influenced our findings. Although this factor may limit generalizability of the findings, the proportion of patients identified as MRSA/VRE on admission is within the range of prevalence reported previously.2,33,34 MGH operates at consistently high patient census, and thus the impact of flag prevalence, combined with hospital structure, may be more pronounced. This study relied on a proxy measures for patient acuity, which are likely to incompletely characterize patient severity of illness. These data are, however, often those most readily available in administrative sources used for large cohort analyses.
We found that MRSA/VRE designation was associated with operational consequences, and additional mechanisms to efficiently identify patients no longer colonized with MRSA/VRE are warranted. This need is especially true as EHRs begin to fulfill the promise of improving exchange of administrative and clinical information across the care continuum, raising the stakes for ensuring the validity of that information.
Acknowledgments
Financial Support
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (K01AI110524 to E.S.S.). This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Authorship and manuscript preparation
The authors would like to thank Katie Capallo, Kathryn M. Turcotte, MBA, and Benjamin Orcutt of the Massachusetts General Hospital Admitting Services Department; Victor Grishkan, Kirill Boyarin and Joy Boulware of Information Systems and eMAR and Aaron Sacco of the MGH Pharmacy Department for their assistance in identifying antibiotic data; William Driscoll, MA and Milcho Nikolov, MSEE of the Department of Anesthesia, Critical Care and Pain Medicine; Jessica Cotter, MPH and Lauren R. West, MPH of the Massachusetts General Hospital Infection Control Unit; and Joseph Newhouse, PhD of the Harvard University Division of Health Policy Research and Education.
Conflict of Interest
All authors report no conflict of interest.
References
- 1.David MZ, Medvedev S, Hohmann SF, Ewigman B, Daum RS. Increasing burden of methicillin-resistant Staphylococcus aureus hospitalizations at US academic medical centers, 2003–2008. Infect Control Hosp Epidemiol. 2012;33:782–789. doi: 10.1086/666640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarvis WR, Jarvis AA, Chinn RY. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at United States health care facilities, 2010. Am J Infect Control. 2012;40:194–200. doi: 10.1016/j.ajic.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2007;35:S165–93. doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Siegel JD, Rhinehart E, Jackson M, Chiarello L. Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:10-S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shenoy ES, Walensky RP, Lee H, Orcutt B, Hooper DC. Resource burden associated with contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: The patient access managers’ perspective. Infect Control Hosp Epidemiol. 2012;33:849–852. doi: 10.1086/666629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLemore A, Bearman G, Edmond MB. Effect of contact precautions on wait time from emergency room disposition to inpatient admission. Infect Control Hosp Epidemiol. 2011;32:298–299. doi: 10.1086/658913. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DW, Schmidt UH, Bittner EA, Christensen B, Levi R, Pino RM. Delay of transfer from the intensive care unit: a prospective observational study of incidence, causes, and financial impact. Crit Care. 2013;17:R128. doi: 10.1186/cc12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryce EA, Tiffin SM, Isaac-Renton JL, Wright CJ. Evidence of delays in transferring patients with methicillin-resistant Staphylococcus aureus or vancomycin-resistant Enterococcus to long-term-care facilities. Infect Control Hosp Epidemiol. 2000;21:270–271. doi: 10.1086/501757. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds C, Kim D, Kaplan SH, et al. Are nursing homes less likely to admit methicillin-resistant Staphylococcus aureus carriers? Am J Infect Control. 2014;42:63–65. doi: 10.1016/j.ajic.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics. Health, United States, 2014: With Special Feature on Adults Aged 55-64. Hyattsville, MD: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2015. [Google Scholar]
- 11.Council of Teaching Hospitals and Health Systems. COTH Quarterly Survey of Hospital Operations & Financial Performance. Washington, DC: Association of American Medical Colleges; 2013. [Google Scholar]
- 12.Shenoy ES, Kim J, Rosenberg ES, et al. Discontinuation of contact precautions for methicillin-resistant Staphylococcus aureus: a randomized controlled trial comparing passive and active screening with culture and polymerase chain reaction. Clin Infect Dis. 2013;57:176–184. doi: 10.1093/cid/cit206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: An alternative to least squares means. American Statstician. 1980;34:216–221. [Google Scholar]
- 14.Singer AJ, Thode HC, Jr, Viccellio P, Pines JM. The association between length of emergency department boarding and mortality. Acad Emerg Med. 2011;18:1324–1329. doi: 10.1111/j.1553-2712.2011.01236.x. [DOI] [PubMed] [Google Scholar]
- 15.Sri-On J, Chang Y, Curley DP, et al. Boarding is associated with higher rates of medication delays and adverse events but fewer laboratory-related delays. Am J Emerg Med. 2014;32:1033–1036. doi: 10.1016/j.ajem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Liu SW, Chang Y, Weissman JS, et al. An empirical assessment of boarding and quality of care: delays in care among chest pain, pneumonia, and cellulitis patients. Acad Emerg Med. 2011;18:1339–1348. doi: 10.1111/j.1553-2712.2011.01082.x. [DOI] [PubMed] [Google Scholar]
- 17.Detsky ME, Etchells E. Single-patient rooms for safe patient-centered hospitals. JAMA. 2008;300:954–956. doi: 10.1001/jama.300.8.954. [DOI] [PubMed] [Google Scholar]
- 18.Bobrow M, Thomas J. Inpatient care facilities. In: Kobus RL, Skaggs RL, Bobrow M, Thomas J, Payette TM, Kliment SA, editors. Building type basics for healthcare facilities. New York: John Wiley and Sons; 2000. pp. 131–192. [Google Scholar]
- 19.Shenoy ES, Lee H, Cotter JA, et al. Impact of rapid screening for discontinuation of methicillin-resistant Staphylococcus aureus contact precautions. Am J Infect Control. 2015 doi: 10.1016/j.ajic.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shenoy ES, Paras ML, Noubary F, Walensky RP, Hooper DC. Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE): a systematic review. BMC Infect Dis. 2014;14:177-2334–14-177. doi: 10.1186/1471-2334-14-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39:971–979. doi: 10.1086/423965. [DOI] [PubMed] [Google Scholar]
- 22.Scanvic A, Denic L, Gaillon S, Giry P, Andremont A, Lucet JC. Duration of colonization by methicillin-resistant Staphylococcus aureus after hospital discharge and risk factors for prolonged carriage. Clin Infect Dis. 2001;32:1393–1398. doi: 10.1086/320151. [DOI] [PubMed] [Google Scholar]
- 23.Cretnik TZ, Vovko P, Retelj M, et al. Prevalence and nosocomial spread of methicillin-resistant Staphylococcus aureus in a long-term-care facility in Slovenia. Infect Control Hosp Epidemiol. 2005;26:184–190. doi: 10.1086/502524. [DOI] [PubMed] [Google Scholar]
- 24.Byers KE, Anglim AM, Anneski CJ, Farr BM. Duration of colonization with vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2002;23:207–211. doi: 10.1086/502036. [DOI] [PubMed] [Google Scholar]
- 25.Park I, Park RW, Lim SK, et al. Rectal culture screening for vancomycin-resistant Enterococcus in chronic haemodialysis patients: false-negative rates and duration of colonization. J Hosp Infect. 2011;79:147–150. doi: 10.1016/j.jhin.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Yoon YK, Lee SE, Lee J, et al. Risk factors for prolonged carriage of vancomycin-resistant Enterococcus faecium among patients in intensive care units: a case-control study. J Antimicrob Chemother. 2011;66:1831–1838. doi: 10.1093/jac/dkr204. [DOI] [PubMed] [Google Scholar]
- 27.Cluzet VC, Gerber JS, Nachamkin I, et al. Duration of colonization and determinants of earlier clearance of colonization with methicillin-resistant Staphylococcus aureus. Clinical Infectious Diseases. 2015;60:1489–1496. doi: 10.1093/cid/civ075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haverkate MR, Derde LP, Brun-Buisson C, Bonten MJ, Bootsma MC. Duration of colonization with antimicrobial-resistant bacteria after ICU discharge. Intensive Care Med. 2014;40:564–571. doi: 10.1007/s00134-014-3225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers C, Sharma A, Rimland D, et al. Duration of colonization with methicillin-resistant Staphylococcus aureus in an acute care facility: a study to assess epidemiologic features. Am J Infect Control. 2014;42:249–253. doi: 10.1016/j.ajic.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Siegel JD, Rhinehart E, Jackson M, Chiarello L, Health Care Infection Control Practices Advisory Committee 2007 guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Glind I, de Roode S, Goossensen A. Do patients in hospitals benefit from single rooms? A literature review Health Policy. 2007;84:153–161. doi: 10.1016/j.healthpol.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhury H, Mahmood A, Valente M. Advantages and disadvantages of single-versus multiple-occupancy rooms in acute care environments: A review and analysis of the literature. Environment and Behavior. 2005;37:760–26. [Google Scholar]
- 33.Furuno JP, Perencevich EN, Johnson JA, et al. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci co-colonization. Emerging Infectious Diseases. 2005;11:1539–1544. doi: 10.3201/eid1110.050508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan DJ, Day HR, Furuno JP, et al. Improving efficiency in active surveillance for methicillin-resistant Staphylococcus aureus or vancomycin-resistant Enterococcus at hospital admission. Infection Control & Hospital Epidemiology. 2010;31:1230–1235. doi: 10.1086/657335. [DOI] [PMC free article] [PubMed] [Google Scholar]


