Abstract
Background
Gastrointestinal (GI) and non-GI disorders are associated with altered intestinal permeability, which can be measured in vivo by urinary excretion after oral lactulose and mannitol ingestion. Inadvertent dietary consumption of 12Carbon (12C, regular) mannitol in food or from other sources may interfere with the test’s interpretation. 13Carbon (13C) constitutes 1% of carbon in nature and 13C mannitol is a stable isotope. Our aim was to determine performance of 13C mannitol for measurement of intestinal permeability.
Methods
Ten healthy volunteers underwent intestinal permeability assay using co-administered 12C mannitol, 13C mannitol and lactulose, followed by timed urine collections. Urinary sugar concentrations were measured using tandem high performance liquid chromatography-mass spectrometry.
Key Results
We found that 13C mannitol can be distinguishable from 12C mannitol on tandem mass spectrometry. Additionally, 13C mannitol had ~20-fold lower baseline contamination compared to 12C mannitol. We describe here the 13C mannitol assay method for measurement of intestinal permeability.
Conclusions & Inferences
In conclusion, 13C mannitol is superior to 12C mannitol for measurement of intestinal permeability. It avoids issues with baseline contamination and erratic excretions during the testing period.
Keywords: barrier function, gastrointestinal, lactulose, irritable bowel syndrome
Introduction
Increased intestinal permeability is implicated in the pathophysiology of a number of disorders such as celiac disease, environmental enteropathy, inflammatory bowel disease, irritable bowel syndrome, obesity, and HIV (1). Currently, one method for in vivo measurement of intestinal permeability involves oral ingestion of sugars or sugar alcohols that are absorbed in the gut and not metabolized in the body or the urine (e.g. lactulose, mannitol, rhamnose, sucralose etc.). Thus, measuring their urinary concentrations provides a measure of passive absorption and hence intestinal permeability (2). Of these, lactulose and mannitol excretion and the derived lactulose: mannitol (L:M) excretion ratio has been one of the most widely used methods (3). Lactulose and mannitol excretion based assays are widely used for both mechanistic studies (4,5) and more recently as treatment efficacy endpoints in clinical trials (6,7).
Typically, study participants are provided guidelines for dietary restrictions and they present after an overnight fast for the testing. After a baseline urine collection, test sugars are administered and urine collections are done over a period of up to 24 hours depending upon the region of interest for permeability measurement and the site’s protocol. Given prior experience that up to 30% of participants have detectable urinary mannitol at baseline (prior to administration of test sugars) or disproportionate excretion relative to the mass of mannitol administered for the test, our overall hypothesis was that inadvertent ingestion of mannitol (in diet or medications) is the most likely reason for this “contamination”. Specifically, since a previous study has shown that only 25% of mannitol was available after an overnight incubation with rat feces suggesting that a significant proportion of orally administered mannitol is expected to be utilized by the colonic microbiota (8).
13Carbon (13C) is stable (non-radioactive) isotope and constitutes only 1% of naturally occurring carbon (9). 13C mannitol has the same molecular tertiary structure as 12Carbon (12C, regular) mannitol. We specifically hypothesized that 13C mannitol should be distinguishable from 12C mannitol on the tandem high performance liquid chromatography-mass spectrometry (HPLC-MS), and can be quantitated in urine, offering a novel biomarker for measurement of intestinal permeability. Our aim was to determine excretion characteristics of 13C mannitol as a measurement of intestinal permeability assay and compare the excretion profiles with those of co-administered 12C (regular) mannitol.
Materials & Methods
We recruited 10 healthy volunteers (5 females) who were given instructions to not consume any steroids (6 weeks prior to the testing), medications affecting gastrointestinal transit or permeability (7 days prior to the testing) and artificial sweeteners, lactulose, mannitol (2 days prior to the testing)and during the 24 hour testing period. After baseline urine collection, three saccharides {100 mg 13C mannitol, 100 mg 12C (regular) mannitol, and 1000 mg lactulose} dissolved in 250 ml of water were administered. Urine collections were pooled for 0-2, 2-8 and 8-24 hours following administration of test sugars and excreted sugar concentrations were measured by HPLC-tandem MS.
HPLC-Tandem MS method
Urine samples (25 µL) are added to a 96, deep-well plate. Samples, quality controls, and calibrators are diluted 11x by the addition of 250 µL of an internal standard (I.S.) consisting of 13C (6) mannitol. The analytes are separated by normal phase HPLC on a CARBOSep CoreGel 87C column (300 × 7.8 mm, 9 um, 8% cross-link, Ca2+,Transgenomics) using isocratic 5% methanol/water containing 0.1 mM ammonium acetate, and detected on tandem mass spectrometer (LC-MS/MS) utilizing electrospray ionization, operating in the multiple-reaction monitoring negative mode. The calibration utilizes two different six point standard curves over a concentration range of: 0-500 µg/mL (for mannitol) and 0-125 µg/mL (for lactulose). The following transitions were monitored: 12C Mannitol (181.05/89), 13C (1) Mannitol (182.05/89), and 13C (6) Mannitol I.S. (186.9/60.9). The API 5000 triple quadrupole mass spectrometer from AB Sciex was used and the Cohesive LC system was from Cohesive Technologies (now ThermoScientific).
Data Analysis
Cumulative baseline, 0-2, 2-8 and 8-24 hour mannitol (13C and 12C) and lactulose excretions were calculated. In a previous study, excretions in the 0-2 hour time-frame and 8-24 hour time-frame have been validated for measurement of small bowel and colonic permeability respectively (10). Non parametric 2 sided tests (Mann Whitney) were used to compare cumulative excretion values of 13C and 12C mannitol at baseline and each time-frame. A p-value of <0.05 was considered statistically significant.
Results
Detection of 13C mannitol using tandem mass spectrometry
The mannitol isotopes co-migrated on liquid chromatography and were separable on mass spectrometry due to the mass shift, as shown from multiple reaction monitoring in Figure 1 (panel A). The Limit of Detection, the lowest analyte concentration likely to be reliably distinguished from the Limit of Blank, was 0.018 and 0.051 pg/mL in two lactulose samples tested and 0.021 pg/mL in the mannitol sample tested in multiple replicates. The limit of quantification (LoQ) for lactulose concentration measured reproducibly was 0.063 pg/mL (n=13) with a coefficient of variation (CV) of 17%. The lowest mannitol concentration measured reproducibly was 0.029 pg/mL with a CV of 14%. For routine testing purposes, our lab has chosen to set the LoQ for both analytes at 0.3 pg/mL.
Figure 1. Panel A: Mass spectrometric resolution of mannitol isotopes.
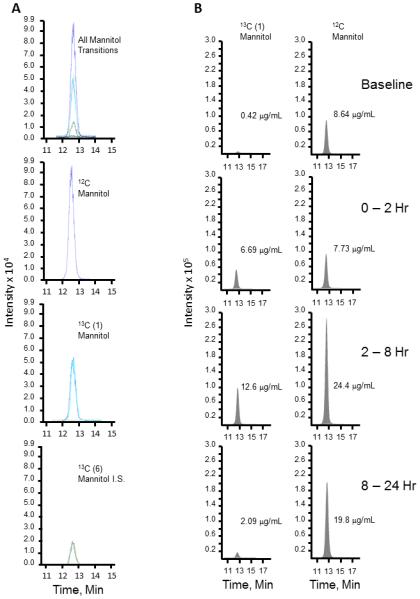
Multiple reaction monitoring (MRM) mass spectrometry resolves the three mannitol isotopes by mass: 12C, the most abundant isotope in nature; 13C (1), one carbon atom substituted with one heavy isotope; 13C (6), all 6 carbon atoms substituted by the heavy isotope.
Panel B: Comparison of 13C and 12C mannitol as probes to measure intestinal permeability. Excretion values in one representative subject. Urine samples were obtained prior to test sugar ingestion (baseline), and at various time points post-ingestion. Cumulatively excreted concentrations of 12C and 13C mannitol used as indexes of permeability. 13C (6) mannitol was used as an internal standard.
Comparison of 13C and 12C mannitol excretions
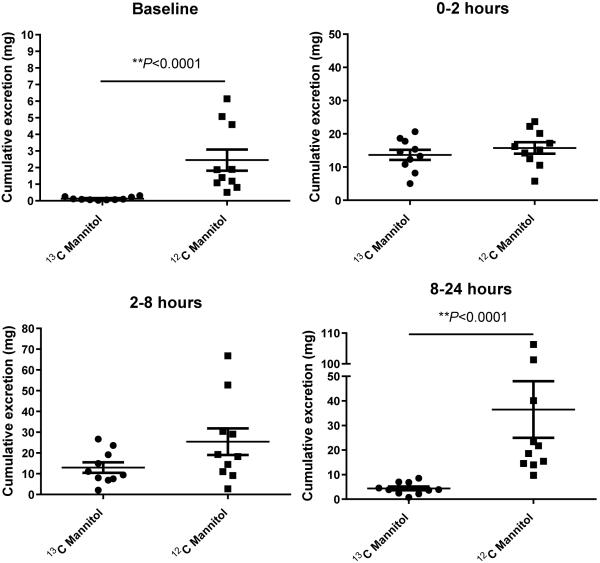
We found a significantly lower baseline cumulative excretion of 13C mannitol than 12C mannitol, with a mean 20-fold lower contamination of 13C mannitol (Table 1). Three of the 10 participants had disproportionately higher baseline excretion of 12C mannitol, whereas none of the participants had high 13C mannitol excretion at baseline (Fig. 2). The 0-2 hour cumulative excretion of 13C mannitol increased 105-fold from baseline in contrast to the 6-fold over baseline excretion for 12C mannitol, but there was no difference in the actual mass of the two mannitol moieties excreted over this time period. After 100 mg of each form of mannitol is ingested, the overall 24 hour cumulative excretion of the 13C mannitol is 31 mg vs 78 mg for the 12C mannitol (Table 1). The excretion of 13C mannitol drops between 8-24 hours, however the 12C mannitol excretion continues to peak in that time frame. This could be possibly due to inadvertent contamination during the study (either from dietary, medications or other sources), baseline sugar competing with absorption or due to production of 12C mannitol by colonic microbiota during the testing (11). Figure 1 (panel B) shows a representative tracing from a urine sample showing the lower baseline and 8-24 hour excretion of 13C mannitol.
Table 1.
Comparison of urinary excretion parameters of 13C and 12C mannitol at various testing intervals and the lactulose excretions
| 13C Mannitol | 12C Mannitol | Lactulose | p-value* | |
|---|---|---|---|---|
|
| ||||
|
Cumulative excretion (mg):
Mean (SEM) |
||||
| Baseline (pre-test dose) | 0.13 (0.03) | 2.57 (0.70) | 0.04 (0.01) | <0.0001 |
| 0-2 hr | 13.65 (1.52) | 15.75 (1.71) | 0.99 (0.11) | 0.4 |
| 2-8 hr | 12.95 (2.51) | 25.41 (6.41) | 1.74 (0.34) | 0.1 |
| 8-24 hr | 4.38 (0.76) | 36.47 (11.52) | 0.42 (0.13) | <0.0001 |
| 0-24 hr | 31.21 (3.40) | 77.63 (13.06) | 3.16 (0.33) | 0.0007 |
For differences in cumulative excretion values of 13C and 12C Mannitol
Figure 2.
Comparison of cumulative excretions of 13C and 12C mannitol at baseline (pre-test dose) and during different time-frames of testing. ** p<0.0001.
Discussion
Our results indicate that 13C mannitol has only 5% of the concentration of 12C mannitol at baseline. This is consistent with the fact that only 1% of the overall carbon in nature is expected to be 13C and in a 6 carbon molecule like mannitol, 6% is expected to be 13C. Three of the ten participants had significantly high baseline excretions of 12C mannitol but none had any significant presence of 13C mannitol at baseline. This lower baseline urinary concentration allows for a greater delta at 0-2 hours which will likely increase the sensitivity of small intestinal permeability testing with 13C mannitol. After 100 mg ingestion of each mannitol type, the mean cumulative excretion of 31 mg with 13C mannitol is physiologically expected as compared to 78 mg excretion with 12C mannitol during the 8-24 hour testing period as reflected by the significantly higher urinary excretion of 12C mannitol between 8-24 hours.
In vivo measurement of intestinal permeability has been historically done using ingestion of non-absorbable saccharides or non-saccharide molecules (e.g. lactulose, mannitol, rhamnose, polyethylene glycol, ethylenediaminetetraacetic acid, etc.). Most of the studies of intestinal permeability do not report or account for baseline excretion of these molecules, which is perhaps most relevant with the saccharides. Despite instructions for dietary restrictions prior to testing, significant quantities of mannitol are detected in the urine at baseline (before administration of test sugar) likely because of “contaminants” in foods that do not specifically document the presence of mannitol or other sugar alcohols. These quantities vary considerably between individuals. The presence of baseline mannitol can interfere with estimation of mannitol excretion after test dose administration. Additionally, inadvertent consumption (in diet or medications), exposure through non-dietary sources, or production by the gut microbiota during the 2-24 hour of testing (during which participants are usually collecting urine samples at their work or home surroundings) further skews measurements. In general, measurement of colonic permeability using saccharides is prone to error considering variable colonic production and consumption of these sugars by the microbiota. Future studies will have to determine possible combination of saccharides poorly consumable by colonic microbiota or non-saccharide molecules for assessment of colonic permeability.
Overall, this study demonstrates that the use of 13C mannitol can eliminate problems with high baseline excretion of mannitol and from inadvertent consumption or exposure of mannitol during the testing period. Further studies are required to evaluate analytical performance of 13C mannitol (intraday and inter day precision, recovery, dilutional linearity, measurement range and sensitivity [limit of detection], specificity, and stability). Subsequently, test performance by response to perturbation of small intestinal and colonic permeability and recovery and validation in disease states such as celiac disease or inflammatory bowel disease will be needed.
Key Messages.
Saccharide (lactulose and mannitol) excretion based assay is commonly used for in vivo measurement of intestinal permeability. However, inadvertent ingesting of test sugars may result in high excretions at baseline (pre-test dose) and during the testing period.
13C mannitol is a stable (non-radioactive) isotope of mannitol. In a standardized intestinal permeability assay, we compared excretion parameters of co-administered 12C (regular) mannitol and 13C mannitol.
13C mannitol had significantly less baseline excretion and a more expected excretion pattern during the testing period.
13C mannitol is a superior saccharide than 12C mannitol for measurement of intestinal permeability.
Acknowledgements
The authors wish to thank Lori R. Anderson and Cindy Stanislav for their assistance in the preparation of the manuscript.
Funding: This work was supported by NIH K23 to MG (DK103911), Pilot and Feasibility Grant to MG from Mayo Clinic Center for Cell Signaling in Gastroenterology (NIH P30 DK084567), 2015 American Gastroenterological Association-Rome Foundation Functional Gastroenterology and Motility Disorders Pilot Research Award to MG, and NIH R01 Dk 92179 to MC. MG is supported by K23 DK103911. MC is supported by R01 DK92179. Studies were supported by Mayo Clinic CTSA grant UL1 TR000135. We acknowledge assistance from the Physiology and Nursing cores of the Clinical Research Unit at Mayo Clinic.
Abbreviations
- GI
gastrointestinal
- 13C
13Carbon
- HPLC-MS
high performance liquid chromatography-mass spectometry
Footnotes
Author Contributions:
Madhusudan Grover: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; study supervision
Michael Camilleri: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Jolaine Hines: acquisition of data; analysis and interpretation of data
Duane Burton: acquisition of data; critical revision of the manuscript for important intellectual content; study supervision
Michael Ryks: acquisition of data
Akhilesh Wadhwa: analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Wendy Sundt: acquisition of data; critical revision of the manuscript for important intellectual content
Roy Dyer: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis
Ravinder Singh: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Disclosures: None
Writing Assistance: None
References
- 1.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–12. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–81. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 3.Kubica P, Kot-Wasik A, Namiesnik J, Namieśnik J, Landowski P. Modern approach for determination of lactulose, mannitol and sucrose in human urine using HPLC-MS/MS for the studies of intestinal and upper digestive tract permeability. J Chromatog B Analyt Technol Biomed Life Sci. 2012;907:34–40. doi: 10.1016/j.jchromb.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Smecuol E, Bai JC, Sugai E, Vazquez H, Niveloni S, Pedreira S, Mauriño E, Meddings J. Acute gastrointestinal permability responses to different non-steroidal anti-inflammatory drugs. Gut. 2001;49:650–5. doi: 10.1136/gut.49.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sequeira IR, Lentle RG, Kruger MC, Hurst RD. The effect of aspirin and smoking on urinary excretion profiles of lactulose and mannitol in young women: toward a dynamic, aspirin augmented, test of gut mucosal permeability. Neurogastroenterol Motil. 2012;24:e401–11. doi: 10.1111/j.1365-2982.2012.01969.x. [DOI] [PubMed] [Google Scholar]
- 6.Kelly CP, Green PH, Murray JA, Dimarino A, Colatrella A, Leffler DA, Alexander T, Arsenescu R, Leon F, Jiang JG, Arterburn LA, Paterson BM, Fedorak RN, Larazotide Acetate Celiac Disease Study Group Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther. 2013;37:252–62. doi: 10.1111/apt.12147. [DOI] [PubMed] [Google Scholar]
- 7.Smith HE, Ryan KN, Stephenson KB, Westcott C, Thakwalakwa C, Maleta K, Cheng JY, Brenna JT, Shulman RJ, Trehan I, Manary MJ. Multiple micronutrient supplementation transiently ameliorates environmental enteropathy in Malawian children aged 12-35 months in a randomized controlled clinical trial. J Nutrition. 2014;144:2059–65. doi: 10.3945/jn.114.201673. [DOI] [PubMed] [Google Scholar]
- 8.Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology. 1998;114:83–92. doi: 10.1016/s0016-5085(98)70636-5. [DOI] [PubMed] [Google Scholar]
- 9.Rosman KJR, Taylor PDP. IUPAC Subcommittee for isotopic abundance measurements. Pure Appl. Chem. 1999:1593–1607. [Google Scholar]
- 10.Rao AS, Camilleri M, Eckert DJ, Busciglio I, Burton DD, Ryks M, Wong BS, Lamsam J, Singh R, Zinsmeister AR. Urine sugars for in vivo gut permability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol. 2011;301:G919–28. doi: 10.1152/ajpgi.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz ME, Raya RR, Mozzi F. Efficient mannitol production by wild-type Lactobacillus reuteri CRL 1101 is attained at constant pH using a simplified culture medium. Appl Microbiol Biotechnol. 2015;99:8717–29. doi: 10.1007/s00253-015-6730-y. [DOI] [PubMed] [Google Scholar]