Abstract
Rationale
Lipoprotein(a) [Lp(a)] is a highly atherogenic low-density lipoprotein (LDL)-like particle characterized by the presence of apoprotein(a) [apo(a)] bound to apolipoprotein B (apoB). Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9) selectively binds LDL, we hypothesized that it can also be associated with Lp(a) in plasma.
Objective
Characterize the association of PCSK9 and Lp(a) in 39 subjects with high Lp(a) levels (range 39-320 mg/dl) and in transgenic mice expressing either human apo(a) only or human Lp(a) (via co-expression of human apo(a) and human apoB).
Methods and Results
We show that PCSK9 is physically associated with Lp(a) in vivo using three different approaches: (i) Analysis of Lp(a) fractions isolated by ultracentrifugation; (ii) Immunoprecipitation of plasma using antibodies to PCSK9 and immunodetection of apo(a); (iii) ELISA quantification of Lp(a)-associated PCSK9. Plasma PCSK9 levels correlated with Lp(a) levels, but not with the number of kringle IV-2 repeats. PCSK9 did not bind to apo(a) only and the association of PCSK9 with Lp(a) was not affected by the loss of the apo(a) region responsible for binding oxidized phospholipids. Preferential association of PCSK9 with Lp(a) vs. LDL (1.7-fold increase) was seen in subjects with high Lp(a) and normal LDL. Finally, Lp(a)-associated PCSK9 levels directly correlated with plasma Lp(a) levels but not with total plasma PCSK9 levels.
Conclusions
Our results show, for the first time, that plasma PCSK9 is found in association with Lp(a) particles in humans with high Lp(a) levels and in mice carrying human Lp(a). Lp(a)-bound PCSK9 may be pursued as a biomarker for cardiovascular risk.
Keywords: Low-density lipoprotein, Lipoprotein (a), Proprotein Convertase Subtilisin/Kexin type 9, lipids and lipoprotein metabolism
INTRODUCTION
Elevated plasma levels of lipoprotein (a) [Lp(a)] are associated with increased risk of myocardial infarction and stroke 1 both in the general population and in individuals with familial hypercholesterolemia (FH) 2. Lp(a) is an LDL-sized particle composed of apolipoprotein (a) [apo(a)] bound to apolipoprotein B-100 (apoB-100) through a single disulfide bond 3. Apo(a) is structurally similar to a portion of plasminogen, and very heterogeneous in size, depending on the copy-number of Kringle IV type 2 repeats, ranging from 1 to >40 3. Recent data suggest that Lp(a) levels in subjects with FH are higher compared with those of non-affected relatives carrying similar apo(a) isoforms 4, and that the LDL receptor (LDLR) is involved in the clearance of Lp(a), at least in the HepG2 cell lines with supra-physiological LDLR density 5. In contrast, several lines of prior evidence suggest that Lp(a) clearance is not regulated by the LDLR: (i) catabolism of Lp(a) in FH subjects is not different than that of controls 6; (ii) Lp(a) levels are not reduced by statin treatment 7; (iii) LDLR deficient mice catabolize human Lp(a) similarly to WT mice 8;(iv) In homozygous FH patients without expression of LDLR, inhibition of PCSK9 did not affect LDL but decreased Lp(a) by about 20% 9.
Due to the lack of understanding of the mechanism of Lp(a) clearance, therapeutic approaches to enhance clearance to reduce Lp(a) have been limited. PCSK9 inhibitors have been shown to reduce Lp(a) ~20-35%, but the mechanism for this effect has not been established 10. Moreover, a recent study has reported reduction in plasma Lp(a) levels by ~80% by means of an antisense oligonucleotide that inhibits apo(a) production 11.
PCSK9 is a serine protease mainly expressed by the liver and secreted into the circulation 12 which binds LDLR and inhibits its recycling to the plasma membrane, thus reducing the number of LDLR on the cell surface and regulating LDL-C clearance and plasma LDL levels 13. In addition, PCSK9 affects the assembly and secretion of apoB-containing lipoproteins 14, 15. Although a significant correlation between plasma PCSK9 and LDL-C levels has been reported 16, the correlation between PCSK9 and Lp(a) levels is less clear 17, 18. Monoclonal antibodies (mAbs) that block PCSK9 have been recently approved for clinical use in Europe and the US as a treatment for severe hypercholesterolemia 19. PCSK9 inhibition reduces LDL-C levels by up to 65%, mainly by increasing hepatic LDLR levels and increasing the efficiency of clearance of LDL particles 20. PCSK9 inhibition also reduces Lp(a) levels by up to 35% in a dose-dependent manner through an unexplained mechanism 21.
Our laboratory was the first to report that plasma PCSK9 associates with LDL-sized particles both in vivo and in vitro 22. Later, several laboratories including ours have refined the knowledge on biology and function of PCSK9-LDL complexes in plasma 14, 23, 24. Quantification of PCSK9 binding shows that up to 40% of plasma PCSK9 can be found in association with LDL, with a Kd of 160-320nM 5, 23, 24. Although PCSK9 is known to associate with LDL in plasma, it remains unknown whether PCSK9 also associates with Lp(a), and whether this has physiological relevance for the production or removal of Lp(a) from the plasma compartment.
To study PCSK9 association with Lp(a), we isolated lipoproteins through various techniques and characterized the interactions between PCSK9 and Lp(a) or apo(a), using plasma from transgenic mice or from subjects with elevated Lp(a) (>30 mg/dl). We show that PCSK9 is physically associated with Lp(a) in mouse and human plasma. Additionally, PCSK9 binding to Lp(a) did not occur through interaction with apo(a) and was not dependent on whether Lp(a) carries oxidized phospholipids or not. Finally, in subjects with high Lp(a), PCSK9 preferentially associated with Lp(a) vs. LDL.
METHODS
Subjects and samples
Thirty-nine subjects from the outpatient clinic or undergoing lipoprotein apheresis at OHSU's Center for Preventive Cardiology (IRB#00010649 and IRB#00015117) were recruited on the basis of Lp(a) levels >30 mg/dl (mass concentration).
Statistical analyses
Data were analyzed with multiple linear regression models adjusting for age, sex, and lipid lowering medication as covariates. The strength of associations between variables was assessed by estimating coefficients and Wald test p-values from the multiple regression models. r = univariate Spearman's correlation; p = p-value from multiple (adjusted) regression.
See the supplemental section for plasma lipids and protein determinations, mass spectrometry protein analysis, lipoprotein isolation, western blotting, immunoprecipitation, animal preparations, quantitative sandwich-based ELISA, detailed statistical analyses, and the detailed description of the lipid-lowering medications taken by each subject.
RESULTS
Characterization of PCSK9 association with Lp(a)
Because PCSK9 binds to LDL likely through its apoB moiety 14, 24, we predicted that plasma PCSK9 would also bind Lp(a), an LDL variant. First, we studied two subjects who regularly undergo lipoprotein apheresis due to extremely high Lp(a) levels (>100 mg/dl). Plasma lipoproteins were isolated using iodixanol-based ultracentrifugation (Figure 1A) and tested for purity using agarose lipid gels (Figure 1B). The isolated LDL fraction produced the expected beta band on agarose electrophoresis. The isolated Lp(a) fraction produced a “sinking” pre-beta band, indicating purity and absence of LDL. Purity was further assessed for apoB (Figure 1C, upper panel) and apo(a) (Figure 1C, middle panel). Whereas both fractions contained apoB-100, the Lp(a) fraction was highly enriched in apo(a), compared with the LDL fraction.
Figure 1. Characterization of PCSK9 association with Lp(a).
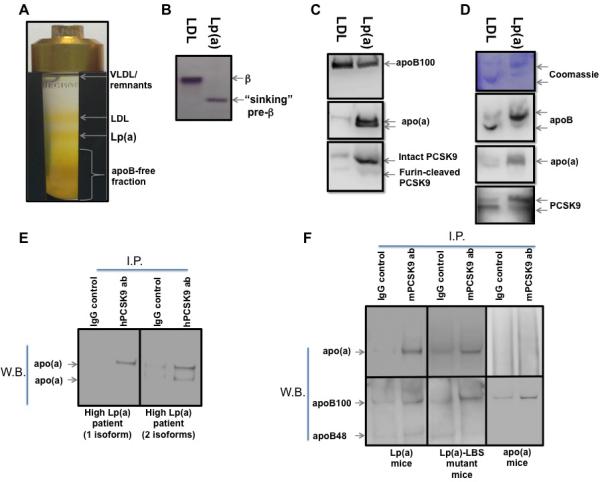
(A) lipoprotein isolation via iodixanol-based gradient ultracentrifugation. (B) agarose gel electrophoresis of LDL and Lp(a) fractions isolated as described in A, followed by neutral lipid staining. (C) Reducing gel immunoblot of LDL and Lp(a) fractions isolated as described in A. (D) Non-reducing PAGE of LDL and Lp(a) fractions isolated as described in A, followed by Coomassie protein staining (upper panel) and immunoblot (lower panel). (E) Immuno-precipitation of apo(a) using anti-PCSK9 antibody from plasma of subjects with one (left panel) or two (right panel) visible apo(a) isoforms. (F) Immuno-precipitation of apo(a) (upper panel) and apoB (lower panel) using anti-PCSK9 antibody from plasma of Lp(a) (left panel), Lp(a)-LBS mutant (middle panel), and apo(a) transgenic mice (right panel).
Figure 1C (lower panel) shows that the intact form of PCSK9 is found in the Lp(a) fraction. To determine whether PCSK9 is physically part of the Lp(a) particle, we used native gel electrophoresis and immunoblotting. The LDL fraction shows an upper band containing both apoB and PCSK9, confirming the previously reported association of PCSK9 with LDL 23, 24. The Lp(a) fraction shows a band containing apoB, apo(a), and PCSK9, suggesting that PCSK9 is also associated with the Lp(a) particle (Figure 1D, middle and lower panels).
Next we performed immunoprecipitation of PCSK9 from plasma of two subjects with either only one or two visible apo(a) isoforms. Figure 1E shows that immunoprecipitation of PCSK9 from human plasma also pulled down apo(a), regardless of the apo(a) isoform. We then studied whether PCSK9 associates with Lp(a) via apo(a) and if the association is linked to the presence of oxidized phospholipids on Lp(a). We used plasma from transgenic mice expressing either recombinant apo(a) containing 8 kringle IV repeats (8K-IV) alone (apo(a) mice), or recombinant (8K-IV) apo(a) together with human apoB-100 (Lp(a) mice), or recombinant (8K-IV) apo(a) with a ligand-binding site (LBS) mutation in KIV-10 that does not allow for the binding of oxidized phospholipids (Lp(a)-LBS mice) 25. Immunoprecipitation of PCSK9 from plasma of Lp(a) mice shows both apoB and apo(a) bands, supporting the concept that PCSK9 associates with Lp(a) (Figure 1F, left panel). The association of PCSK9 with Lp(a) was not affected by the ability of Lp(a) to bind oxidized phospholipids (Figure 1F, middle panel). In contrast, immunoprecipitation of PCSK9 from plasma of apo(a) mice pulled down apoB but not apo(a), suggesting that PCSK9 does not directly interact with apo(a) (Figure 1F, right panel).
Quantitative assessments of PCSK9 levels and correlations in subjects with high Lp(a) levels
We studied thirty-nine subjects with high Lp(a) levels (Lp(a) mass concentration range 39-320 mg/dl). A summary of the relevant data is shown in Table 1. Within our cohort, PCSK9 correlated with LDL-C only in subjects not taking lipid-lowering medications (Figure 2A). We also show a significant correlation between total plasma PCSK9 and Lp(a) levels after adjusting for age and sex (r=0.5, p<0.0001) (Figure 2B), however the number of kringle IV-2 repeats did not correlate with PCSK9 levels (Figure 2C). ApoB significantly correlated with LDL-C levels (Figures 2D) but not with Lp(a) levels (not shown). It was recently suggested that the calculated LDL-C levels represent a combination of the cholesterol concentration on LDL and Lp(a), with up to 30% of Lp(a) mass concentration being reflected in the LDL-C calculation 26. LDL-C correlated with Lp(a) levels only in subjects not on lipid-lowering medications (Figure 2E). Plasma PCSK9 and Lp(a) levels did not correlate with apoA-I, apoA-II, apoA-IV, apoC-I, apoC-II, apoC-III, apoE, or apoM levels (not shown).
Table 1.
Characteristics of High-Lp(a) subjects: Number of subjects: 39 (females = 25, on lipid-lowering medications = 25). RLU=Relative Light Units.
| Minimum | Median | Maximum | Mean | Std. Deviation | Std. Error of Mean | |
|---|---|---|---|---|---|---|
| Age | 20 | 62 | 83 | 58.9 | 16 | 2.3 |
| Lp(a) (mg/dl) | 39 | 98 | 320 | 132 | 78 | 13 |
| Apo(a) nM | 117 | 219 | 664 | 270 | 146 | 25 |
| Average Kringle IV2 repeats | 3.8 | 10 | 27 | 11 | 5.2 | 0.8 |
| ApoA-I (mg/dl) | 65 | 118 | 208 | 117 | 27 | 4.3 |
| ApoA-II (mg/dl) | 13 | 30 | 86 | 32 | 16 | 2.5 |
| ApoA-IV (mg/dl) | 5.5 | 13 | 45 | 16 | 10 | 1.7 |
| apoB (mg/dl) | 52 | 173 | 313 | 183 | 71 | 11 |
| ApoC-I (mg/dl) | 0.2 | 2.8 | 12 | 3.2 | 2.7 | 0.4 |
| ApoC-II (mg/dl) | 1.0 | 2.9 | 11 | 3.5 | 1.9 | 0.3 |
| ApoC-III (mg/dl) | 2.9 | 10 | 17 | 9.8 | 3.8 | 0.6 |
| ApoE (mg/dl) | 3.5 | 9.7 | 18 | 9.3 | 3.3 | 0.5 |
| ApoM (mg/dl) | 0.5 | 1.2 | 2.0 | 1.3 | 0.4 | 0.06 |
| LDL (mg/dl) | 54 | 141 | 257 | 140 | 48.5 | 7.8 |
| PCSK9 (ng/ml) | 168 | 438 | 916 | 464 | 197 | 31.6 |
| Lp(a)-bound PCSK9 (RLU) | 713 | 1834 | 8924 | 2622 | 1943 | 343 |
| Fold increase in PCSK9 on Lp(a) compare to LDL | 0.2 | 1.7 | 9.2 | 2.4 | 2.1 | 0.4 |
Figure 2. Quantitative assessments of PCSK9 levels and correlations in subject with high Lp(a) levels.
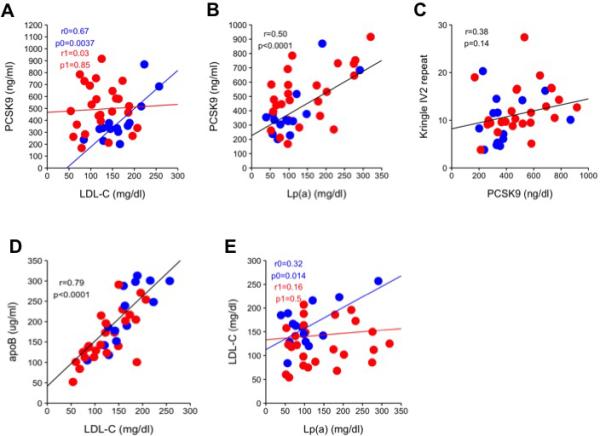
(A) Linear regression analysis of LDL-C and PCSK9 levels, adjusted to age, sex, and interaction with lipid-lowering medications. Blue regression analysis (r0, p0) = subjects without lipid-lowering medication, red regression analysis (r1, p1) = subjects on lipid-lowering medication. (B) Linear regression analysis of Lp(a) and PCSK9 levels, adjusted to age and sex. (C) Linear regression analysis of PCSK9 levels and the average number of kringle IV-2 repeats, adjusted to age and sex. (D) Linear regression analysis of LDL-C and apoB levels, adjusted to age and sex. (E) Linear regression analysis of LDL-C and Lp(a) levels, adjusted to age, sex, and interaction with lipid-lowering medications. Blue regression analysis (r0, p0) = without lipid-lowering medication, red regression analysis (r1, p1) = on lipid-lowering medication. For all panels, blue = subjects without lipid-lowering medication, red = subjects on lipid-lowering medication, black regression lines represents the combined regression of both blue and red dots and indicate no interaction with lipid-lowering medications.
Association of PCSK9 with Lp(a) in subjects with high Lp(a) levels
To validate the association of PCSK9 with Lp(a), and to quantify Lp(a)-associated PCSK9 in these subjects, we developed a sensitive ELISA method, illustrated in Figure 3A and described in the Methods. 27 In this assay, PCSK9 was first bound to a monoclonal anti-PCSK9 antibody on a microtiter well plate, then Lp(a) was detected with an anti-apo(a) antibody. Lp(a)-associated PCSK9 levels strongly correlated with plasma Lp(a) levels (r=0.65, p<0.0001) (Figure 3B), but not with total plasma PCSK9 levels (Figure 3C). Next, we wanted to quantify the relative distribution of plasma PCSK9 between Lp(a), LDL, and the apoB-free compartment in these subjects. Using an ELISA to measure apoB-associated PCSK9 assay we compared the amount of PCSK9 found in Lp(a) to the amount of PCSK9 in LDL, as illustrated in Figure 3D. Our results showed a significant (p<0.001) 1.7-fold (median) increase in PCSK9 associated with Lp(a)-apoB vs. LDL-apoB (after normalization to total apoB in each fraction; Figure 2E). To examine whether PCSK9 association with Lp(a) affects the absolute amount of PCSK9 in the apoB compartment, we compared apoB-associated and apoB-free PCSK9 in 12 subjects with low levels of Lp(a) (Lp(a) mass concentration of <2-15 mg/dl), as explained in the Methods. We saw no differences in the levels of apoB-associated and apoB-free PCSK9 between the two groups (Figure 3F). We have estimated the proportion of Lp(a) particles that contains PCSK9 based on the following results in our subjects with extremely elevated Lp(a) levels:(i) 37% of plasma PCSK9 is associated with apoB (Figure 3F); (ii) 25% of plasma PCSK9 is associated with the Lp(a) (based on PCKS9 distribution between LDL and Lp(a) as shown in Figure 3E); (iii) Mean plasma PCSK9 level was 464 ng/ml (6.19 nM); and (iv) mean plasma apo(a) level was 270 nM. A calculation based on these data (0.25×6.19/270) allows us to estimate that, on average, only one in every 175 Lp(a) particles carries PCSK9.
Figure 3. Association of PCSK9 with Lp(a) in subjects with high Lp(a) levels.
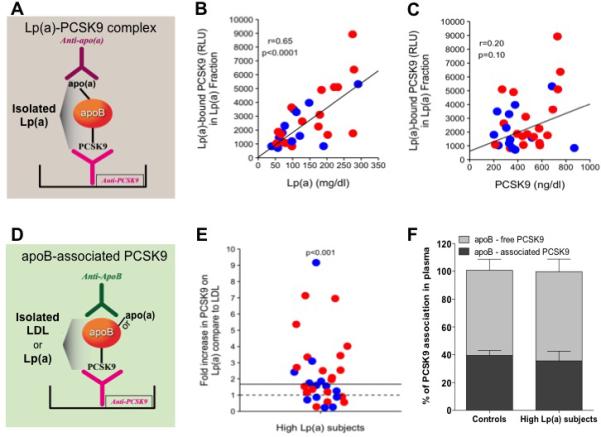
(A) Illustration of the ELISA method used to detect Lp(a)-bound PCSK9. (B) Linear regression analysis of levels of Lp(a) and Lp(a)-bound PCSK9, adjusted for age and sex. (C) Linear regression analysis of levels of PCSK9 and Lp(a)-bound PCSK9, adjusted for age and sex. (D) Illustration of the ELISA method used to detect apoB-associated PCSK9 in LDL and Lp(a) fractions. (E) Fold increase in levels of Lp(a)-bound PCSK9 over LDL-bound PCSK9, measured as apoB-bound PCSK9 (ELISA) and normalized for apoB level in each fraction. (F) Percentage of apoB-associated PCSK9 in plasma of subjects with high Lp(a) levels vs. those with low Lp(a) levels (groups matched for plasma LDL-C levels). All panels: blue = subjects without lipid-lowing medication, red = subjects on lipid-lowering medication, black regression lines represent the combined regression of both blue and red dots and indicate no interaction with lipid-lowering medications.
DISCUSSION
PCSK9 is found in association with LDL particles in plasma 22, 23. Since PCSK9 binds apoB but only associates with LDL 14, 24, we hypothesized that Lp(a), a particle of size and composition similar to LDL, may also be a carrier of PCSK9 in the circulation. PCSK9 is found in plasma mainly in two monomeric forms: (i) a 62-kDa protein representing the full-length (intact) protein (minus the pro-domain); and (ii) a 55-kDa fragment, product of cleavage of the 62-kDa form by the protease furin. Furin-cleaved PCSK9 is generally considered less active than the intact form 28. We and others have shown that a significant portion of intact PCSK9 is found associated with LDL, whereas most of the furin-cleaved form is in the apoB-free fraction 29, 30. Here, we show for the first time that the intact form of PCSK9 is also found associated with the Lp(a) particle. Our results show that PCSK9 is associated with Lp(a) both in plasma of subjects with elevated Lp(a) levels and in transgenic mice expressing human Lp(a), though the association is not related to the presence of oxidized phospholipids and does not occur through apo(a).
PCSK9 and LDL-C levels are directly correlated both because PCSK9 increases LDL-C through the degradation of hepatic LDLR 31 and because PCSK9 is bound to LDL and may leave the circulation concomitantly with LDL 22. In contrast, the correlation of PCSK9 with Lp(a) is not well established 17, 18. In our cohort, we found a correlation between PCSK9 and Lp(a) levels but not with the size of main apo(a) repeat, kringle IV-2 of plasminogen. Thus, the correlation between PCSK9 and Lp(a) is driven by the concentration of Lp(a), not the size of apo(a). It was previously shown that statins and ezetimibe both increase plasma PCSK9 levels while lowering LDL-C, thus interfering with the correlation between PCSK9 and LDL-C levels 32. In our cohort, LDL-C levels correlated with PCSK9 and Lp(a) levels only in subjects not on lipid-lowering medication. A positive correlation between the concentration of Lp(a) and LDL cholesterol has been reported previously 33, but the mechanism underlying this association is unknown.
It was previously reported that up to 40% of PCSK9 is associated with LDL whereas the rest is found in the apoB-free compartment 23, 24. In subjects with high Lp(a) levels, PCSK9 shows a preferential association with Lp(a) relative to LDL. However, it appears that Lp(a)-associated PCSK9 has an LDL derivation rather than being recruited from the apoB-free compartment. One explanation for the preferential association of PCSK9 with Lp(a) is linked to the approximately 1.5-fold longer half-life of Lp(a) compared with LDL 6.
In conclusion, our results support a scenario where: (i) PCSK9 is found in association with Lp(a) in subjects with high Lp(a) levels (>30 mg/dl); (ii) the association does not depend on the capacity of Lp(a) to carry oxidized phospholipids; (iii) apo(a) alone is not sufficient for the association of Lp(a) with PCSK9; and (iv) PCSK9 preferentially associates with Lp(a) at the expense of LDL. It is currently being debated whether plasma PCSK9 levels are predictive of CVD events 17, 34. Since PCSK9 bound to Lp(a) is mainly in the intact and active form, its plasma level should be investigated as a potential biomarker for CVD.
Limitations of the study
This study is based on data from 39 unique patients with extremely elevated Lp(a) levels. It remains to be determined whether PCSK9 binds Lp(a) in subject with normal Lp(a) levels. The study does not allow us to attribute a functional role to the PCSK9 that is part of Lp(a) or to provide a mechanistic insight into why therapeutic antibodies blocking PCSK9 also lower Lp(a) levels.
Supplementary Material
Novelty and Significance.
What Is Known?
Elevated plasma levels of Lp(a) are associated with increased risk of MI and stroke.
Plasma PCSK9 controls degradation of hepatic LDLR and regulates plasma cholesterol levels.
Anti-PCSK9 antibodies effectively reduce both LDL cholesterol and Lp(a) levels.
A significant portion of plasma PCSK9 associates with the LDL particle.
What New Information Does This Article Contribute?
PCSK9 is associated with plasma Lp(a) in both humans with elevated Lp(a) levels and in Lp(a)-expressing transgenic mice.
PCSK9 levels correlate with Lp(a) levels, but not with the number of kringle IV-2 repeats.
PCSK9 does not directly bind apo(a), and its association with Lp(a) is not related to the presence of oxidized phospholipids.
In subjects with extremely elevated Lp(a), PCSK9 is found more on Lp(a) than on LDL.
LDL-bound PCSK9 represents a large portion of plasma PCSK9. Here we show that PCSK9 is also associated with Lp(a) in subjects with high Lp(a) levels, but this association is not due to direct binding to apo(a), and the capacity of Lp(a) to carry oxidized phospholipids does not play a role in this association . We also show that PCSK9 preferentially associates with Lp(a) than LDL, and that Lp(a)-bound PCSK9 is mainly in the intact and active form. These findings may inform studies to assess the value of Lp(a)-bound PCSK9 in CVD prediction.
ACKNOWLEDGMENTS
The authors thank Cathleen Moscibrocki and Carol Marsh for their technical assistance.
SOURCES OF FUNDING
This study was supported by the National Institutes of Health (NHLBI) through grant R01-HL106845 to SF and NIH R01-HL119828 to ST. Alirocumab was provided by Regeneron, Inc. to ST.
Nonstandard Abbreviations and Acronyms
- apo
apolipoprotein
- FH
familial hypercholesterolemia
- LDL
low-density lipoprotein
- Lp(a)
lipoprotein (a)
- PCSK9
proprotein convertase subtilisin/kexin 9
Footnotes
DISCLOSURES
ST is a co-inventor of and receives royalties from patents or patent applications owned by the University of California San Diego on antibodies used in biotheranostic applications and has a dual appointment at Ionis Pharmaceuticals, Inc. and UCSD.
REFERENCES
- 1.Gurdasani D, Sjouke B, Tsimikas S, Hovingh GK, Luben RN, Wainwright NW, Pomilla C, Wareham NJ, Khaw KT, Boekholdt SM, Sandhu MS. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: The epic-norfolk prospective population study. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:3058–3065. doi: 10.1161/ATVBAHA.112.255521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes DT, Schick BA, Humphries KH, Frohlich J. Lipoprotein(a) is an independent risk factor for cardiovascular disease in heterozygous familial hypercholesterolemia. Clin Chem. 2005;51:2067–2073. doi: 10.1373/clinchem.2005.055228. [DOI] [PubMed] [Google Scholar]
- 3.Marcovina SM, Koschinsky ML. Lipoprotein(a) concentration and apolipoprotein(a) size: A synergistic role in advanced atherosclerosis? Circulation. 1999;100:1151–1153. doi: 10.1161/01.cir.100.11.1151. [DOI] [PubMed] [Google Scholar]
- 4.Alonso R, Andres E, Mata N, Fuentes-Jimenez F, Badimon L, Lopez-Miranda J, Padro T, Muniz O, Diaz-Diaz JL, Mauri M, Ordovas JM, Mata P, Investigators S. Lipoprotein(a) levels in familial hypercholesterolemia: An important predictor of cardiovascular disease independent of the type of ldl receptor mutation. J Am Coll Cardiol. 2014;63:1982–1989. doi: 10.1016/j.jacc.2014.01.063. [DOI] [PubMed] [Google Scholar]
- 5.Romagnuolo R, Scipione CA, Boffa MB, Marcovina SM, Seidah NG, Koschinsky ML. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J Biol Chem. 2015;290:11649–11662. doi: 10.1074/jbc.M114.611988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rader DJ, Mann WA, Cain W, Kraft HG, Usher D, Zech LA, Hoeg JM, Davignon J, Lupien P, Grossman M, et al. The low density lipoprotein receptor is not required for normal catabolism of lp(a) in humans. J Clin Invest. 1995;95:1403–1408. doi: 10.1172/JCI117794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostner GM, Gavish D, Leopold B, Bolzano K, Weintraub MS, Breslow JL. Hmg coa reductase inhibitors lower ldl cholesterol without reducing lp(a) levels. Circulation. 1989;80:1313–1319. doi: 10.1161/01.cir.80.5.1313. [DOI] [PubMed] [Google Scholar]
- 8.Cain WJ, Millar JS, Himebauch AS, Tietge UJ, Maugeais C, Usher D, Rader DJ. Lipoprotein [a] is cleared from the plasma primarily by the liver in a process mediated by apolipoprotein [a]. J Lipid Res. 2005;46:2681–2691. doi: 10.1194/jlr.M500249-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA, Investigators T. Inhibition of pcsk9 with evolocumab in homozygous familial hypercholesterolaemia (tesla part b): A randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:341–350. doi: 10.1016/S0140-6736(14)61374-X. [DOI] [PubMed] [Google Scholar]
- 10.Desai NR, Kohli P, Giugliano RP, O'Donoghue ML, Somaratne R, Zhou J, Hoffman EB, Huang F, Rogers WJ, Wasserman SM, Scott R, Sabatine MS. Amg145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: An analysis from the ldl-c assessment with proprotein convertase subtilisin kexin type 9 monoclonal antibody inhibition combined with statin therapy (laplace)-thrombolysis in myocardial infarction (timi) 57 trial. Circulation. 2013;128:962–969. doi: 10.1161/CIRCULATIONAHA.113.001969. [DOI] [PubMed] [Google Scholar]
- 11.Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, Burkey JL, Yang Q, Marcovina SM, Geary RS, Crooke RM, Witztum JL. Antisense therapy targeting apolipoprotein(a): A randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472–1483. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 12.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (narc-1): Liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton JD, Cohen JC, Hobbs HH. Molecular biology of pcsk9: Its role in ldl metabolism. Trends Biochem Sci. 2007;32:71–77. doi: 10.1016/j.tibs.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, Samarghandi A, Zhang N, Yao Z, Xiong M, Teng BB. Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein b and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1585–1595. doi: 10.1161/ATVBAHA.112.250043. [DOI] [PubMed] [Google Scholar]
- 15.Rashid S, Tavori H, Brown PE, Linton MF, He J, Giunzioni I, Fazio S. Proprotein convertase subtilisin kexin type 9 promotes intestinal overproduction of triglyceride-rich apolipoprotein b lipoproteins through both low-density lipoprotein receptor-dependent and -independent mechanisms. Circulation. 2014;130:431–441. doi: 10.1161/CIRCULATIONAHA.113.006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raal F, Panz V, Immelman A, Pilcher G. Elevated pcsk9 levels in untreated patients with heterozygous or homozygous familial hypercholesterolemia and the response to high-dose statin therapy. J Am Heart Assoc. 2013;2:e000028. doi: 10.1161/JAHA.112.000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridker PM, Rifai N, Bradwin G, Rose L. Plasma proprotein convertase subtilisin/kexin type 9 levels and the risk of first cardiovascular events. European heart journal. 2015 doi: 10.1093/eurheartj/ehv568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nekaies Y, Baudin B, Kelbousi S, Sakly M, Attia N. Plasma proprotein convertase subtilisin/kexin type 9 is associated with lp(a) in type 2 diabetic patients. J Diabetes Complications. 2015;29:1165–1170. doi: 10.1016/j.jdiacomp.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Therapeutic monoclonal antibodies approved or in review in the european union or united states. 2015 [Google Scholar]
- 20.Stein EA, Raal FJ. Insights into pcsk9, low-density lipoprotein receptor, and low-density lipoprotein cholesterol metabolism: Of mice and man. Circulation. 2013;127:2372–2374. doi: 10.1161/CIRCULATIONAHA.113.003360. [DOI] [PubMed] [Google Scholar]
- 21.Gaudet D, Kereiakes DJ, McKenney JM, Roth EM, Hanotin C, Gipe D, Du Y, Ferrand AC, Ginsberg HN, Stein EA. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am J Cardiol. 2014;114:711–715. doi: 10.1016/j.amjcard.2014.05.060. [DOI] [PubMed] [Google Scholar]
- 22.Fan D, Yancey PG, Qiu S, Ding L, Weeber EJ, Linton MF, Fazio S. Self-association of human pcsk9 correlates with its ldlr-degrading activity. Biochemistry. 2008;47:1631–1639. doi: 10.1021/bi7016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavori H, Fan D, Blakemore JL, Yancey PG, Ding L, Linton MF, Fazio S. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: Evidence for a reciprocal regulation. Circulation. 2013;127:2403–2413. doi: 10.1161/CIRCULATIONAHA.113.001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosenko T, Golder M, Leblond G, Weng W, Lagace TA. Low-density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (pcsk9) in human plasma and inhibits pcsk9-mediated ldl receptor degradation. J Biol Chem. 2013;288:8279–8288. doi: 10.1074/jbc.M112.421370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider M, Witztum JL, Young SG, Ludwig EH, Miller ER, Tsimikas S, Curtiss LK, Marcovina SM, Taylor JM, Lawn RM, Innerarity TL, Pitas RE. High-level lipoprotein [a] expression in transgenic mice: Evidence for oxidized phospholipids in lipoprotein [a] but not in low density lipoproteins. J Lipid Res. 2005;46:769–778. doi: 10.1194/jlr.M400467-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Yeang C, Witztum JL, Tsimikas S. 'Ldl-c' = ldl-c + lp(a)-c: Implications of achieved ultra-low ldl-c levels in the proprotein convertase subtilisin/kexin type 9 era of potent ldl-c lowering. Curr Opin Lipidol. 2015;26:169–178. doi: 10.1097/MOL.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 27.Yeang C, Choi Y, Lee S, Bertoia M, Rimm E, Yang X, Witztum J, Tsimikas S. Novel assays for quantification of lipoprotein-associated (pcsk9-apob, pcsk9-lp(a)) proprotein covertase subtilisin/kexin type 9 (pcks9). Circulation. 2015;132:A14697. [Google Scholar]
- 28.Benjannet S, Rhainds D, Hamelin J, Nassoury N, Seidah NG. The proprotein convertase (pc) pcsk9 is inactivated by furin and/or pc5/6a: Functional consequences of natural mutations and post-translational modifications. J Biol Chem. 2006;281:30561–30572. doi: 10.1074/jbc.M606495200. [DOI] [PubMed] [Google Scholar]
- 29.Tavori H, Giunzioni I, Linton MF, Fazio S. Loss of plasma proprotein convertase subtilisin/kexin 9 (pcsk9) after lipoprotein apheresis. Circ Res. 2013;113:1290–1295. doi: 10.1161/CIRCRESAHA.113.302655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hori M, Ishihara M, Yuasa Y, Makino H, Yanagi K, Tamanaha T, Kishimoto I, Kujiraoka T, Hattori H, Harada-Shiba M. Removal of plasma mature and furin-cleaved proprotein convertase subtilisin/kexin 9 (pcsk9) by low-density lipoprotein-apheresis in familial hypercholesterolemia: Development and application of a new assay for pcsk9. The Journal of clinical endocrinology and metabolism. 2014:jc20143066. doi: 10.1210/jc.2014-3066. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell KN, Breslow JL. Adenoviral-mediated expression of pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci U S A. 2004;101:7100–7105. doi: 10.1073/pnas.0402133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konrad RJ, Troutt JS, Cao G. Effects of currently prescribed ldl-c-lowering drugs on pcsk9 and implications for the next generation of ldl-c-lowering agents. Lipids Health Dis. 2011;10:38. doi: 10.1186/1476-511X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contois JH, Lammi-Keefe CJ, Vogel S, McNamara JR, Wilson PW, Massov T, Schaefer EJ. Plasma lipoprotein(a) distribution in the framingham offspring study as determined with a commercially available immunoturbidimetric assay. Clin Chim Acta. 1996;253:21–35. doi: 10.1016/0009-8981(96)06341-3. [DOI] [PubMed] [Google Scholar]
- 34.Leander K, Malarstig A, Van't Hooft FM, Hyde C, Hellenius ML, Troutt JS, Konrad RJ, Ohrvik J, Hamsten A, de Faire U. Circulating pcsk9 predicts future risk of cardiovascular events independently of established risk factors. Circulation. 2016 doi: 10.1161/CIRCULATIONAHA.115.018531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


