Abstract
Purpose
Racial disparities in uterine cancer-related outcomes have been reported. The goal of this study was to determine if race, pre-operative body mass index (BMI) and medical comorbidities are predictors of loss of functional independence after surgery for uterine cancer.
Methods
Loss of independence was defined as a change from pre-operative functional independence, to a post-operative requirement of discharge to a post-care facility or death within the first 30 days following uterine cancer surgery. Demographic factors, comorbidities, BMI, intra-operative and post-operative outcomes and discharge status were abstracted from the 2011 and 2012 American College of Surgeons National Surgical Quality Improvement Program (NSQIP). Statistical analyses included multivariable logistic regression and Wald tests for interaction.
Results
4,005 patients had uterine cancer and were functionally independent pre-operatively. After adjusting for clinical features and comorbidities, Black women were not significantly more likely to lose functional independence than non-Black women. However, a significant interaction (OR=1.17, p<0.001) was found between race and BMI for loss of functional independence. Interaction plots revealed worsening functional outcomes for Black women with BMI>40 but not in non-Blacks.
Conclusions
The interaction suggests a 17% increased odds of losing independence for each unit of BMI difference for Black uterine cancer patients, or 170% increased odds of losing independence for a 10-point increase in BMI, given a linear association. To reduce the likelihood of losing post-operative functional independence, Black, high BMI patients with or at-risk for uterine cancer may especially benefit from weight loss or interventions to optimize physical function.
Keywords: uterine cancer, racial/ethnic disparities, functional dependence, survivorship
Introduction
Uterine cancer is the most common gynecologic malignancy in the United States[1]. Black women have more aggressive tumors and worse histology (Type 2 endometrial cancer) [2], and are significantly more likely to die from uterine cancer than any other racial group[3], warranting exploration of outcomes for Blacks in comparison with all other racial groups. Black women with uterine cancer are more obese[4], younger at diagnosis[2], and have more comorbidities prior to diagnosis[5] than White women, all of which may be associated with the woman’s ability to return to her pre-surgery level of functioning and need for institutional care. For example, a Black uterine cancer patient who was functional enough to go into the hospital unaided may be less likely than a White cancer patient to be able to return home from the hospital with the same degree of functioning as when she left for the hospital, or may be less likely to return home at all. Black women have poorer survival outcomes after uterine cancer, which are not fully explained by a higher number of comorbidities[6], and may include longer hospital stay[7], treatment complications[7,8] that lead to longer operative times, worse prognosis[9,10], histology[11], and greater risk of recurrence and death[2,12]. Many of these factors may be related to ability to return to activities of daily living after uterine cancer surgery.
Functional independence can be defined broadly as the ability to perform activities of daily living without assistance[13,14], the loss of which has been associated with further risk of decline[15], lower quality of life, less autonomy, increased mortality[16,17], increased healthcare needs and higher costs[18]. In a study of older adults who lost independence, 41% died within 1 year of discharge and 29% remained disabled at 1 year [16]. In one safety net hospital setting, loss of independence after surgery was estimated to occur in 27.4% of those ages 55–59, 22.2% of those ages 60–64, 17.4 % of those ages 65–69, 30.3 % of those ages 70–79, and 61.7% in patients over 80 [19]. Although these estimates were not specific to cancer populations, they are inclusive of cancer populations, meaning that some uterine cancer patients will complete treatment and be discharged to return to home without need for further follow-up care, while others may be discharged to rehabilitation services, nursing homes, care facilities, or may not survive. The life changes can have profound effects for patients [18], which may be especially salient for Black uterine cancer patients, who are already predisposed to poorer outcomes after surgery [6] and are more likely to have scarcer access to health resources to address symptoms and issues in follow-up [20]. Yet there has been no published assessment on who is more likely to experience loss of functional independence after uterine cancer surgery or whether or not there are differences by race.
The purpose of this study was to explore predictive factors associated with loss of functional independence after uterine cancer surgery, including race, pre-operative body mass index (BMI), co-morbidities, and level of surgical complexity. In this analysis, and similar to previous studies [19,17,18], loss of functional independence was defined as a patient who could not return home after her surgery due to discharge to a post-care facility or death within the first 30 days following uterine cancer surgery.
Methods
Data Sources and Study Sample
Gynecologic surgeries performed between 2011 and 2012 were identified using the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database. ACS NSQIP is a national, validated, risk-adjusted data set submitted by participating hospitals, based on patient medical charts, for the purpose of improving the quality of surgical care. It includes data on pre-operative risk factors, intra-operative variables, and 30-day post-operative mortality and morbidity outcomes for over 100,000 patients over 16 years of age undergoing major surgical procedures in both the inpatient and outpatient setting. Data were collected by trained surgical clinical reviewers provided by the American College of Surgeons. Peri-operative outcomes within NSQIP have been validated by the trained surgical clinical reviewers of individual patient charts per standard registry protocols. This study used de-identified secondary data only, thus met the qualifications for exemption from IRB review.
Sample
Inclusion criteria included patients with a diagnosis of uterine cancer (ICD-9 179.x, 182.x, and 233.2) who underwent hysterectomy (under the assumption that <5% of uterine malignancies would be non-endometrial), including those who had lymphadenectomy, bilateral salpingo-oophorectomy (BSO), and were physically independent prior to surgery. Exclusion criteria included patients who: 1) were non-functionally independent within the 30 days prior to surgery (partially dependent, totally dependent pre-operatively, diagnosis of quadriplegia, impaired sensorium, and coma); 2) had an unknown discharge destination; 3) had no race data reported; 4) weighed greater than 600lbs or unknown weight or height; 5) had secondary procedural codes for a major concurrent procedure, such as gastrointestinal, genitourinary or other surgeries (under the presumption that they had extreme advanced stage disease, or additional surgical conditions beyond uterine cancer); and 6) had an operative time less than 40 minutes or greater than 600 minutes (under the presumption that an unusually short operative time indicated that the patient did not have a complete hysterectomy or an aborted procedure, or an unusually long operative time due to additional health conditions and surgical complexity).
Key Variables
The outcome of loss of functional independence was defined as: a change from baseline pre-operative functional independence to a post-operative status requiring any of the following: 1) discharge to a post-care facility, including skilled care, unskilled facility, separate acute care, or rehabilitation facilities, or 2) death within the immediate post-operative 30 day period. Pre-operative functional independence was defined by NSQIP as “the patient’s abilities to perform activities of daily living in the 30 days prior to surgery. Activities of daily living are defined as ‘the activities usually performed in the course of a normal day in a person’s life… bathing, feeding, dressing, toileting, and mobility. ’” The best functional status within the 30 days prior to surgery is reported based on the following criteria: “(1) Independent: The patient does not require assistance from another person for any activities of daily living. This includes a person who is able to function independently with prosthetics, equipment, or devices.; (2) Partially dependent: The patient requires some assistance from another person for activities of daily living. This includes a person who utilizes prosthetics, equipment, or devices but still requires some assistance from another person for activities of daily living; or (3) Totally dependent: The patient requires total assistance for all activities of daily living.” [21]
Demographic factors of age at time of surgery and self-reported race, comorbidities, body mass index (BMI), intra-operative and post-operative outcomes, and discharge status data were included for analysis. Demographic and clinical data were collected including the following: age at time of surgery, body mass index (BMI), race, pre-operative health conditions, comorbidities, intra-operative and post-operative outcomes, and discharge destination. Age was dichotomized at age 60 because that is the average age of endometrial cancer diagnosis[22], and to account for the NSQIP age variable that grouped all patients over the age of 90 into one category. Pre-operative BMI was calculated based on reported weight and height. Due to no height or weight listed, 39 patients were missing BMI and were excluded from the analysis. Comorbidities included diabetes, dyspnea, chronic obstructive pulmonary disorder (COPD), cardiovascular disease, hypertension, transient ischemic attacks, and stroke. We used the count of comorbidities in our regression models. Worse pre-operative conditions were defined as having ascites, disseminated cancer, prior operation within 30 days, emergent surgical case, baseline renal failure or dialysis. Pre-operative hospital stay was defined as days of hospital admission prior to the surgery date.
Major complications included: organ surgical site infection, wound disruption, stroke, coma, myocardial infarction, cardiac arrest requiring cardiopulmonary resuscitation (CPR), pulmonary embolism, unplanned intubation, on ventilator dependence greater than 48 hours, acute renal failure, sepsis, re-operation, and deep vein thrombosis (DVT) requiring therapy post-operatively. Pre-operative hospital stay was defined as days of hospital admission prior to the surgery date. Type of surgical approach was classified as open abdominal versus minimally invasive surgery. Minimally invasive surgery included all types of laparoscopy-assisted supracervical hysterectomy (LASH), Laparoscopy-Assisted Vaginal Hysterectomy and Total Laparoscopic Hysterectomy.
Statistical Analysis
Cases were excluded from analysis when data were missing on discharge destination, pre-admission function, height, weight or race. When these variables were present, analyses were run even if there were other missing data; results were unchanged when run as complete-case analysis. Mann-Whitney and Chi-square tests were used for comparison tests. Multivariable logistic regression with a dichotomous outcome for whether or not the patient was classified as having loss of functional independence, was performed based on the aforementioned definition. To assess whether or not results were driven by differences in surgical approaches, a sensitivity analysis was performed among those patients who underwent open abdominal surgery. Wald tests were used to assess the interaction of race and BMI. Stata/MP 13.0 was used for statistical analysis. A two-sided P value of 0.05 was considered statistically significant.
Results
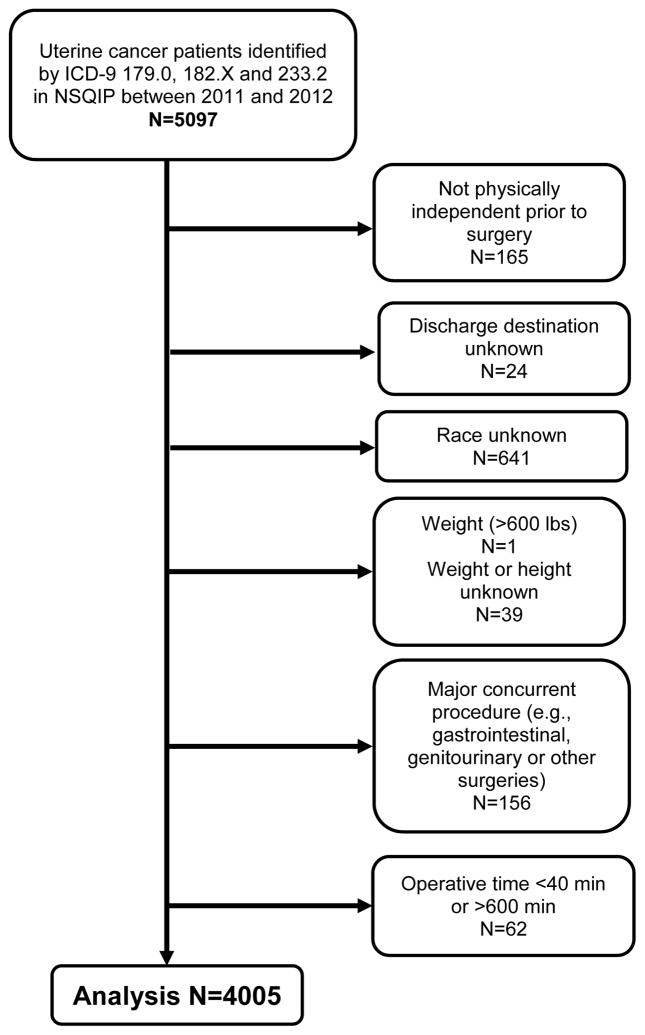
As shown in Figure 1, 4,005 patients of the total NSQIP patient sample met inclusion/exclusion criteria and were identified as having a diagnosis of uterine cancer and who were functionally independent pre-operatively (Figure 1. Flow Chart). Demographic information of these patients appear in Table 1. Of the 4,005 total patients, 305 (7.6%) were Black and 3,700 (92.4%) were non-Black. Non-Black included White (n=3,544), Asian (n=138), American Indian/Alaskan Native (n=8), and Native Hawaiian/Pacific Islander (n=10). Compared with non-Black, Black uterine cancer patients had significantly higher average BMI (35.5 vs. 32.4, p<0.001), were more likely to have one or more comorbidities (77.7% vs. 59.6%, p<0.001), and had longer operative times (175 minutes vs. 163 minutes, p<0.001). Black women were more likely than non-Black women to have open abdominal surgery (52.5% vs. 33.9%, p<0.001), but all of the Black women who were discharged to a facility had open abdominal surgery. There were no significant differences by race, age category of patient at time of surgery, major complication during surgery, operative time, worse pre-operative condition, pre-operative hospital stay, or likelihood of discharge to a post-acute facility (2.3% for Black women versus 3.2% for non-Black women, p=0.38).
Figure 1.
Patient Selection Flow Diagram
Table 1.
Demographics
| All (N=4005 ) | Black (n=305 ) | Non-Black (n=3700 ) | p-value | |
|---|---|---|---|---|
| Age | 62 (IQR: 56–70) | 63(IQR: 56–68) | 62(IQR: 56–70) | 0.37 |
| Age<60 | 1543(38.5%) | 113(37.1%) | 1430(38.6%) | 0.58 |
| Age≥60 | 2462(61.5%) | 192(62.9%) | 2270(61.4%) | |
| BMI | 32.8 (IQR: 26.9–40.4) | 35.5 (IQR: 30.8–42.4) | 32.4 (IQR: 26.6–40.2) | <0.0001 |
| Comorbidity | 1 (IQR:0–1) | 1(IQR:1–2) | 1(IQR:0–1) | <0.0001 |
| 0 | 1561 (39.0%) | 68(22.3%) | 1493(40.4%) | <0.0001 |
| 1 | 1541 (38.5%) | 127(41.6%) | 1414(38.2%) | |
| ≥2 | 903 (22.5%) | 110(36.1%) | 793(21.4%) | |
| Major Complication | 195 (4.9%) | 15(4.9%) | 180(4.9%) | 0.97 |
| Worse Pre-Operative Condition | 179 (4.5%) | 20(6.6%) | 159(4.3%) | 0.07 |
| Operative Time (minutes) | 164 (IQR: 121–214) | 175 (IQR: 132.5–242.5) | 163 (IQR: 120–213) | <0.0001 |
| Pre-Operative Hospital Stay | ||||
| 0 | 3878 (96.8%) | 289(94.8%) | 3589(97%) | 0.07 |
| 1 day | 74 (1.9%) | 8(2.6%) | 66(1.8%) | |
| ≥ 2 days | 53 (1.3%) | 8(2.6%) | 45(1.2%) | |
| Open abdominal surgery | 1416(33.4%) | 160(52.5%) | 1256(33.9%) | <0.0001 |
| Expired | 5(0.12%) | 1(0.33%) | 4(0.11%) | 0.33 |
| Discharge to facility | 126(3.2%) | 7(2.3%) | 119(3.2%) | 0.38 |
After adjusting for race, BMI, age, number of comorbidities, worse pre-operative condition, major complications and days hospitalized prior to surgery, Black women were not significantly more likely to lose functional independence overall during the post-operative period than non-Black women (results not presented). However, a significant interaction (Table 2) was found between Black and BMI for loss of functional independence, with an OR=1.17 (p<0.001) indicating a 17% increased odds of losing functional independence for each increasing unit of BMI. Being over 60 (OR=4.18, p<0.001), having more comorbid conditions (OR=1.64, p<0.001), worse pre-operative condition (OR=2.53, p<0.001), experiencing major complication during surgery (OR=2.57, p<0.001), and pre-surgical admission (OR=1.19, p<0.001) were significantly and positively associated with increased odds of loss of functional independence. The significant interaction between Black race and BMI (OR=1.14; p<0.001) remained in the subsample of participants (n=1416) with open abdominal surgery only (Table 3).
Table 2.
Logistic Regression Results for Loss of Functional Independence Outcome (N=3583)
| Unadjusted ORs | No interaction | With interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p-value | OR | 95%CI | p-value | OR | 95%CI | p-value | |
| Black* BMI | -- | -- | -- | -- | -- | -- | 1.17 | 1.08–1.26 | <0.0001 |
| Black | 0.72 | 0.31–1.65 | 0.437 | 0.50 | 0.20–1.23 | 0.132 | -- | -- | -- |
| BMI | 1.01 | 0.99–1.03 | 0.196 | 1.01 | 0.99–1.03 | 0.382 | -- | -- | -- |
| Age>60 | 3.95 | 2.32–6.73 | <0.001 | 3.89 | 2.18–6.91 | <0.0001 | 4.18 | 2.29–7.63 | <0.0001 |
| Comorbid | 1.76 | 1.47–2.12 | <0.001 | 1.57 | 1.27–1.93 | <0.0001 | 1.64 | 1.33–2.01 | <0.0001 |
| Worse Pre-op Condition | 3.45 | 2.30–5.17 | <0.001 | 2.59 | 1.58–4.26 | <0.0001 | 2.53 | 1.53–4.19 | <0.0001 |
| Major Complication | 2.64 | 2.10–3.32 | <0.001 | 2.53 | 1.97–3.25 | <0.0001 | 2.57 | 1.99–3.33 | <0.0001 |
| Operative Time* | 1.00 | 0.10–1.00 | 0.373 | 1.00 | 0.99–1.00 | 0.493 | 1.00 | 0.99–1.00 | 0.4 |
| Pre-Operative Hospital Stay | 1.23 | 1.09–1.39 | 0.001 | 1.18 | 1.07–1.31 | 0.001 | 1.19 | 1.08–1.32 | 0.001 |
Wald test for interaction: p<0.0001; component elements of interaction term not reported because they are not directly interpretable
Only patients with operative times in the 5th–95th percentile (78.3–311 minutes) were included in the regression model.
Table 3.
Sensitivity Analysis: Logistic Regression for Loss of Functional Independence Outcome among Patients with Open Abdominal Surgery (n=1238)
| Unadjusted ORs | With Interaction | |||||
|---|---|---|---|---|---|---|
| Regression Odds Ratio | 95%CI | p-value | Regression Odds Ratio | 95%CI | p-value | |
| Black* BMI | -- | -- | 1.15 | 1.07–1.24 | <0.0001 | |
| Black | 0.54 | 0.23–1.27 | 0.159 | -- | -- | -- |
| BMI | 1.01 | 0.99–1.03 | 0.279 | -- | -- | -- |
| Age>60 | 6.09 | 3.03–12.25 | <0.001 | 8.76 | 3.80–20.23 | <0.0001 |
| Comorbid | 1.70 | 1.37–2.10 | <0.001 | 1.62 | 1.27–2.06 | <0.0001 |
| Worse Pre-op Condition | 2.15 | 1.41–3.23 | <0.001 | 1.68 | 1.01–2.84 | 0.050 |
| Major Complication | 2.25 | 1.72–2.95 | <0.001 | 2.39 | 1.73–3.31 | <0.0001 |
| Operative Time* | -- | 0.10–1.00 | 0.538 | 1.00 | 0.99–1.00 | 0.486 |
| Pre-operative Hospital Stay | 0.54 | 1.01–1.24 | 0.036 | 1.13 | 1.03–1.26 | 0.024 |
Wald test for interaction: p=0.0002; component elements of interaction term not reported because they are not directly interpretable
Only patients with operative times in the 5th–95th percentile (78.3–311 minutes) were included in the regression model.
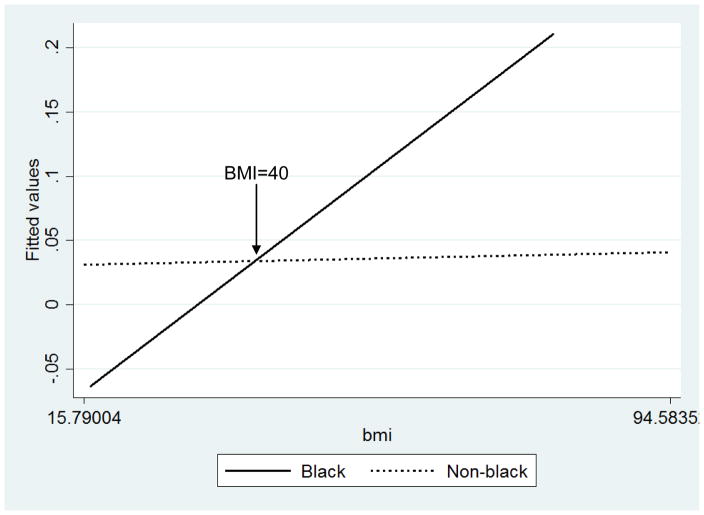
An interaction plot (Figure 2) revealed worse functional outcomes per unit change in BMI for Black women but not for non-Black women, with a cross-over effect at BMI=40. Based on examination of the plot, Black and non-Black women have the same odds of loss of functional independence at BMI of 40. Black women with BMI<40 experienced lower odds of loss of functional independence compared with non-Black women, but higher odds of loss of functional independence with BMI>40 compared with non-Black women. Sensitivity analysis that excluded all race groups other than White and Black revealed similar results; however, the sample size for other races (<5%) may have been too small to detect differences by the “other races” group members.
Figure 2.
Plot of Race*Body Mass Index (BMI) Interaction for Loss of Functional Independence after Uterine Cancer Surgery
Discussion
A significant interaction between Black race and BMI suggested that each unit increase in BMI would confer a 17% increase in odds of losing functional independence, making Black women with high BMIs at high-risk for losing functional independence. Given a linear relationship between BMI and loss of independence, this would translate to a 170% increase in odds of losing functional dependence for a 10-point increase in BMI. At a BMI of less than 40, Black women have less loss of function than non-Black women, but non-Black women still have relatively low risk of loss of function across all BMIs. Other key contributing factors to loss of independence were being over 60 years of age, higher number of co-morbid conditions, worse pre-operative condition, experiencing a major complication, and greater length of pre-operative hospital stay. Of these, age and worse pre-operative condition had the strongest association, which have been previously associated with functional decline after hospital admission[17] and higher post-discharge mortality risk[23], respectively.
The initial multivariable regression result aligns with a recent study suggesting no worse post-surgical 30-day outcomes for Black compared to White endometrial cancer patients, when accounting for pre- and post-operative complications[24]. This analysis reinforced that race in and of itself does not explain the disparities in why Black patients have differential outcomes after treatment. It is the combination of the factors associated with the race, including higher BMI, that drive why Black patients experience worse outcomes for functional independence. While the initial multivariable results suggested that adjusting for clinical factors could overshadow the effect of race on functional loss disparities, the experience is that Black uterine cancer patients will still present in clinic with both higher BMIs and higher numbers of co-morbidities, not just one or the other, and then may go on to experience higher levels of complications [7,8]. Factors operating at the physician, hospital, and community level may also contribute to both higher BMI and lower physical functioning in overweight Black women, especially given that Black cancer patients are more likely to be seen in low-resource and low-volume healthcare settings [25]. Reductions in BMI may decrease the number of comorbidities and related negative health factors that Black women experience. Weight loss and exercise programs have not been well-studied in patients prior to hysterectomy, but have been studied among the endometrial cancer survivor population [26–28].
Targeting patients for weight loss prior to surgery may be the most effective method to prevent functional loss after surgery; however, one of the challenges is that there may be insufficient time between diagnosis and surgery for patients to achieve weight loss significant enough to reduce the disparity in functional loss, which based on this single analysis, would be a goal of a BMI of under 40. Surgical intervention for weight loss at the time of hysterectomy has not been explored, but there is a current ongoing pilot study to explore the feasibility of a brief pre-surgery weight loss intervention to improve surgical outcomes in endometrial cancer patients [29]. Future studies should explore the effectiveness of weight loss and physical activity interventions both prior to surgery and after surgery for uterine cancer survivors, and should specifically target Black women with BMI>40.
Weight loss through changes in diet and increased physical activity has been shown to be achievable for endometrial cancer survivors[26–28] and endometrial cancer survivors show strong desire to participate in exercise interventions[30]. Studies in functional decline for other conditions have shown that a lack of regular vigorous physical activity nearly doubled the odds of further declines over time, and suggests that physical activity can reduce potential declines by as much as 32%[15]. Exercise does not worsen pre-operative conditions such as ascites[31,32] and has been associated with improved outcomes for patients on dialysis[33,34]. Exercise may have maximal benefits by improving pre-operative conditions which are the precursors for post-surgery loss of functional independence. Weight loss studies targeting Black women specifically have found success when interventions involve community members and leaders in planning the trajectory and content of the intervention [35], promote walking and resistance training [36], and focus on goal-setting [37]. Studies should include measures to assess actual changes in physical functioning pre-and post-surgery, as well as account for factors like health insurance and modality of surgery, which may lead to tailored referrals most appropriate for recovery from open versus minimally invasive surgery. Given the multi-level influences on BMI, studies should consider multi-level weight reduction intervention approaches that target the individual, the healthcare setting, and the health-promoting resources available in an individual’s daily environment.
Although the outcome was present in only 3% of the study population, uterine cancer is the most common gynecologic malignancy[1], and thus may constitute a large absolute number of patients who are at risk for loss of functional independence. Due to the limitations of the NSQIP dataset, this analysis may underestimate the degree of functional losses experienced by uterine cancer patients, as the data has limited follow-up after discharge from the hospital. An alternative would have been to assess any new disabilities that arose in the first 30 days after surgery, but this data set did not allow for differentiation of whether or not a disability arose due to the uterine cancer surgery itself. Not being able to return home after surgery may represent the most extreme form of loss of independence, which would have the greatest impact on a patient’s life. All of the Black patients in the sample who had experienced loss of function had also had abdominal surgery, which could have been an artifact of the data sample; however the data set used is of the largest available to assess differences in functional loss and has been demonstrated over time to successfully predict outcomes in surgical patients better than other administrative datasets[38]. Sensitivity analysis also supported that loss of independence was more likely for Black uterine cancer patients of high BMI, regardless of the modality of the surgical procedure. Data were not available on cancer history, including cancer stage, grade and history, or social and environmental factors, such as insurance status, socioeconomic position, relationship status, stairs in the home, or urban or rural population, which may affect whether or not patients are referred to care facilities [19]. It is possible that different hospitals have different surgical protocols, and some are more likely to treat patients aggressively, which may lead to longer hospital stay and higher likelihood to be discharged to facility for recovery.
The findings from this study highlight the need to address disparities in post-operative functioning, and to identify race as a characteristic for determining who is most at risk to experience loss of functional independence after uterine cancer surgery. To prevent functional losses after surgery, clinicians should emphasize healthy weight maintenance or weight loss for Black women who are at high risk for uterine cancer, especially for those with a BMI of >40. Prescriptions for exercise, physician-led counseling [39], and expanded insurance coverage for fitness facilities could be integrated into primary care and oncology practice to assist in weight loss and maintenance across the health and disease continuum. Recent advancements in this area have shown that one-on-one physician-led counseling combined with telemedicine and text messaging based weight loss techniques can produce significant weight loss for endometrial cancer patients [40]. Together these efforts may improve disease- specific as well as overall health outcomes in Black women with uterine cancer.
Acknowledgments
Funding: This work was supported by the National Institutes of Health and National Cancer Institute (grant number 1K01CA184288).
Footnotes
The abstract and results of this paper have been presented in a poster presentation at the American Association for Cancer Research Annual Meeting on April 18–22, 2015 in Philadelphia, Pennsylvania. The full manuscript has never been published, in whole, to any audience or entity.
Author’s Disclosures of Potential Conflict of Interests: None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Fiorelli J, Schiff PB, Burke WM, Kansler AL, Cohen CJ, Herzog TJ. Racial disparities for uterine corpus tumors. Cancer. 2009;115(6):1276–1285. doi: 10.1002/cncr.24160. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control. [Accessed 17 December 2014];Uterine Cancer Rates by Race and Ethnicity. 2014 http://www.cdc.gov/cancer/uterine/statistics/race.htm.
- 4.Park SL, Goodman MT, Zhang ZF, Kolonel LN, Henderson BE, Setiawan VW. Body size, adult BMI gain and endometrial cancer risk: the multiethnic cohort. International Journal of Cancer. 2010;126(2):490–499. doi: 10.1002/ijc.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahdi H, Lockhart D, Moslemi-Kebria M, Rose PG. Racial disparity in the 30-day morbidity and mortality after surgery for endometrial cancer. Gynecologic Oncology. 2014;134(3):510–515. doi: 10.1016/j.ygyno.2014.05.024. http://dx.doi.org/10.1016/j.ygyno.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Long B, Liu FW, Bristow RE. Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecologic Oncology. 2013;130(3):652–659. doi: 10.1016/j.ygyno.2013.05.020. http://dx.doi.org/10.1016/j.ygyno.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allard JE, Maxwell GL. Race disparities between black and white women in the incidence, treatment, and prognosis of endometrial cancer. Cancer Control. 2009;16(1):53–56. doi: 10.1177/107327480901600108. [DOI] [PubMed] [Google Scholar]
- 8.Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. American Journal of Obstetrics and Gynecology. 2014;210(3):194–199. doi: 10.1016/j.ajog.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Wahab ZR, Kumar S, Mutch DG, Dowdy SC, Hensley SA, Wang Y, Mahdi H, Ali-Fehmi R, Morris RT, Elshaikh M. Racial disparities in uterine clear cell carcinoma: a multi-institution study. International Journal of Gynecological Cancer. 2014;24(3):541–548. doi: 10.1097/IGC.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 10.Erickson BK, Doo DW, Zhang B, Huh WK, Leath CA., III Black race independently predicts worse survival in uterine carcinosarcoma. Gynecologic oncology. 2014;133(2):238–241. doi: 10.1016/j.ygyno.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 11.Rauh-Hain JA, Clemmer JT, Bradford LS, Clark RM, Growdon WB, Goodman A, Boruta DM, Schorge JO, Carmen MG. Racial disparities in cervical cancer survival over time. Cancer. 2013;119(20):3644–3652. doi: 10.1002/cncr.28261. [DOI] [PubMed] [Google Scholar]
- 12.Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee Y-C, Futoran RJ, Cohn DE, Ioffe OB. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I–IV carcinosarcoma (CS) of the uterus. Gynecologic oncology. 2007;107(2):177–185. doi: 10.1016/j.ygyno.2007.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milte R, Crotty M. Musculoskeletal health, frailty and functional decline. Best Practice & Research Clinical Rheumatology. 2014;28(3):395–410. doi: 10.1016/j.berh.2014.07.005. http://dx.doi.org/10.1016/j.berh.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Beaton K, Grimmer K. Tools that assess functional decline: systematic literature review update. Clinical Interventions in Aging. 2013;8:485. doi: 10.2147/CIA.S42528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunlop DD, Semanik P, Song J, Manheim LM, Shih V, Chang RW. Risk factors for functional decline in older adults with arthritis. Arthritis & Rheumatism. 2005;52(4):1274–1282. doi: 10.1002/art.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyd CM, Ricks M, Fried LP, Guralnik JM, Xue QL, Xia J, Bandeen-Roche K. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: the Women’s Health and Aging Study I. Journal of the American Geriatrics Society. 2009;57(10):1757–1766. doi: 10.1111/j.1532-5415.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, Burant CJ, Landefeld CS. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. Journal of the American Geriatrics Society. 2003;51(4):451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 18.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. Jama. 2011;306(16):1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 19.Chodos AH, Kushel MB, Greysen SR, Guzman D, Kessell ER, Sarkar U, Goldman LE, Critchfield JM, Pierluissi E. Hospitalization-Associated Disability in Adults Admitted to a Safety-Net Hospital. Journal of General Internal Medicine. 2015;30(12):1765–1772. doi: 10.1007/s11606-015-3395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haynes-Maslow L, Allicock M, Johnson L-S. Cancer support needs for African American breast cancer survivors and caregivers. Journal of Cancer Education. 2016;31(1):166–171. doi: 10.1007/s13187-015-0832-1. [DOI] [PubMed] [Google Scholar]
- 21.National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data. Chicago, IL: 2013. [Google Scholar]
- 22.American Cancer Society. Detailed Guide: Endometrial Cancer: What are the Risk Factors for Endometrial Cancer? [Accessed 3 December 2014];2005 http://www.cancer.org/cancer/endometrialcancer/detailedguide/endometrial-uterine-cancer-risk-factors.
- 23.Chen C, Sia I, Ma HM, Tai BC, Cheong A, Fong NP, Tan SY, Chan KM, Tan BY, Menon E, Ee CH, Lee KK, Ng YS, Teo YY, Ma S, Heng D, Koh GC. The synergistic effect of functional status and comorbidity burden on mortality: a 16-year survival analysis. PloS one. 2014;9(8):e106248. doi: 10.1371/journal.pone.0106248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cote ML, Ruterbusch JJ, Ahmed Q, Bandyopadhyay S, Alosh B, Abdulfatah E, Seward S, Morris R, Ali-Fehmi R. Endometrial cancer in morbidly obese women: Do racial disparities affect surgical or survival outcomes? Gynecologic Oncology. 2014;133(1):38–42. doi: 10.1016/j.ygyno.2014.01.013. http://dx.doi.org/10.1016/j.ygyno.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray BH, Schlesinger M, Siegfried SM, Horowitz E. Racial and ethnic disparities in the use of high-volume hospitals. INQUIRY: The Journal of Health Care Organization, Provision, and Financing. 2009;46(3):322–338. doi: 10.5034/inquiryjrnl_46.03.322. [DOI] [PubMed] [Google Scholar]
- 26.von Gruenigen V, Frasure H, Kavanagh MB, Janata J, Waggoner S, Rose P, Lerner E, Courneya KS. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial. Gynecol Oncol. 2012;125(3):699–704. doi: 10.1016/j.ygyno.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 27.Basen-Engquist K, Carmack C, Brown J, Jhingran A, Baum G, Song J, Scruggs S, Swartz MC, Cox MG, Lu KH. Response to an exercise intervention after endometrial cancer: differences between obese and non-obese survivors. Gynecol Oncol. 2014;133(1):48–55. doi: 10.1016/j.ygyno.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarroll ML, Armbruster S, Frasure HE, Gothard MD, Gil KM, Kavanagh MB, Waggoner S, von Gruenigen VE. Self-efficacy, quality of life, and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): a randomized controlled trial. Gynecol Oncol. 2014;132(2):397–402. doi: 10.1016/j.ygyno.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Ko E. Evaluating the Effect of Perioperative Caloric Restriction Program on Perioperative Outcomes in Patients With Obesity and Endometrial Cancer ( NCT02665962) Abramson Cancer Center, University of Pennsylvania; Philadelphia, PA: 2015. [Google Scholar]
- 30.Karvinen KH, Courneya KS, Campbell KL, Pearcey RG, Dundas G, Capstick V, Tonkin KS. Exercise preferences of endometrial cancer survivors: a population-based study. Cancer Nurs. 2006;29(4):259–265. doi: 10.1097/00002820-200607000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Salo J, Guevara M, Fernandez-Esparrach G, Bataller R, Gines A, Jimenez W, Gines P, Rivera F, Arroyo V, Rodes J. Impairment of renal function during moderate physical exercise in cirrhotic patients with ascites: relationship with the activity of neurohormonal systems. Hepatology. 1997;25(6):1338–1342. doi: 10.1002/hep.510250606. [DOI] [PubMed] [Google Scholar]
- 32.Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55(suppl 6):vi1–vi12. doi: 10.1136/gut.2006.099580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansen KL. Exercise in the end-stage renal disease population. Journal of the American Society of Nephrology: JASN. 2007;18(6):1845–1854. doi: 10.1681/ASN.2007010009. [DOI] [PubMed] [Google Scholar]
- 34.Delgado C, Johansen KL. Barriers to exercise participation among dialysis patients. Nephrology Dialysis Transplantation. 2012;27(3):1152–1157. doi: 10.1093/ndt/gfr404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong A, Tussing-Humphreys L, Odoms-Young A, Stolley M, Fitzgibbon M. Systematic review of behavioural interventions with culturally adapted strategies to improve diet and weight outcomes in African American women. Obesity Reviews. 2014;15(S4):62–92. doi: 10.1111/obr.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paxton RJ, Nayak P, Taylor WC, Chang S, Courneya KS, Schover L, Hodges K, Jones LA. African-American breast cancer survivors’ preferences for various types of physical activity interventions: a Sisters Network Inc. web-based survey. Journal of Cancer Survivorship. 2014;8(1):31–38. doi: 10.1007/s11764-013-0307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitt-Glover MC, Kumanyika SK. Systematic review of interventions to increase physical activity and physical fitness in African-Americans. American Journal of Health Promotion. 2009;23(6):S33–S56. doi: 10.4278/ajhp.070924101. [DOI] [PubMed] [Google Scholar]
- 38.Atherly A, Fink AS, Campbell DC, Mentzer RM, Jr, Henderson W, Khuri S, Culler SD. Evaluating alternative risk-adjustment strategies for surgery. American journal of surgery. 2004;188(5):566–570. doi: 10.1016/j.amjsurg.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 39.Kumanyika S, Fassbender J, Phipps E, Tan-Torres S, Localio R, Morales KH, Sarwer DB, Harralson T, Allison K, Wesby L. Design, recruitment and start up of a primary care weight loss trial targeting African American and Hispanic adults. Contemporary Clinical Trials. 2011;32(2):215–224. doi: 10.1016/j.cct.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haggerty AF, Huepenbecker S, Sarwer DB, Spitzer J, Raggio G, Chu CS, Ko E, Allison KC. The use of novel technology-based weight loss interventions for obese women with endometrial hyperplasia and cancer. Gynecologic oncology. 2015;140(2):239–244. doi: 10.1016/j.ygyno.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]