Abstract
Rationale
Fibrosis is an important structural contributor to formation of atrial fibrillation (AF) substrate in heart failure (HF). TGF-β signaling is thought to be intricately involved in creation of atrial fibrosis.
Objective
We hypothesized that gene-based expression of dominant-negative type II TGF-β receptor (TGF-β-RII-DN) in the posterior left atrium (PLA) in a canine HF model will sufficiently attenuate fibrosis induced changes in atrial conduction and/or restitution to decrease AF. Since AF electrograms (EGMs) are thought to reflect AF substrate, we further hypothesized that TGF-β-RII-DN would lead to increased fractionation and decreased organization of AF EGMs.
Methods and Results
21 dogs underwent injection + electroporation in the PLA of plasmid expressing a dominant negative TGF-β type II receptor (pUBc-TGFβ-DN-RII) (N=9) or control vector (pUBc-LacZ) (N=12), followed by 3–4 weeks of right ventricular tachypacing (VTP) (240 bpm). Compared to controls, dogs treated with pUBC-TGFβ-DN-RII demonstrated an attenuated increase in conduction inhomogeneity (CI), flattening of restitution slope and decreased duration of induced AF, with AF EGMs being more fractionated and less organized in pUBc-TGFβ-DN-RII versus pUBc-LacZ dogs. Tissue analysis revealed a significant decrease in replacement/interstitial fibrosis, pSMAD2/3 and pERK1/2.
Conclusions
Targeted, gene-based reduction of TGF-β signaling in the PLA – with resulting decrease in replacement fibrosis – led to beneficial remodeling of both conduction and restitution characteristics of the PLA, translating into a decrease in AF and increased complexity of AF EGMs. In addition to providing mechanistic insights, this data may have important diagnostic and therapeutic implications for AF.
Keywords: Atrial fibrillation, fibrosis, gene therapy, congestive heart failure
INTRODUCTION
Atrial fibrillation (AF) is the most common heart rhythm disorder and is a major cause of stroke and heart failure (HF)1. Unfortunately, current therapies for AF, including drugs and ablation are suboptimal in the setting of persistent AF and structural heart disease (e.g. HF)2. Recent investigations have therefore attempted to better understand the pathophysiological mechanisms underlying AF, in order to develop more ‘mechanism’ guided approaches to treat AF.
The development of AF is heralded by electrophysiological and structural alterations which serve to maintain, promote and propagate AF. Of the structural changes that occur in AF, fibrosis is considered especially important in the generation of the substrate leading to AF, especially in the setting of HF, with fibrosis likely contributing to reentry by causing inhomogeneous conduction in the atrium3–5. Although the underlying molecular mechanisms that lead to the development of fibrosis are complex, several recent studies suggest that the Transforming Growth Factor-β1 (TGF-β-1) signaling pathway may be an important contributor to the development of atrial fibrosis6–9. TGF- β-1 is an inflammatory, pro-fibrotic cytokine that stimulates the production of extracellular matrix proteins such as collagen, fibronectin, and proteoglycans in a number of different organ systems, including the heart.10,11 TGF-β is also known to cause generation of reactive oxygen species, with NADPH oxidase 4 (NOX4) generated reactive oxygen species thought to at least partially mediate TGF-β effects in the atrium12. Serum levels of TGF-β have been shown to be increased in patients with AF undergoing defibrillation10. Similarly, tissue expression of TGF-β is increased in the atria of patients with AF secondary to valvular heart disease13. Moreover, transgenic over-expression of TGF-β in the mouse causes selective fibrosis of atrial, but not ventricular myocardium14, resulting in inhomogenous atrial conduction and increased AF inducibility11.
We and others have recently reported the use of gene-based approaches to target key mechanisms underlying the development of AF substrate, with a resulting reduction in spontaneous and/or inducible AF15–18. Since structural remodeling, specifically fibrosis, is key to the development of AF substrate in the setting of structural heart disease, we now report a novel gene-based approach to decrease the development of atrial fibrosis in the setting of HF. We hypothesized that: a) targeted reduction of fibrosis in the atrium in a canine HF model of AF by selective, gene-based inhibition of pro-fibrotic TGF-β signaling would significantly attenuate fibrosis-induced conduction heterogeneity, with a resulting decrease in inducible AF, b) AF triggers and drivers have been shown to originate in a majority of patients with AF in the posterior left atrium (PLA)4, with this region having been shown to have unique molecular, structural and electrophysiological attributes that are thought to contribute to the genesis of AF. We therefore hypothesized that targeted reduction of fibrosis in the PLA would be sufficient to decrease inducible AF in this model of AF and c) in light of clinical studies that indicate that altered action potential duration (APD) restitution characteristics of the atrium predispose to AF, targeted inhibition of fibrosis in the PLA would decrease the restitution slope (of the PLA). In order to test our hypotheses, we employed a novel non-viral gene therapy approach in a well characterized canine model of HF, in which fibrosis-induced heterogeneity of conduction is thought to underlie AF substrate. Specifically, we performed targeted injection in the canine PLA of a trans-gene expressing a kinase-deficient, dominant negative TGF-β type II receptor (TGF-β-RII DN) under the control of a long acting human polyubiquitinin C (UBc) promoter. We report that such a strategy is highly efficacious in reducing atrial fibrosis in the HF atrium, with the resulting attenuation in heterogeneous conduction and improved restitution slope in the PLA translating into a significant reduction in inducible AF. Lastly, since some clinical studies suggest that AF substrate may be reflected in the characteristics of AF electrograms (EGMs), we also assessed how targeted reduction of fibrosis in the PLA affects AF EGM characteristics in this region.
METHODS
Detailed Methods are provided in the Data Supplement. An overview of the Methods used is given below.
In-Vitro Studies
Cloning of plasmid vectors
The TGFβ-DN-RII was cloned into a human polyubitiquin C (pUBc) backbone. The final product was pUB6/HA-V5-His/ TGFβ-DN-RII. Chemically competent E. coli were transformed with the plasmid. DNA was purified and re-suspended in sterile saline for injection. The control plasmid used was pUBc-LacZ.
Studies in fibroblasts and myocytes
NIH 3T3 cells were transfected with pUBc-TGFβ-DN-RII and pUBc-LacZ in the absence and presence of TGF-β. Western blotting was performed for pSMAD2 and type I collagen to assess for gene transfection.
In addition, canine atrial fibroblasts as well as atrial myocytes were isolated and transfected in the absence and presence of TGF-β with a) retrovirus/lentivirus expressing TGFβ-DN-RII or GFP and b) plasmid expressing TGFβ-DN-RII or LacZ. Western blotting was performed for pSMAD2/3 and pERK1/2 to assess for signaling resulting from gene transfection. See Expanded Methods for full details.
In-Vivo Studies in Intact Animals
Three groups of dogs were studied: 1) HF dogs (n=12) that underwent injection with LacZ expressing plasmid (pUBc-LacZ) (i.e. ‘pUBc-LacZ’ group) 2) HF dogs injected with plasmid expressing TGFβ-DN-RII (pUBc-TGFβ-DN-RII) (n=9) (i.e. ‘pUBc-TGFβ-DN-RII group’) and 3) sham controls (n=3; two of these animals received pUBc-LacZ and one received pUBc-TGFβ-DN-RII). During an initial procedure, a left lateral thoracotomy was performed. The animals underwent epicardial electrophysiological mapping followed by gene injection and by epicardial implantation of a left ventricular pacemaker. After animals were allowed to recover for 3–5 days, the first two groups of animals (pUBc-LacZ group and pUBc-TGFβ-DN-RII group) were subjected to ventricular tachypacing at 240 beats/min. The sham controls were not subjected to ventricular tachypacing. Clinical status assessment and pacing was verified daily. Three weeks after the initial procedure, animals underwent repeat open-chest electrophysiological mapping (terminal study).
Echocardiography
Comprehensive echocardiography was performed prior to the baseline study and then immediately prior to the terminal study. Echocardiographic data included left ventricular end-diastolic and systolic dimensions, ejection fraction and left atrial volumes.
Open-chest electrophysiological mapping
Effective Refractory Periods (ERPs)
ERPs were obtained using two rectangular, 21-electrode plaques epicardially positioned on the PLA and left atrial appendage (LAA). ERPs were obtained from five evenly distributed sites in the both the PLA and LAA.
AF inducibility
AF was induced with burst pacing as previously described.19,20 AF was defined as episodes lasting more than 3 seconds. AF inducibility was defined as the percentage of burst pacing attempts that induced AF. Sustained AF was defined as AF>60 seconds.
Activation Mapping
High density epicardial mapping was performed sequentially in the LAA and at two adjacent sites in the PLA (so as to encompass the entire PLA). At each site, 10 second recordings were made during sinus rhythm and pacing with cycle lengths of 400, 300 and 200 msec. See Expanded Methods for full details.
AF Electrogram Mapping
AF EGMs were recorded in order to determine the following EGM characteristics: 1) Dominant Frequency (DF), 2) Organization Index (OI), 3) Fractionation Interval (FI) and 4) Shannon’s Entropy (ShEn). 21 Each of these EGM characteristics has been described in detail in the Expanded Methods.
Monophasic Action Potential (MAP) Recordings
MAPs were recorded from the LAA and the PLA. A dynamic restitution pacing protocol was performed as previously described22.
Electrophysiological data analysis
Analysis of each of the above-mentioned electrophysiological parameters was performed as described in Expanded Methods (see Data Supplement).
Gene injection/transfer
After the completion of the initial electrophysiological study, plasmid was injected sub-epicardially in the PLA to encompass the entire PLA and electroporation was performed at each site of gene injection. See Expanded Methods for full details.
Tissue Analysis
After the completion of the terminal electrophysiological study, the heart was removed and the atria snap frozen as previously described by us15,23. The explanted left atrium (PLA/PVs and LAA) were subjected to the following analysis: Real-time PCR, Western Blot, Immunofluroscnece, Masson-Trichrome staining, Immunohistochemical analysis for macrophages and Protein oxidation (Carbonylation). See Expanded Methods for full details.
Statistical Methods
All data is reported as mean±standard error. Comparison of conduction parameters (at different pacing cycle lengths) before and after gene injection in pUBc-TGFβ-DN-RII and pUBc-LacZ dogs was performed using two-way repeated measures ANOVA; individual means were compared using the Holm-Sidak method. The change in conduction parameter(s) with chronic ventricular tachypacing was compared between the two gene groups using a linear model, ANOVA analysis with study group, cycle length and dog as fixed effects.
Comparison of ERPs before and after gene injection in pUBc-TGFβ-DN-RII and pUBc-LacZ dogs was performed using paired t-tests. Comparisons of all other electrophysiology parameters (restitution parameters, AF duration, AF EGMs) and tissue parameters (e.g. fibrosis, gene expression) between pUBc-TGFβ-DN-RII and pUBc-LacZ dogs were performed by unpaired t-tests (continuous variables) or by the chi-square test (categorical variables). Comparisons of tissue characteristics between the PLA and LAA in the same animals were performed using paired t-tests. In addition, for the occurrence of AF events (AF inducibility), we used hierarchical logistic regression where pacing attempt within each dog and within each study group was the unit of analysis.
RESULTS
Efficacy of pUBc-TGFβ-DN-RII in disrupting canonical TGF-β signaling in fibroblasts
The schematic in Figure 1 shows the canonical TGF-β signaling cascade and how the cytoplasmic (kinase) deficient pUBc-TGFβ-DN-RII blocks this signaling cascade. Online figure I (Panel A and B) shows that both the pUBc-TGFβ-DN-RII and pUBc-Lac Z plasmids efficiently transfected NIH 3T3 fibroblasts. The fusion product of pUBc-TGFβ-DN-RII was 25kD and of pUBc-Lac Z was 100 kD (corresponding to predicted sizes for each). In addition, TGF-β activated fibroblasts transfected with pUBc-TGFβ-DN-RII showed an additional band ~ 45 kD (Online figure I) – in addition to a predicted 25 kD band, a band in the 45 kD range has been previously reported for TGFβ-DN-RII 24,25, likely representing a glycosylated form of the receptor. As previously reported with a similar TGFβ-DN-RII construct25, pUBc-TGFβ-DN-RII significantly attenuated TGF-β induced p-SMAD2 and collagen production in fibroblasts (Online figure I, Panel C and D).
Figure 1.
Schematic of the TGF-β signaling pathway and how a dominant negative TGF-β type II receptor lacks the cytoplasmic domain necessary for TGF-β signaling.
Similar attenuation of canonical TGF-β signaling was noted in isolated canine atrial fibroblasts. As shown in Online Figure II (panel A), pUBc-TGFβ-DN-RII attenuated TGF-β induced p-SMAD2/3 production in canine atrial fibroblasts. In contrast, there was no discernible evidence of canonical TGF-β signaling in isolated canine atrial myocytes; as shown in Online Figure II, panel B, TGF-β stimulation did not significantly increase p-SMAD2/3 generation in the atrial myocytes.
Efficacy of pUBc-TGFβ-DN-RII in disrupting non-canonical TGF-β – pERK1/2 signaling in atrial fibroblasts and myocytes
pUBc-TGFβ-DN-RII attenuated TGF-β induced pERK1/2 generation (a well described non-canonical pathway mediating TGF-β induced pro-fibrotic signaling26–29) in both canine atrial fibroblasts and myocytes (Online figure II, panels C and D respectively).
Taken together, these data indicate that the pUBc-TGFβ-DN-RII may be exerting its in-vivo effects on AF substrate via its action on both canonical and non-canonical TGF-β signaling.
Echocardiography
Online figure III shows that as previously demonstrated by us30 and by others in this HF model there was a significant increase in left ventricular dimensions and decrease in left ventricular systolic function (ejection fraction) after 3 weeks of ventricular tachypacing. There was also an expected increase in left atrial size. There was no difference in ventricular dimensions, systolic function or left atrial size between the pUBc-TGFβ-DN-RII and the pUBc-LacZ groups.
Effects of TGFβdnRII on conduction inhomogeneity and conduction velocity
We determined the effects of pUBc-TGFβ-DN-RII gene transfer on atrial conduction using high-density epicardial plaques.
Previous reports have repeatedly demonstrated that fibrosis contributes to the formation of AF substrate in HF primarily by increasing conduction inhomogeneity (heterogeneity) in the atrium, as measured by the Conduction Inhomogeneity Index (CI)5,31. Changes in overall conduction velocity (CV) in the presence of fibrosis in this model have been shown to much less pronounced5. Our primary electrophysiological end-point of interest was therefore CI, with CV being a secondary endpoint. In PLA injected with pUBc-LacZ, the expected increase in CI with HF was significantly attenuated in pUBc-TGFβ-DN-RII injected PLA (Figure 2A). Figure 2A, panel I provides a representative example of a lack of significant increase in CI seen in PLA injected with pUBc-TGFβ-DN-RII, compared with pUBc-LacZ injected PLA, where there is a marked increase in CI after 3 weeks of ventricular tachypacing. Figure 2A, panels II shows in pUBc-LacZ PLA, the expected increase in CI that is known to occur in the HF atrium5 was seen at all cycle lengths. Figure 2A, Panel III shows that the expected increase in CI with HF was significantly attenuated in pUBc-TGFβ-DN-RII injected PLA (no significant increase in CI at any cycle length). Online figure IV shows that the increase CI with ventricular tachypacing was significantly lower in pUBc-TGFβ-DN-RII compared to pUBc-LacZ.
Figure 2. Effect of pUBc-TGFβ-DN-RII on conduction inhomogeneity (CI) and conduction velocity (CV) in PLA.
A. Panel I: Examples of isochronal maps showing effect of gene injection on CI in the PLA. Top two panels show an example of significant increase in CI in a pUBc-LacZ injected PLA. Bottom two panels show the lack of an increase in CI in a pUBc-TGFβ-DN-RII injected PLA. Panel II: pUBc-LacZ injected PLA shows significant increase in CI after 3 weeks of ventricular tachypacing. Panel III: In pUBc-TGFβ-DN-RII injected PLA there is no significant increase in CI after 3 weeks of ventricular tachypacing. B. Panel I: pUBc-LacZ injected PLA shows significant decrease in CV after 3 weeks of ventricular tachypacing. Panel II: In pUBc-TGFβ-DN-RII injected PLA a significant decrease in CV is only noted with the fastest pacing cycle length i.e. 200 msec.
In the un-injected LAA, there was no significant increase in CI in either the pUBc-TGFβ-DN-RII or the pUBc-LacZ groups (Online figure V). Online figure VI, panel A shows that sham controls had no significant change in CI after gene injection.
Conduction velocity (CV) was also assessed in both groups of animals. As shown in Figure 2B, HF led to a decrease in CV in pUBc-LacZ PLA (slower CV noted both during sinus rhythm as well as during pacing). However, in pUBc-TGFβ-DN-RII injected PLA a significant decrease in CV was only noted at the fastest pacing cycle length i.e. 200 msec. In the LAA, there was no significant change in CV with HF (in either gene group) (Online Figure VII). Online figure VI, panel A shows that sham controls had no significant change in CV after gene injection.
Effects of TGFβ-DN-RII on atrial repolarization
Effective refractory periods
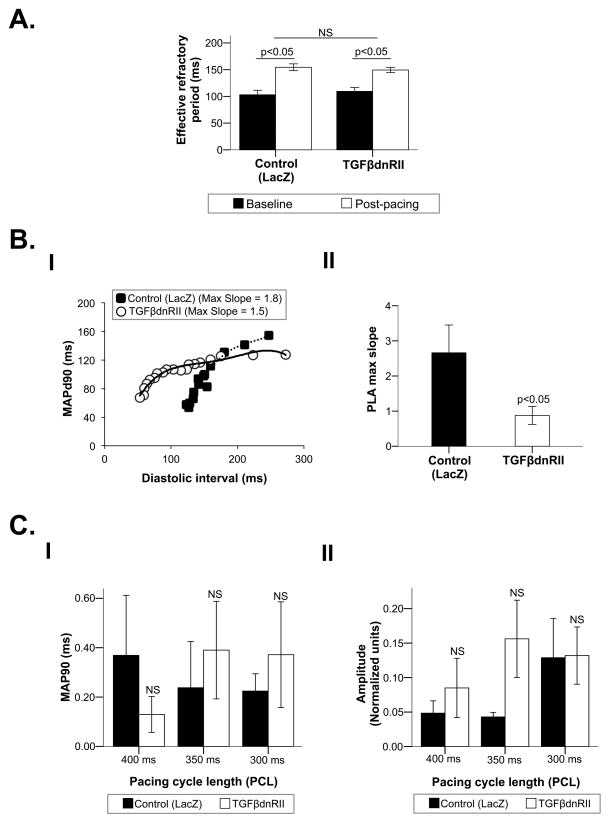
ERPs lengthened significantly after 3 weeks of pacing when compared to baseline in both pUBc-TGFβ-DN-RII and pUBc-LacZ animals, with the magnitude of ERP increase being similar in both groups (Figure 3A). Similar changes were noted in the LAA, with no difference between the pUBc-TGFβ-DN-RII and pUBc-LacZ animals (Online figure VIII).
Figure 3. Effect of pUBc-TGFβ-DN-RII on repolarization characteristics of the PLA.
A. ERP change in pUBc-TGFβ-DN-RII versus pUBc-LacZ injected PLA after 3 weeks of ventricular tachypacing. B. Effect of pUBc-TGFβ-DN-RII on restitution slope. Panel I: An example of flattening of restitution slope in a pUBc-TGFβ-DN-RII versus a pUBc-LacZ injected PLA. Panel II: Restitution slope of pUBc-TGFβ-DN-RII versus pUBc-LacZ injected PLA. C. Effect of pUBc-TGFβ-DN-RII on alternans of MAPd90 and MAP amplitude (Panels I and II).
APD restitution characteristics
An example from one animal from each group is shown in Figure 3B, panel I. The maximum slope of the of the APD restitution curve was steeper in pUBc-LacZ injected PLA when compared with pUBc-TGFβ-DN-RII injected PLA (3.10 ±0.78 vs 1.09 ±0.17; p<0.05) (Figure 3B, panel II). There was no significant change in APD restitution slope in the LAA in either group (Online figure IX).
There was no significant difference in alternans of MAPd90 and MAP amplitude between the pUBc-LacZ and pUBc-TGFβ-DN-RII injected groups in either the PLA (Figure 3C, panels II and II) or the LAA (Online figure X)
As shown in Online figure VI, panel B, the PLA of sham control dogs had an APD restitution slope close to pUBc-TGFβ-DN-RII injected PLA.
Effects of TGFβ-DN-RII on AF inducibility and AF EGM characteristics
AF inducibility
The duration of induced AF (defined as AF > 3 seconds) was markedly lower in pUBc-TGFβ-DN-RII injected animals compared to pUBC-Lac Z injected animals (Figure 4A, panel I). AF inducibility (i.e. percentage of burst pacing attempts that induced AF) was also significantly lower in pUBc-TGFβ-DN-RII injected animals compared to pUBc-Lac Z injected animals (Figure 4A, panel II). The decrease in AF pUBc-TGFβ-DN-RII continued to be significant (p = 0.023) after hierarchical logistic regression was performed to determine the effect of variability between animals on AF inducibility. No AF was noted in the sham controls.
Figure 4. Effect of pUBc-TGFβ-DN-RII on AF duration, inducibility and characteristics.
A. Decrease in AF with pUBc-TGFβ-DN-RII. Panel I: Effect of pUBc-TGFβ-DN-RII versus pUBc-LacZ on duration of induced AF. Panel II: Effect of pUBc-TGFβ-DN-RII versus pUBc-LacZ on AF inducibility (i.e. the percentage of burst pacing episodes that induced AF). B. An example of AF EGMs being slower and less fractionated in pUBc-LacZ injected PLA (panel I) as compared to pUBc-TGFβ-DN-RII injected PLA (panel II). C. Effect of pUBc-TGFβ-DN-RII versus pUBc-LacZ on frequency characteristics of induced AF i.e. DF (panel I), OI (panel II), ShEn (panel III) and FI (panel IV).
AF EGM characteristics
In previously published data from our laboratory, we compared AF EGM characteristics in dogs with HF and dogs without any structural heart disease19. We discovered that AF in dogs with HF was significantly slower and more organized that AF in dogs without structural heart disease (in which AF was induced by vagal stimulation). AF EGMs in HF demonstrate the following changes in AF EGM characteristics compared to vagal induced AF in normal dogs: decrease in DF (a frequency domain measure of activation rate), increase in OI (a frequency domain measure of temporal organization or regularity), increase in FI (the mean interval between deflections detected in the EGM segment and decrease in ShEn (a statistical measure of complexity. Moreover, percent (%) fibrosis in the HF atrium was negatively correlated with DF and positively correlated with FI19.
Since pUBc-TGFβ-DN-RII injected animals demonstrated a significant decrease in atrial fibrosis (see histological evaluation in next section), we compared AF EGM characteristics between pUBc-Lac Z and pUBc-TGFβ-DN-RII injected atria, in order to determine if there is an association between fibrosis and AF EGM characteristics. Figure 4B shows an example of atrial EGMs in pUBc-Lac Z and pUBc-TGFβ-DN-RII injected PLA. As shown in figure 4C, AF EGMs from pUBc-TGFβ-DN-RII injected PLA were significantly faster and less organized (higher DF, lower OI, lower FI and higher ShEn) than AF EGMs recorded from pUBc-Lac Z injected PLA. In contrast, there was no difference in AF EGM characteristics in the LAA between the pUBc-TGFβ-DN-RII and pUBc-Lac Z dogs (Online figure XI). Taken together, these data indicate that atrial fibrosis contributes to both the frequency and organizational characteristics of AF EGMs, with increased fibrosis leading to slowing and organization of AF EGMs.
Fibrosis and inflammation
To assess differences in structural remodeling between groups, we systematically assessed atrial fibrosis in both groups of animals. pUBc-TGFβ-DN-RII injected PLAs were much less likely to harbor regions of dense, interstitial fibrosis as compared to pUBc-Lac Z injected PLAs (figure 5). Figure 5A, panel I shows representative micrographs of dense, interstitial fibrosis in two pUBc-Lac Z injected PLAs. The sections clearly show clear evidence of myocyte dropout, indicating this is replacement fibrosis, because of the broad geographic areas of dense collagen, without embedded or intervening myocytes. In comparison, panel II shows a lack of myocyte dropout and a paucity of interstitial fibrosis in two pUBc-TGFβ-DN-RII injected PLAs. The fibrosis seen here is seen to surround myocytes. No broad geographic areas of dense collagen are seen (and there is no myocyte drop out).
Figure 5. Effect of pUBc-TGFβ-DN-RII on fibrosis and inflammation.
A. Panel I: Example of dense, interstitial fibrosis in PLA from two dogs receiving pUBc-LacZ. Panel II: PLA from two dogs receiving pUBc-TGFβ-DN-RII shows very little interstitial fibrosis. Fibrosis is stained blue. III. % dense, interstitial fibrosis in pUBc-LacZ versus pUBc-TGFβ-DN-RII injected PLA.
B. Macrophage staining in pUBc-LacZ versus pUBc-TGFβ-DN-RII injected PLA. Panel I: Shows examples of nuclear, macrophage, and merged staining in pUBc-LacZ and pUBc-TGFβ-DN-RII injected atria (top and bottom subpanels respectively). Macrophage (MΦ) staining is green. Nuclei were detected with DAPI (blue). Panel II: bar graph shows quantitation of percent macrophage positive nuclei in experimental groups.
Panel III shows that % dense interstitial fibrosis respectively was significantly lower in pUBc-TGFβ-DN-RII compared to pUBc-Lac Z injected PLAs. As expected, there was no significant difference in interstitial fibrosis in the LAA between the two groups of animals (Online figure XII). As shown in Online figure VI, panel C, sham control dogs had no evidence of atrial fibrosis.
To assess whether the fibrosis noted in this model was inflammatory in nature, macrophage staining was performed in gene-injected PLA in both pUBc-TGFβ-DN-RII and pUBc-Lac Z injected dogs. As shown in figure 5B, there was no difference in macrophage staining between pUBc-TGFβ-DN-RII and pUBc-Lac Z injected animals. Furthermore, we did not detect any evidence of apoptotic bodies in the either group of animals.
The absence of macrophage infiltration, along with the nature of the fibrosis as described above (dense fibrosis with myocyte dropout) indicate that fibrosis seen in the HF model is replacement in character, with the beneficial effects of pUBc-TGFβ-DN-RII being mediated by a reduction in this replacement fibrosis.
In-vivo gene expression
PCR
Gene expression by PCR was noted in the PLA of both pUBc-TGFβ-DN-RII and pUBc-Lac Z injected animals (plasmid copy number = 2.17 x 108 ± 7.3 x 107). Transcription product was either absent or was barely detectable in the adjoining, uninjected LAA.
Western blotting
As shown in figure 6A, pUBc-TGFβ-DN-RII transfected PLA demonstrated His-tagged TGF-β-DN-RII protein close to its predicted size of ~25kDa. In addition, an additional band was noted at ~45 kD as previously described for this truncated receptor24,25. Prolonged exposure was required – likely because of relatively low and/or inhomogeneous gene expression (see Discussion) - to discern bands at these expected sizes; as shown in figure 6A, prolonged exposure revealed two specific, expected bands at ~25 kD and 45 kD respectively (bands only seen in tissue injected with active gene, and not in any control lane). Prolonged exposure unfortunately also led to the appearance of several non-specific bands, which were seen in both gene injected tissue and controls. pUBc-Lac Z transfected PLA demonstrated expression of the corresponding His-tagged β-galactosidase protein at the expected size of ~100kD (Online figure XIII).
Figure 6. Gene expression in myocardium.
A. Western blot for His-tagged fusion protein. In pUBc-TGFβ-DN-RII injected PLA, His-tagged fusion protein is noted at the expected sizes (25kD and 45kD); also see arrows. No His tag is noted at these sizes in control i.e. pUBc-LacZ injected PLA. Prolonged exposure (see text) led to the appearance of several non-specific bands, which were seen in both pUBc-TGFβ-DN-RII and pUBc-LacZ lanes. B. Immunofluorescence (confocal microscopy) for V5-tagged fusion protein. Panel I: Expression of V5-tagged fusion protein is noted in multiple, randomly selected panels (each 40X magnification) in a pUBc-LacZ injected PLA. The V5 tag is green. Panel II. In neighboring, un-injected LAA there is no evidence of V5 expression.
Immunofluorescence
We also evaluated the expression and distribution of TGFβ-DN-RII using immunofluorescence (Figure 6B). Panel I shows expression of His-tagged TGFβ-DN-RII protein, with no expression being seen in un-injected (control) PLA (Figure 6B; panel II). As panel I demonstrates, even though gene expression was noted in most randomly selected 20X panels, it was not entirely homogeneous in all of these panels.
Effect of TGFβdnRII on canonical and non-canonical TGF-β signaling and on protein oxidation
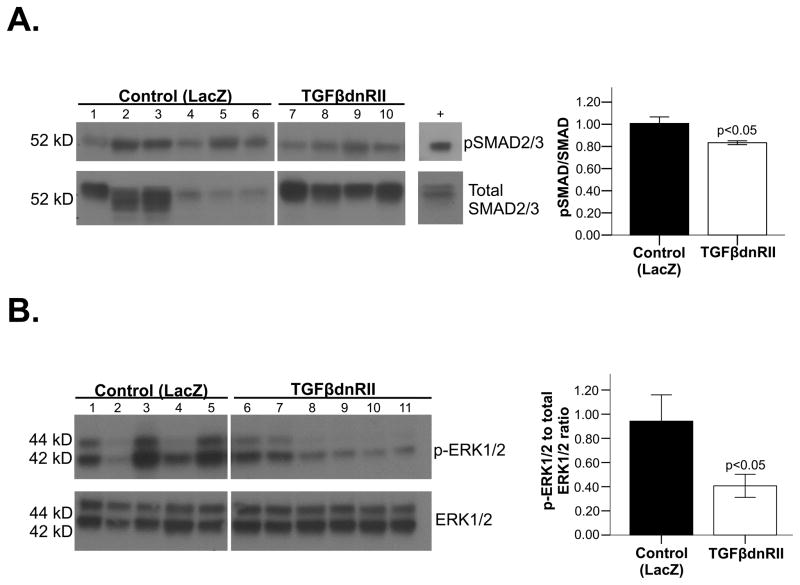
Next we assessed the signaling mechanisms downstream of TGF-β inhibition by a TGFβ-DN-RII approach. TGF-β knockdown by pUBc-TGFβ-DN-RII led to a decrease in p-SMAD 2/3 in the injected PLA (compared to pUBc-Lac Z transfected PLA) (figure 7A). Sham controls, as expected, had significantly less p-SMAD 2/3 compared to pUBc-Lac Z transfected PLA (Online figure VI, panel D).
Figure 7. Effect of pUBc-TGFβ-DN-RII on downstream signaling.
A. Western blot showing p-SMAD 2/3 in pUBc-TGFβ-DN-RII versus pUBc-LacZ injected PLA. B. Western blot showing pERK1/2 in pUBc-LacZ injected PLA versus PLA from sham control dogs.
Non-canonical TGF-β signaling was also assessed, by examining expression of phosphorylated TGF-β activated kinase (pTAK1), pERK1/2 and phospho-p38MAP kinase. pERK1/2 was markedly reduced in pUBc-TGFβ-DN-RII compared to pUBc-Lac Z (figure 7B). Phospho-p38MAP kinase trended to be lower in TGFβ-DN-RII injected atria, while pTAK1 was not changed (Online figure XIV A and B respectively).
Assessment of carbonylation revealed no attenuation of protein oxidation by pUBc-TGFβ-DN-RII compared to pUBc-Lac Z (Online figure XV).
DISCUSSION
In this study, we demonstrate that gene-based targeting of pro-fibrotic signaling in the atrium is not only feasible, but helps limit adverse fibrotic remodeling in the HF atrium, with a resulting decrease in inducible AF. More specifically, we demonstrate that: a) gene-based targeting of TGF-β signaling in the canine atrium is feasible, with a plasmid expressing TGF-β-RII-DN under the control of a long-acting UBc promoter, b) inhibition of TGF-β signaling in the PLA by TGF-β-RII-DN resulted in a significant decrease in HF-induced atrial fibrosis, leading to a significant decrease in the conduction heterogeneity that characterizes fibrosis-induced AF substrate, c) TGF-β-RII-DN expression results in flattening of the restitution slope in the PLA and d) the improvement in conduction and restitution characteristics translates into a significant decrease in the duration of inducible AF. In addition, our results demonstrate that a decrease in atrial fibrosis by TGF-β inhibition is accompanied by a significant change in the complexity and organizational characteristics of induced AF.
TGF-β signaling is a therapeutic target to reduce fibrosis in the HF atrium
Of the morphological changes that occur in the fibrillating atrium - fibrosis, hypertrophy, necrotic and apoptotic cell loss, and dilation 4,32 - fibrosis is considered especially important in the creation of AF substrate – both in the absence and presence of HF4. Patients with AF display increased atrial fibrous tissue content, along with increased expression of collagen I and III33. Atrial extracellular matrix remodeling in AF patients with HF is characterized by increased collagen type I fraction, up-regulation of MMP-2 protein, and down-regulation of the tissue inhibitor of metalloproteinase, TIMP-133. Expression of the active form of MMP-9 and of monocyte chemoattractant protein-1, an inflammatory mediator, is increased in AF patients34. The regional distribution of extracellular matrix remodeling in humans has been studied, and suggests that the left atrial free wall around the PV area presents particularly strong interstitial fibrotic changes35,36.
Although the underlying molecular mechanisms that lead to the development of fibrosis are complex, recent work suggests that the TGF-β pathway may be an important contributor to fibrosis both within and outside the heart6,37. TGF- β1 has been implicated in the development of diabetic nephropathy, ulcerative colitis, hepatic, pulmonary and skin fibrosis10. TGF-β1 appears to also be a major signaling pathway underlying induction of cardiac fibrosis, as evidenced by over-expression and knockout models.10,11 TGF-β stimulates fibroblast production of collagen, fibronectin, proteoglycans and promotes apoptosis, which can indirectly lead to replacement fibrosis10. Both SMAD2/3 mediated canonical TGF-β signaling and non-canonical signaling (predominantly by ERK1/2, p38 MAP kinase)26–29 have been implicated in TGF-β induced fibrosis in the heart. TGF-β also activates ROS, with redox-sensitive signaling pathways mediating TGF-β-induced cardiac hypertrophy, fibrosis, and structural remodeling in chronic disease states38. Cucoranu et al39 reported that NADPH oxidase – specifically Nox4 - mediates TGF-β induced conversion of fibroblasts to myofibroblasts by regulating Smad 2/3 activation. Other studies also indicate a close relationship between TGF-β1 and ROS production by NAPDH oxidase40, suggesting the presence of an intricate feedback loop between these molecules41.
Lately, TGF-β has also been shown to be involved in creation of atrial fibrosis. Serum levels of TGF-β have been shown to be increased in patients with AF undergoing defibrillation10. Moreover, transgenic over-expression of TGF-β in the mouse causes selective fibrosis of atrial, but not ventricular myocardium14. Later studies in this murine model showed that TGF-β over-expression elicited marked atrial fibrosis, altered conduction characteristics and increased AF inducibility11. Recently, Lamirault and coworkers, using microarray analysis, illustrated that gene expression of TGF-β is up-regulated in patients with AF secondary to valvular heart disease in right atrial preparations13.
In view of the above, TGF-β signaling in the atrium appears to be a viable therapeutic target in AF. Lee et al42 attempted to target TGF-β signaling in AF by using used pirfenidone - a non-specific blocker of TGF-β, TNF-α and multiple other cytokines – and showed that this approach could prevent the development of fibrosis (and resulting AF) in a canine model of HF. We hypothesized that a more targeted strategy that selectively inhibits canonical TGF-β signaling in the atrium – and more specifically in the region of the PVs and PLA - would be sufficient to attenuate formation of fibrotic substrate in AF.
Previous attempts at selectively targeting TGF-β signaling have used small molecules or monoclonal antibodies that are specifically targeted to the TGF-β signaling pathway e.g. in the setting of metastatic cancer43,44 and renal fibrosis45. There have also been attempts at targeting TGF-β signaling more selectively in a single organ by using a gene-based approach. A gene-based approach using a soluble, dominant negative TGF-β type II receptor – as a competitive inhibitor of TGF-β - when used in the post-myocardial mouse ventricle, mitigated cardiac remodeling by affecting cardiac fibrosis and infarct tissue dynamics (apoptosis inhibition and infarct contraction)46. In this study, we employed a related dominant negative gene therapy approach to decrease TGF-β signaling in the HF atrium. To our knowledge, this is the first report of a targeted gene-therapy approach to decrease atrial fibrosis (with a view towards modifying AF substrate). This is also the first attempt, to our knowledge, of using such a gene-based approach to target cardiac fibrosis in a clinically relevant large animal model of AF. TGF-β-RII-DN not only decreased atrial fibrosis in the current study, but appeared to do so by affecting both canonical and non-canonical TGF-β signaling. Indeed, TGF-β-RII-DN had a greater effect on non-canonical pERK1/2 than canonical pSMAD2/3 in gene-transfected atria. Since TGF-β-RII-DN was found to attenuate pERK1/2 not only in atrial fibroblasts but also in atrial myocytes, it is possible that at least some of the beneficial effects of TGF-β-DN-RII on atrial fibrosis may be mediated by paracrine cross-talk between myocytes and fibroblasts (as described previously by several investigators47–50), with TGF-β having been shown to participate in bidirectional regulatory signaling between fibroblasts and cardiomyocytes51.
Electrophysiological mechanisms underlying attenuation of AF substrate by TGF-β-RII-DN expression in the PLA
In addition to causing structural remodeling (fibrosis) in the atrium, HF is also known to promote alterations in atrial ionic currents52 e.g. decrease in atrial Ito, ICaL, and IKs and increase in INCX (i.e. electrophysiological remodeling). The contribution of TGF-β signaling to formation of atrial fibrosis has been discussed earlier. In addition, it appears that TGF-β signaling also be contributing to electrophysiological remodeling in the atrium. TGF-β1 released by myofibroblasts has been shown to differentially regulate transcription and function of ion channels involved in cardiac activation and repolarization e.g. INa, Ito, ICaL in both atrial and ventricular myocytes53,54. While individual ion channels were not examined in this study, we did examine for evidence of electrical remodeling by assessing the restitution characteristics of the left atrium. Indeed, inhibition of TGF-β signaling in the atrium, in addition to attenuating fibrosis, also flattened the restitution slope of the PLA. A steepening of the APD restitution slope with atrial APD alternans has been shown to correlate with increased vulnerability to AF55,56, with the slope of the restitution curve thought to affect propensity for wavebreak and substrate for reentry57, including in the PVs58. A decrease in restitution slope in the PVs and PLA may therefore attenuate arrhythmogenic substrate in the atrium. Since TGF-β signaling can affect INa, it is possible some of the salutary effects of TGF-β-RII-DN on conduction inhomogeneity are also mediated at least partially by the ion-channel effects of TGF-β signaling (in addition to the improvement in conduction resulting from a decrease in fibrosis).
Limitations of current therapies for AF and need for new ‘mechanism guided’ therapies for AF
Since a majority of AF triggers and drivers originate in the PVs and PLA, catheter ablation to electrically isolate the PVs/PLA has recently emerged as a viable therapy for AF. Nonetheless, high ablation success rates have only been achieved in selected patients59,60. Even with extensive, linear ablation in the atrium – which can lead to a decrease in atrial contractility and an increase in the incidence of complications – ablation success rates in the setting of structural heart disease do not appear to exceed 50–60%61. The limited efficacy of current treatment options has led to a major research effort to better understand the mechanisms underlying this arrhythmia. A better understanding of the molecular mechanisms underlying electrophysiological remodeling in the atrium has also led to recent efforts to selectively target some of these mechanisms by using a biological i.e. gene-based approach. We have previously shown that vagal induced AF in normal dogs can be successfully prevented by atrial injection of plasmid expressing C-terminal Gαi and/or Gαo.15,62 Amit et al16 and Soucek et al63 showed that refractory period shortening in AF can be prevented in a rapid atrial pacing model of AF by over-expression of dominant negative mutants of the IKr channel, with a resulting decrease in AF16. Since conduction changes also contribute to the creation of AF substrate, Igarashi et al18 and Bikou et al17 showed that gene transfer of Connexin 40 and/or 43 led to improved conduction and reduced AF relative to controls in the same rapid atrial pacing model of AF. More recently, Trappe et al64 have shown that knockdown of caspase 3 by atrial Ad-siRNA-Cas3 gene transfer suppresses or delays the onset of persistent AF by reduction in apoptosis and prevention of intra-atrial conduction delay (in the above-mentioned porcine model of AF).
Our approach differs from the ones mentioned above in that it is the first, to our knowledge, that has specifically targeted fibrosis as AF substrate. Fibrosis is not only a major contributor to AF substrate in the setting of structural heart disease, but is also thought to contribute to the maintenance of AF in patients with long standing AF in the absence of any overt valvular heart disease or HF65. Even more importantly, it is thought to be a major determinant of the failure of ablative approaches to AF 66, with increasing fibrosis correlating directly with the decreasing success of AF ablation. As a result, any therapeutic approach that can prevent or attenuate the progression of fibrotic remodeling would likely have benefit in reducing the ability of the atria to sustain AF. Our approach targeting TGF-β signaling in the atrium – performed in a model of AF where fibrosis is thought to the major substrate underlying AF – successfully attenuated the formation of fibrosis in the HF atrium, with resulting attenuation of the adverse electrophysiological remodeling – specifically the increasing in conduction inhomogeneity - that is characteristic of the HF atrium and a concomitant decrease in the duration of induced AF. A gene-based approach that targets a key molecular signaling pathway underlying fibrosis may have significant therapeutic potential in patients with AF. Nonetheless, to conclusively demonstrate its therapeutic potential, this targeted gene-based approach would have to be systematically compared with systemically administered small molecule inhibitors of TGF-β signaling. Furthermore, the benefits of such an approach would to be weighed against the possible risks associated with the invasive delivery approaches required for targeted gene delivery in the atrium.
Effect of fibrosis on AF EGM characteristics - insights gained from inhibition of TGF-β signaling in the PLA
Several investigators have suggested that the characteristics of electrical signals (electrograms or EGMs) recorded from the atria reflect the pathophysiological substrate underlying AF67,68. The need for a better understanding of the mechanisms underlying AF EGM formation is heightened by emerging data that mapping AF may improve ablation outcomes. 69,70 The ability to use such EGMs as a marker of key AF mechanisms may translate into an increase in the specificity and success of current ablation procedures, as well as provide suitable targets for novel biological therapies15,23.
In order to determine if fibrosis contributes to AF EGM characteristics, we compared in the present study AF EGM characteristics in dogs that received active TGF-β-DN RII (with a resulting decrease in fibrosis) with dogs that received empty vector. We discovered that TGF-β-DN RII dogs (that had mild-moderate interstitial fibrosis compared to the more severe fibrosis seen in dogs receiving empty vector) had significantly more fractionated and disorganized AF EGMs than dogs that received empty vector. These results are consistent with our recent findings, where we discovered that in the setting of HF, AF EGMs over regions of dense fibrosis were more organized and less fractionated compared to AF EGMs overlying lesser degrees of fibrosis19. While the finding that AF EGMs are slower and more organized in HF may seem contradictory to some clinical studies that indicate that complex fractional atrial electrograms (CFAEs) % are higher in patients with persistent than in paroxysmal AF71, the precise structural substrate underlying AF EGMs has not characterized in these prior studies. Indeed, the observation that f-wave frequency of AF on surface ECGs decreases with increasing age72 further supports the notion that increasing atrial fibrosis – a phenomenon well described in the aging heart – may also be contributing to slowing and organization of AF in the HF atrium. It is therefore entirely possible that dense replacement fibrosis as seen in advanced HF may lead to a coalescing and organization of activation wavefronts in the atrium, with an increased organization of AF EGMs. In comparison, a lesser degree of fibrosis – as noted in the current case with injection of TGF-β-DN RII - may be more likely to set up microscopic conduction barriers (and resulting anisotropy) that may be conducive to the creation of disorganized EGMs. Regardless of the precise mechanisms underlying the differences in AF EGM characteristics in the presence of dense versus milder degrees of fibrosis, the findings of this study may have translational significance, in that they may allow for potential use of AF EGMs to detect fibrosis burden in the atrium, as well as to potentially ‘hone in’ on regions of dense fibrosis in the atrium. Future studies are therefore necessary in order understand the precise contribution of fibrosis to AF inducibility, duration and organization.
Study limitations
The study was performed in a model that is known to have easily inducible AF – in large part because of atrial fibrosis - but does not harbor spontaneous AF. It is therefore not known if TGF-β inhibition would have inhibited spontaneous AF. Further testing of this targeted gene-based approach would ideally have to be performed in the setting of spontaneous AF. Future studies will also need to systematically examine how TGF-β contributes to not just structural but also electrophysiological remodeling in AF.
In our study, gene expression in the region of injection was not entirely homogeneous. While inhomogenous gene expression may theoretically enhance heterogeneity of conduction and/or repolarization and therefore increase potential for pro-arrhythmia, we did not find this to be the case in our study. Future studies need to more systematically examine if homogenous gene expression is indeed required for a beneficial therapeutic effect.
Supplementary Material
Novelty and Significance.
What Is Known?
Clinical and experimental evidence has shown that structural remodeling, specifically atrial fibrosis, creates a substrate which serves to maintain, promote, and propagate atrial fibrillation (AF).
Transforming growth factor β1 (TGF-β1) signaling is central to the genesis of atrial fibrosis.
Current pharmacological and ablative approaches to AF have limited efficacy and the potential to cause adverse effects; consequently, there has been an emphasis to develop more mechanism-based therapies for AF that target the specific molecular mechanisms underlying the genesis of AF.
What New Information Does This Article Contribute?
TGF-β inhibition in the failing left atrium can be successfully achieved with a dominant negative trans-gene.
Targeted down regulation of TGF-β in the posterior left atrium with a non-viral gene-based approach results in decreased atrial fibrosis and inducible AF.
Our results highlight the contribution of TGF-β signaling to the genesis of AF and demonstrate the feasibility of a targeted, non-viral gene-based approach for prevention of structural substrate for AF.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and accounts for significant morbidity and mortality. Present therapeutic approaches to AF have major limitations, including limited efficacy and potentially serious adverse effects. Clinical and experimental studies have demonstrated that structural remodeling (fibrosis) is an important feature of AF; the resulting alteration in atrial tissue composition and function serves to maintain, promote, and propagate AF. Although the precise mechanisms that lead to formation of atrial fibrosis are complex, recent work suggests that the transforming growth factor- β1 (TGF-β1) signaling pathway may be an important contributor to the development of fibrosis. TGF- β1 is an inflammatory, profibrotic cytokine that is a potent stimulator of collagen-producing cardiac fibroblasts. Using a novel non-viral gene-based approach, we demonstrate that targeted inhibition of TGF-β signaling can be successfully achieved in the intact atrium, with a resulting decrease in fibrosis. This decrease in atrial fibrosis attenuates the conduction heterogeneity that is known occur in heart failure, thereby leading to a decrease in inducible AF. In addition to providing mechanistic insights into the pathophysiology of AF, this study demonstrates that gene-based approaches targeting key AF mechanisms (e.g. pro-fibrotic signaling pathways) may have significant therapeutic potential in AF.
Acknowledgments
We thank Ashish Ghosh, Lauren Beussink-Nelson, Brandon Benefeld, Chunmeng (Michelle) Zhang, and Alfred Rademaker for assistance with the study.
SOURCES OF FUNDING
NIH - RO1 HL093490, NIH - RO1 HL093490-1S1, NIH - RO1 HL107577, Zoe foundation, Northwestern Memorial Foundation.
Nonstandard Abbreviations and Acroynms
- HF
Heart Failure
- TGF-β-RII DN
Dominant negative TGF-β type II receptor
- PLA
Posterior left atrium
- LAA
Left atrial appendage
- CI
Conduction Inhomogeneity index
- EGMs
Electrograms
Footnotes
DISCLOSURES
Rhythm Therapeutics, Inc.
References
- 1.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 3.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–99. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- 4.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 5.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 6.Khan R. Examining potential therapies targeting myocardial fibrosis through the inhibition of transforming growth factor-beta 1. Cardiology. 2007;108:368–80. doi: 10.1159/000099111. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Jian Z, Yang ZY, Chen L, Wang XF, MARY, Xiao YB. Increased expression of connective tissue growth factor and transforming growth factor-beta-1 in atrial myocardium of patients with chronic atrial fibrillation. Cardiology. 2013;124:233–40. doi: 10.1159/000347126. [DOI] [PubMed] [Google Scholar]
- 8.Rahmutula D, Marcus GM, Wilson EE, Ding CH, Xiao Y, Paquet AC, Barbeau R, Barczak AJ, Erle DJ, Olgin JE. Molecular basis of selective atrial fibrosis due to overexpression of transforming growth factor-beta1. Cardiovasc Res. 2013;99:769–79. doi: 10.1093/cvr/cvt074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanna N, Cardin S, Leung TK, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res. 2004;63:236–44. doi: 10.1016/j.cardiores.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 10.Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verheule S, Sato T, Everett Tt, Engle SK, Otten D, Rubart-von der Lohe M, Nakajima HO, Nakajima H, Field LJ, Olgin JE. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–65. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh YH, Kuo CT, Chang GJ, Qi XY, Nattel S, Chen WJ. Nicotinamide adenine dinucleotide phosphate oxidase 4 mediates the differential responsiveness of atrial versus ventricular fibroblasts to transforming growth factor-beta. Circ Arrhythm Electrophysiol. 2013;6:790–8. doi: 10.1161/CIRCEP.113.000338. [DOI] [PubMed] [Google Scholar]
- 13.Lamirault G, Gaborit N, Le Meur N, Chevalier C, Lande G, Demolombe S, Escande D, Nattel S, Léger JJ, Steenman M. Gene expression profile associated with chronic atrial fibrillation and underlying valvular heart disease in man. Journal of molecular and cellular cardiology. 2006;40:173–84. doi: 10.1016/j.yjmcc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima H, Nakajima HO, Salcher O, Dittiè AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–9. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 15.Aistrup GL, Cokic I, Ng J, Gordon D, Koduri H, Browne S, Arapi D, Segon Y, Goldstein J, Angulo A, Wasserstrom JA, Goldberger JJ, Kadish AH, Arora R. Targeted nonviral gene-based inhibition of Galpha(i/o)-mediated vagal signaling in the posterior left atrium decreases vagal-induced atrial fibrillation. Heart Rhythm. 2011;8:1722–9. doi: 10.1016/j.hrthm.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amit G, Kikuchi K, Greener ID, Yang L, Novack V, Donahue JK. Selective molecular potassium channel blockade prevents atrial fibrillation. Circulation. 2010;121:2263–70. doi: 10.1161/CIRCULATIONAHA.109.911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bikou O, Thomas D, Trappe K, Lugenbiel P, Kelemen K, Koch M, Soucek R, Voss F, Becker R, Katus HA, Bauer A. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc Res. 2011;92:218–25. doi: 10.1093/cvr/cvr209. [DOI] [PubMed] [Google Scholar]
- 18.Igarashi T, Finet JE, Takeuchi A, Fujino Y, Strom M, Greener ID, Rosenbaum DS, Donahue JK. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation. 2012;125:216–25. doi: 10.1161/CIRCULATIONAHA.111.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koduri H, Ng J, Cokic I, Aistrup GL, Gordon D, Wasserstrom JA, Kadish AH, Lee R, Passman R, Knight BP, Goldberger JJ, Arora R. Contribution of fibrosis and the autonomic nervous system to atrial fibrillation electrograms in heart failure. Circ Arrhythm Electrophysiol. 2012;5:640–9. doi: 10.1161/CIRCEP.111.970095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng J, Villuendas R, Cokic I, Schliamser JE, Gordon D, Koduri H, Benefield B, Simon J, Murthy SN, Lomasney JW, Wasserstrom JA, Goldberger JJ, Aistrup GL, Arora R. Autonomic remodeling in the left atrium and pulmonary veins in heart failure: creation of a dynamic substrate for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:388–96. doi: 10.1161/CIRCEP.110.959650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng J, Borodyanskiy AI, Chang ET, Villuendas R, Dibs S, Kadish AH, Goldberger JJ. Measuring the complexity of atrial fibrillation electrograms. J Cardiovasc Electrophysiol. 2010;21:649–55. doi: 10.1111/j.1540-8167.2009.01695.x. [DOI] [PubMed] [Google Scholar]
- 22.Koller ML, Riccio ML, Gilmour RF., Jr Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol. 1998;275:H1635–42. doi: 10.1152/ajpheart.1998.275.5.H1635. [DOI] [PubMed] [Google Scholar]
- 23.Aistrup GL, Villuendas R, Ng J, Lynch TW, Gordon D, Cokic I, Mottl S, Zhou R, Dean DA, Wasserstrom JA, Goldberger JJ, Kadish AH, Arora R. Targeted G-protein inhibition as a novel approach to decrease vagal atrial fibrillation by selective parasympathetic attenuation. Cardiovasc Res. 2009;83:481–92. doi: 10.1093/cvr/cvp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanzler S, Meyer E, Lohse AW, Schirmacher P, Henninger J, Galle PR, Blessing M. Hepatocellular expression of a dominant-negative mutant TGF-beta type II receptor accelerates chemically induced hepatocarcinogenesis. Oncogene. 2001;20:5015–24. doi: 10.1038/sj.onc.1204544. [DOI] [PubMed] [Google Scholar]
- 25.Marquez-Aguirre A, Sandoval-Rodriguez A, Gonzalez-Cuevas J, Bueno-Topete M, Navarro-Partida J, Arellano-Olivera I, Lucano-Landeros S, Armendariz-Borunda J. Adenoviral delivery of dominant-negative transforming growth factor beta type II receptor up-regulates transcriptional repressor SKI-like oncogene, decreases matrix metalloproteinase 2 in hepatic stellate cell and prevents liver fibrosis in rats. J Gene Med. 2009;11:207–19. doi: 10.1002/jgm.1303. [DOI] [PubMed] [Google Scholar]
- 26.Tae HJ, Petrashevskaya N, Marshall S, Krawczyk M, Talan M. Cardiac remodeling in the mouse model of Marfan syndrome develops into two distinctive phenotypes. Am J Physiol Heart Circ Physiol. 2016;310:H290–9. doi: 10.1152/ajpheart.00354.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Z, Zhao W, Zhang X, Wang B, Wang J, Sun X, Liu X, Feng S, Yang B, Lu Y. Scutellarin alleviates interstitial fibrosis and cardiac dysfunction of infarct rats by inhibiting TGFbeta1 expression and activation of p38-MAPK and ERK1/2. Br J Pharmacol. 2011;162:688–700. doi: 10.1111/j.1476-5381.2010.01070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao MW, Zhang XJ, Li L, Cai Z, Liu X, Wan N, Hu G, Wan F, Zhang R, Zhu X, Xia H, Li H. Cardioprotective role of growth/differentiation factor 1 in post-infarction left ventricular remodelling and dysfunction. The Journal of pathology. 2015;236:360–72. doi: 10.1002/path.4523. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Philip JL, Xu X, Theccanat T, Abdur Razzaque M, Akhter SA. beta-Arrestins regulate human cardiac fibroblast transformation and collagen synthesis in adverse ventricular remodeling. J Mol Cell Cardiol. 2014;76:73–83. doi: 10.1016/j.yjmcc.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng J, Villuendas R, Cokic I, Schliamser JE, Gordon D, Koduri H, Benefield B, Simon J, Murthy SN, Lomasney JW, Wasserstrom JA, Goldberger JJ, Aistrup GL, Arora R. Autonomic remodeling in the left atrium and pulmonary veins in heart failure: creation of a dynamic substrate for atrial fibrillation. Circulation: Arrhythmia and Electrophysiology. 2011;4:388–96. doi: 10.1161/CIRCEP.110.959650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinagawa K, Shi YF, Tardif JC, Leung TK, Nattel S. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation. 2002;105:2672–8. doi: 10.1161/01.cir.0000016826.62813.f5. [DOI] [PubMed] [Google Scholar]
- 32.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–9. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 33.Polyakova V, Miyagawa S, Szalay Z, Risteli J, Kostin S. Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med. 2008;12:189–208. doi: 10.1111/j.1582-4934.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellman J, Lyon RC, Sheikh F. Extracellular matrix remodeling in atrial fibrosis: mechanisms and implications in atrial fibrillation. J Mol Cell Cardiol. 2010;48:461–7. doi: 10.1016/j.yjmcc.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corradi D, Callegari S, Maestri R, Benussi S, Bosio S, De Palma G, Alinovi R, Caglieri A, Goldoni M, Mozzoni P, Pastori P, Manotti L, Nascimbene S, Dorigo E, Rusconi R, Astorri E, Alfieri O. Heme oxygenase-1 expression in the left atrial myocardium of patients with chronic atrial fibrillation related to mitral valve disease: its regional relationship with structural remodeling. Hum Pathol. 2008;39:1162–71. doi: 10.1016/j.humpath.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Corradi D, Callegari S, Benussi S, Nascimbene S, Pastori P, Calvi S, Maestri R, Astorri E, Pappone C, Alfieri O. Regional left atrial interstitial remodeling in patients with chronic atrial fibrillation undergoing mitral-valve surgery. Virchows Arch. 2004;445:498–505. doi: 10.1007/s00428-004-1040-2. [DOI] [PubMed] [Google Scholar]
- 37.Edgley AJ, Krum H, Kelly DJ. Targeting fibrosis for the treatment of heart failure: a role for transforming growth factor-beta. Cardiovascular therapeutics. 2012;30:e30–40. doi: 10.1111/j.1755-5922.2010.00228.x. [DOI] [PubMed] [Google Scholar]
- 38.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–8. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 39.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–7. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 40.Zhao W, Zhao T, Chen Y, Ahokas RA, Sun Y. Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol Cell Biochem. 2008;317:43–50. doi: 10.1007/s11010-008-9803-8. [DOI] [PubMed] [Google Scholar]
- 41.Yeh YH, Kuo CT, Chan TH, Chang GJ, Qi XY, Tsai F, Nattel S, Chen WJ. Transforming growth factor-beta and oxidative stress mediate tachycardia-induced cellular remodelling in cultured atrial-derived myocytes. Cardiovasc Res. 2011;91:62–70. doi: 10.1093/cvr/cvr041. [DOI] [PubMed] [Google Scholar]
- 42.Lee KW, Everett THt, Rahmutula D, et al. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114:1703–12. doi: 10.1161/CIRCULATIONAHA.106.624320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohn A, Lahn MM, Williams KE, Cleverly AL, Pitou C, Kadam SK, Farmen MW, Desaiah D, Raju R, Conkling P, Richards D. A phase I dose-escalation study to a predefined dose of a transforming growth factor-beta1 monoclonal antibody (TbetaM1) in patients with metastatic cancer. International journal of oncology. 2014;45:2221–31. doi: 10.3892/ijo.2014.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M, Kleber S, Rohrich M, Timke C, Han N, Tuettenberg J, Martin-Villalba A, Debus J, Peschke P, Wirkner U, Lahn M, Huber PE. Blockade of TGF-beta signaling by the TGFbetaR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer research. 2011;71:7155–67. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- 45.Moon JA, Kim HT, Cho IS, Sheen YY, Kim DK. IN-1130, a novel transforming growth factor-beta type I receptor kinase (ALK5) inhibitor, suppresses renal fibrosis in obstructive nephropathy. Kidney Int. 2006;70:1234–43. doi: 10.1038/sj.ki.5001775. [DOI] [PubMed] [Google Scholar]
- 46.Okada H, Takemura G, Kosai K, Li Y, Takahashi T, Esaki M, Yuge K, Miyata S, Maruyama R, Mikami A, Minatoguchi S, Fujiwara T, Fujiwara H. Postinfarction gene therapy against transforming growth factor-beta signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation. 2005;111:2430–7. doi: 10.1161/01.CIR.0000165066.71481.8E. [DOI] [PubMed] [Google Scholar]
- 47.Zhang P, Su J, Mende U. Cross talk between cardiac myocytes and fibroblasts: from multiscale investigative approaches to mechanisms and functional consequences. Am J Physiol Heart Circ Physiol. 2012;303:H1385–96. doi: 10.1152/ajpheart.01167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panse KD, Felkin LE, Lopez-Olaneta MM, Gómez-Salinero J, Villalba M, Muñoz L, Nakamura K, Shimano M, Walsh K, Barton PJ, Rosenthal N, Lara-Pezzi E. Follistatin-like 3 mediates paracrine fibroblast activation by cardiomyocytes. J Cardiovasc Transl Res. 2012;5:814–26. doi: 10.1007/s12265-012-9400-9. [DOI] [PubMed] [Google Scholar]
- 49.Tian Y, Morrisey EE. Importance of myocyte-nonmyocyte interactions in cardiac development and disease. Circ Res. 2012;110:1023–34. doi: 10.1161/CIRCRESAHA.111.243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ottaviano FG, Yee KO. Communication signals between cardiac fibroblasts and cardiac myocytes. J Cardiovasc Pharmacol. 2011;57:513–21. doi: 10.1097/FJC.0b013e31821209ee. [DOI] [PubMed] [Google Scholar]
- 51.Cartledge JE, Kane C, Dias P, Tesfom M1, Clarke L1, Mckee B1, Al Ayoubi S2, Chester A1, Yacoub MH1, Camelliti P1, Terracciano CM3. Functional crosstalk between cardiac fibroblasts and adult cardiomyocytes by soluble mediators. Cardiovasc Res. 2015;105:260–70. doi: 10.1093/cvr/cvu264. [DOI] [PubMed] [Google Scholar]
- 52.Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, Nattel S. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–8. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 53.Kaur K, Zarzoso M, Ponce-Balbuena D, Guerrero-Serna G, Hou L, Musa H, Jalife J. TGF-beta1, released by myofibroblasts, differentially regulates transcription and function of sodium and potassium channels in adult rat ventricular myocytes. PLoS One. 2013;8:e55391. doi: 10.1371/journal.pone.0055391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramos-Mondragon R, Vega AV, Avila G. Long-term modulation of Na+ and K+ channels by TGF-beta1 in neonatal rat cardiac myocytes. Pflugers Arch. 2011;461:235–47. doi: 10.1007/s00424-010-0912-3. [DOI] [PubMed] [Google Scholar]
- 55.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123:2922–30. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krummen DE, Bayer JD, Ho J, Ho G, Smetak MR, Clopton P, Trayanova NA, Narayan SM. Mechanisms of human atrial fibrillation initiation: clinical and computational studies of repolarization restitution and activation latency. Circ Arrhythm Electrophysiol. 2012;5:1149–59. doi: 10.1161/CIRCEP.111.969022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franz MR, Jamal SM, Narayan SM. The role of action potential alternans in the initiation of atrial fibrillation in humans: a review and future directions. Europace. 2012;14(Suppl 5):v58–v64. doi: 10.1093/europace/eus273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narayan SM, Kazi D, Krummen DE, Rappel WJ. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: a mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol. 2008;52:1222–30. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Macdonald MR, Connelly DT, Hawkins NM, Steedman T, Payne J, Shaw M, Denvir M, Bhagra S, Small S, Martin W, McMurray JJ, Petrie MC. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011;97:740–747. doi: 10.1136/hrt.2010.207340. [DOI] [PubMed] [Google Scholar]
- 60.Wilton SB, Fundytus A, Ghali WA, Veenhuyzen GD, Quinn FR, Mitchell LB, Hill MD, Faris P, Exner DV. Meta-analysis of the effectiveness and safety of catheter ablation of atrial fibrillation in patients with versus without left ventricular systolic dysfunction. Am J Cardiol. 2010;106:1284–91. doi: 10.1016/j.amjcard.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 61.Estner HL, Hessling G, Ndrepepa G, Wu J, Reents T, Fichtner S, Schmitt C, Bary CV, Kolb C, Karch M, Zrenner B, Deisenhofer I. Electrogram-guided substrate ablation with or without pulmonary vein isolation in patients with persistent atrial fibrillation. Europace. 2008;10:1281–7. doi: 10.1093/europace/eun244. [DOI] [PubMed] [Google Scholar]
- 62.Aistrup GL, Villuendas R, Ng J, Gilchrist A, Lynch TW, Gordon D, Cokic I, Mottl S, Zhou R, Dean DA, Wasserstrom JA, Goldberger JJ, Kadish AH, Arora R. Targeted G-protein inhibition as a novel approach to decrease vagal atrial fibrillation by selective parasympathetic attenuation. Cardiovascular Research. 2009;83:481–92. doi: 10.1093/cvr/cvp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soucek R, Thomas D, Kelemen K, Bikou O, Seyler C, Voss F, Becker R, Koenen M, Katus HA, Bauer A. Genetic suppression of atrial fibrillation using a dominant-negative ether-a-go-go-related gene mutant. Heart Rhythm. 2012;9:265–72. doi: 10.1016/j.hrthm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Trappe K, Thomas D, Bikou O, Kelemen K, Lugenbiel P, Voss F, Becker R, Katus HA, Bauer A. Suppression of persistent atrial fibrillation by genetic knockdown of caspase 3: a pre-clinical pilot study. Eur Heart J. 2013;34:147–57. doi: 10.1093/eurheartj/ehr269. [DOI] [PubMed] [Google Scholar]
- 65.Corradi D, Callegari S, Manotti L, Ferrara D, Goldoni M, Alinovi R, Pinelli S, Mozzoni P, Andreoli R, Asimaki A, Pozzoli A, Becchi G, Mutti A, Benussi S, Saffitz JE, Alfieri O. Persistent lone atrial fibrillation: clinicopathologic study of 19 cases. Heart Rhythm. 2014;11:1250–8. doi: 10.1016/j.hrthm.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 66.McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, Wilson B, Cates J, Harrison A, Ranjan R, Burgon NS, Greene T, Kim D, Dibella EV, Parker D, Macleod RS, Marrouche NF. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol. 2014;7:23–30. doi: 10.1161/CIRCEP.113.000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciaccio EJ, Biviano AB, Whang W, Gambhir A, Garan H. Different characteristics of complex fractionated atrial electrograms in acute paroxysmal versus long-standing persistent atrial fibrillation. Heart Rhythm. 2010;7:1207–15. doi: 10.1016/j.hrthm.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ciaccio EJ, Biviano AB, Whang W, Vest JA, Gambhir A, Einstein AJ, Garan H. Differences in repeating patterns of complex fractionated left atrial electrograms in longstanding persistent atrial fibrillation as compared with paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:470–7. doi: 10.1161/CIRCEP.110.960153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayward RM, Upadhyay GA, Mela T, Ellinor PT, Barrett CD, Heist EK, Verma A, Choudhry NK, Singh JP. Pulmonary vein isolation with complex fractionated atrial electrogram ablation for paroxysmal and nonparoxysmal atrial fibrillation: A meta-analysis. Heart Rhythm. 2011;8:994–1000. doi: 10.1016/j.hrthm.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Narayan SM, Krummen DE, Clopton P, Shivkumar K, Miller JM. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on-treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation) J Am Coll Cardiol. 2013;62:138–47. doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solheim E, Off MK, Hoff PI, Schuster P, Ohm OJ, Chen J. Characteristics and distribution of complex fractionated atrial electrograms in patients with paroxysmal and persistent atrial fibrillation. J Interv Card Electrophysiol. 2010;28:87–93. doi: 10.1007/s10840-010-9479-3. [DOI] [PubMed] [Google Scholar]
- 72.Xi Q, Sahakian AV, Frohlich TG, Ng J, Swiryn S. Relationship between pattern of occurrence of atrial fibrillation and surface electrocardiographic fibrillatory wave characteristics. Heart Rhythm. 2004;1:656–63. doi: 10.1016/j.hrthm.2004.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.