Abstract
The purpose of this work was to isolate and identify native bacteria from plants collected in the State of Yucatán, México with the ability to promote growth of chili pepper (Capsicum annuum L. cv Jalapeño). We identified nine bacterial isolates that belong to five species of Bacillus (i.e. Bacillus subtilis, B. flexus, B. cereus, B. megaterium and B. endophyticus) that produced indoleacetic acid (4.0–24.3 µg/mL) with solubilization index of 1.3–1.6. All the bacterial isolates were evaluated based on their ability to promote growth of chili pepper. Plants inoculated with B. subtilis ITC-N67 showed an increase in stem diameter and root volume, whereas inoculation with B. cereus ITC-BL18 increased the number of flower buds, fresh biomass of roots and total fresh biomass. Conversely, B. flexus ITC-P4 and B. flexus ITC-P22 showed deleterious effect on root volume and total biomass. In summary, our data showed that native B. cereus TC-BL18 and B. subtilis ITC-N67 have potential to be used as growth promoting microorganism for chili pepper, particularly in the state of Yucatán, México.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-016-0582-8) contains supplementary material, which is available to authorized users.
Keywords: Bacillus spp., Growth-promoting, Indolacetic acid, Phosphate solubilization, Capsicum annuum, Yucatán
Plant growth promoting bacteria (e.g. Bacillus species) represent a variety of rhizospheric and endophytic microorganisms, which stimulate host growth through the activation of different mechanisms in the plant cell [1]. In particular, Bacillus species have the ability to solubilize different chemicals, they are also involved in the synthesis of auxins , productions of siderophores, nitrogen fixation, and in the synthesis of bacteriocins [2–4].
About one-third of total production of pepper in Mexico comes from Capsicum annuum cv. Jalapeño. This variety of pepper is cultivated in highly technical production systems in Central and Northern Mexico, and in low-input agroecosystems in southern Mexico and the Yucatan Peninsula [5–7]. Several strains of the genus Bacillus have shown growth promoting effects on chili pepper (C. annuum) [8, 9]. Inoculation of plants with different strains of Bacillus spp. increased not only the fruit weight, but also the number of fruits per plant [10]. Because of the importance of C. annuum in México, the purpose of this work was to isolate and identify native bacteria from plants collected in the State of Yucatán México that have the ability to promote growth of chili pepper (C. annuum L. cv Jalapeño). Our data shown that Bacillus cereus TC-BL18 and B. subtilis ITC-N67 have potential to be used as a C. annuum L. cv Jalapeño growth promoting probiotic, particularly in the state of Yucatán, México.
To carry out this work, bacteria were isolated from roots of different plants (Cenchrus echinatus, Leucaena leucocephala and Sansevieria trifasciata) collected in the State of Yucatán México using the nitrogen-free semisolid malate (NFb) enrichment medium. Bacterial strains that were Gram-positive, catalase positive and endospore forming were used. To detect the Indoleacetic acid (IAA) production, approximately 1 × 108 CFU/mL of each bacterium was inoculated in Luria–Bertani (LB) supplemented with 40 µg/mL tryptophan as precursor of IAA. Samples were incubated (30 °C, 180 rpm, 72 h), centrifuged (3000×g) for 15 min, and 1 mL of each supernatant was mixed with 1 mL of Salkowski reagent (1.5 mL of 0.5 M FeCl3·6H2O in 80 mL of 60 % H2SO4). Samples were incubated in the dark at room temperature for 30 min, a pink color indicated a positive test. IAA concentrations were determined at 535 nm using a standard curve of IAA (Sigma, St. Louis, MO, USA). To evaluate the phosphate solubilizing activity, 0.8 µL of bacterial culture at 1 × 10−8 CFU/mL was streaked onto Pikovskaya’s medium and incubated at 30 °C for 7 days [11]. Isolates that acidified the medium (change in color from blue to yellow) and produced clear zones around colonies were chosen. Phosphate solubilizing capacity of each isolated was calculated using the solubilization index (SI), SI = A/B, where A is the total diameter (colony diameter + halo diameter), and B is the colony diameter.
To perform the growth-promoting test on Capsicum annuum L. cv Jalapeño, seeds were surface disinfected. Seeds were inoculated by immersion in a bacterial suspension (1 × 10−8 CFU/mL) with continuous shaking at 200 rpm for 90 min. For negative control, seeds treated with 0.9 % (w/v) NaCl were used. The seeds were placed in plastic trays with 128 cavities and mixed with a commercial substrate called Cosmopeat (Cosmocel S.A. San Nicolás de los Garza, Nuevo León, México). After 15 days, each sample was inoculated for a second time with 3 mL of the bacterial suspension as carried out in the first inoculation. The negative control was treated with 3 mL of 0.9 % (w/v) NaCl instead of bacterial suspensions. Once a week, 1 g/L of the commercial fertilizer 17-17-17 (N-P-K) (Royal Garden’s®, Guadalajara, México) and 1 g/L of ammonium sulfate, were applied to all samples. Plants were kept for 43 days in a greenhouse at 20–35 °C, 55–75 % relative humidity, and natural light cycles (approximately 11 h light, 13 h dark). After this period, different variables were evaluated (e.g. plant height, stem diameter, etc.). The experiment was set in a completely randomized design, using in each treatments 10 replicates (plants). Data were subject to analysis of variance (ANOVA, StatSoft Inc., Tulsa, OK, USA) and mean comparison by Tukey´s Multiple Range Test (P < 0.05) using the SAS program for Windows 9.1.
To identify bacterial isolates by 16S rDNA, bacterial DNA was isolated using the Wizard® Genomic DNA Purification Kit (Promega, Madison MI, USA). 16S rDNA was amplified using the primers Eubact27F (5′-AGAGTTTGATCMTGGCTCAG-3′ and Eubact1492R (5′-TACGGYTACCTTGTTACGACTT-3′ (https://www.macrogenusa.com/support/seq/primer.jsp). PCR amplifications were carried out as follows: 32 cycles of 94 °C for 1 min, 52 °C for 1 min, 72 °C for 1 min 45 s, each, followed by a 7-min termination step at 72 °C. Amplicons were sequencing at Macrogen, USA (Rockville, MD, USA), and the nucleotide sequences were compared using GenBank data from NCBI (www.ncbi.nlm.nlh.gov).
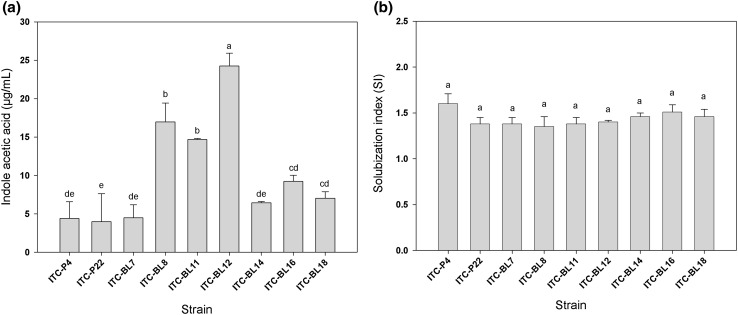
Although we did not know the total microbiota associated to the collected roots in this work, as we would need to carry out metagenomic analysis, we were able to isolated different Bacillus species that might be involved in promoting growth of chili plants. In this regard, we isolated 46 bacterial strains. From the bacterial isolates, 9 were selected because they grew in NFB medium, were bacilli, Gram-positive, catalase positive, and spore-formers. Bacteria were obtained from 9 different localities from the State of Yucatán, México. One of the key aspects of plant growth promoting rhizobacteria is the production of plant hormones. In this regard, one of the most common and naturally-occurring plant hormone is the indoleacetic acid (IAA). Recently, it has been demonstrated that Bacillus strains isolated from rice synthesized IAA in concentrations from 0.1 to 30 µg/mL [3]. Bacterial strains isolated in this work produced IAA in a range from 4.0 to 24.3 µg/mL, which are within the range reported for Bacillus spp. isolated from rice roots. B. megaterium ITC-BL8, B. subtilis ITC-BL11 and B. subtilis ITC-BL12 showed the highest IAA production, with values of 16.9 and 24.3 µg/mL, respectively, which were significantly higher than those of the other isolates (Fig. 1).
Fig. 1.
a Production of indole acetic acid by Bacillus spp. after 72 h of incubation in nutrient broth supplemented with l-trypthophan. b Phosphate solubilization of different strains of Bacillus spp. after 7 days of incubation in Pikovskaya medium. Standard errors were calculated based on ten replicates. In each figure, bars with different letter (a–e) are significantly different as determined by Tukey’s multiple range test (P < 0.05)
Other factor that contributes to the plant growth promoting effect is phosphate solubilization, where the production of organic acids may be linked to this effect [12]. The nine Bacillus (100 %) isolates described in this study did not show significant difference in the phosphate solubilization index, from 1.3 to 1.6 (Fig. 1). This index is higher compared with that reported in Bacillus isolates from rice [2]. Nevertheless, the solubilization index of Bacillus strains in this work were lower than those obtained with species of Bacillus that promote growth of cotton, i.e. 1.3–1.6 versus 3.3–3.8 [13]. Interestingly, some strains (e.g. ITC-LB8, ITC-BL11 and ITC-BL12) that produce IAA and solubilize phosphate were not able to promote growth of chili pepper. This result suggests that other factors, and not only those determined here, might be responsible for the growth-promoting effect observed [14].
The effects of the 11 bacterial isolates (9 strains isolated in this work and 2 strains from our stock collection) on the growth of Capsicum annum plants were evaluated. Significant differences in some of the variables were observed. Plants inoculated with isolate ITC-N67 showed significantly higher (P < 0.05) stem diameter and root volume, whereas plants inoculated with isolate ITC-BL18 showed higher number of flower buds, fresh biomass of roots and total fresh biomass, compared with the control (Table S1; Fig. 2). In contrast, we observed a negative effect of bacterial isolates ITC-P4 and ITC-P22 on root volume and total biomass (Table S1). Previous studies have shown the effect of Bacillus spp. to promote the growth and yield of C. annuum. For example, inoculation of B. subtilis MA12 and MA17 of C. annuum seedling increased biomass by 37 and 16 %, respectively [12]. In other studies, inoculation of Bacillus sp. BECS7 and B. lincheniformis BECL5 increased the number of flower buds, the root length and leaf area in C. annuum. We have found similar results with the inoculation of B. cereus ITC-BL18 and B. subtilis ITC-N67, and the effect might be attributed to the synthesis of IAA [8, 9, 14].
Fig. 2.

Comparative growth of C. annuum L. cv Jalapeño seedling, untreated (C) or treated with B. endophyticus (ITC-BL14), B. subtilis (ITC-N67) and B. cereus (ITC-BL18) after 43 days under greenhouse conditions
B. subtilis and B. pumilus are the most frequently isolated rhizobacteria followed by B. licheniformis, B. cereus and B. amiloliquefascens [15]. Here, in order to identify the rhizospheric bacterial strains, we amplified the 16S rDNA obtaining amplicons of ~1.5 kbp, which were sequenced and compared with GenBank data in NCBI. As such, we identified B. subtilis (one isolate), B. flexus (two isolates), B. cereus (three isolates), B. megaterium (two isolates), and B. endophyticus (one isolates). The bacterial isolates CBRF12 and ITC-N67, which belong to our stock collection, were identified as B. subtilis (Table S2). Those reports and our results confirm the high diversity of Bacillus spp. in the rhozosphere, in particular among grass species. In conclusion, we isolated and identified nine native Bacillus spp. strains, which synthesize IAA and solubilize phosphate, both parameters that appear to be linked to the growth promoting effect on C. annuum. Our results showed that B. cereus ITC-BL18 and B. subtilis ITC-N67 have a high potential to be used as a probiotic promoting biomass of C. annuum L. cv Jalapeño, particularly in the state of Yucatán, México.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This research was supported by DGEST–Mexico (5067.13-P), Grant to Arturo Reyes-Ramírez. Laura Peña-Yam is a graduate student supported by CONACyT, México. We thank Dr. Dennis K. Bideshi from California Baptist University for his critical reading of the manuscript.
References
- 1.Khan MS, Ahemad M. Functional aspects of plant growth promoting rhizobacteria: recent advancements. Insight Microbiol. 2011;1:39–54. doi: 10.5567/IMICRO-IK.2011.39.54. [DOI] [Google Scholar]
- 2.Badía MMR, Hernández BT, Murrel JAL, Mahillon J, Perez MH. Isolation and characterization of strain of the Bacillus associated to rice (Oryza sativa L.) crop. Rev Bras Agroecol. 2011;6:90–99. [Google Scholar]
- 3.Beneduzi A, Peres D, Vargas LK, Bodanese-Zanettini MH, Passaglia LMP. Evaluation of genetic diversity and plant growth promoting activities of nitrogen-fixing bacilli isolated from rice fields in South Brazil. Appl Soil Ecol. 2008;39:11–32. doi: 10.1016/j.apsoil.2008.01.006. [DOI] [Google Scholar]
- 4.Lee KD, Gray EJ, Mabood F, Jung W-J, Charles T, Clarck SRD, Ly A, Souleimanov A, Zhou X, Smith DL. The class IId bacteriocins thurincin-17 increases plant growth. Planta. 2009;229:747–755. doi: 10.1007/s00425-008-0870-6. [DOI] [PubMed] [Google Scholar]
- 5.Powis TG, Gallaga-Murrieta E, Lesure R, Lopez-Bravo R, Grivetti L, Kucera H, Gaikwad NW. Prehispanic use of chili peppers in Chiapas, México. PLoS ONE. 2013;8:e79013. doi: 10.1371/journal.pone.0079013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caro-Encalada M, Leyva-Morales C, Ríos-Santana J. Competitividad mundial de la producción de chile verde de México. Rev de Econ. 2014;31:95–128. [Google Scholar]
- 7.Borges-Gómez L, Moo-Kauil C, Ruíz-Novelo J, Osalde-Balam M, González-Valencia C, Yam-Chimal C, Can-Puc F. Soils used for habanero chili production in Yucatan: predominant physical and chemical characteristics. Agrociencia. 2014;48:347–359. [Google Scholar]
- 8.Amaresan N, Jayakumar V, Thajuddin N. Isolation and characterization of endophytic bacteria associated with chilli (Capsicum annuum) grown in coastal agricultural ecosystem. Indian J Biotechnol. 2014;13:247–255. [Google Scholar]
- 9.Luna-Martínez L, Martínez-Peniche RA, Hernández-Iturriaga M, Arvizu-Mendrano SM, Pacheco-Aguilar JR. Characterization of rhizobacteria isolated from tomato and their effect on tomato and bell pepper growth. Rev Fitotec Mex. 2013;36:63–69. [Google Scholar]
- 10.Datta M, Palit R, Sengupta C, Pandit MK, Banerjee S. Plant growth promoting rhizobacteria enhance growth and yield of chilli (Capsicum annuum L.) under field conditions. Aust J Crop Sci. 2011;5:531–536. [Google Scholar]
- 11.Pradhan N, Sukla LB. Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr J Biotechnol. 2005;5:850–854. [Google Scholar]
- 12.Alikhani HA, Saleh-Rastin N, Antoun H (2007) Phosphate solubilization activity of rhizobia native to Iranian soils. In: First international Meeting on microbial phosphate solubilization, pp 35–41. doi:10.1007/978-1-4020-5765-6_4
- 13.Qureshi MA, Ahmad ZA, Akhtar N, Iqbal A, Mujeeb F, Shakir MA. Role of phosphate solubilizing bacteria (PSB) in enhancing P availability and promoting cotton growth. J Anim Plant Sci. 2012;22:204–210. [Google Scholar]
- 14.López-Bucio J, Campos-Cuevas JC, Valencia-Cantero E, Velázquez-Becerra C, Farias-Rodríguez R, Macías-Rodríguez LI. Bacillus megaterium modifica la arquitectura de la raíz de Arabidopsis independientemente de auxinas y etileno. Rev Biol. 2009;11:1–8. [Google Scholar]
- 15.Figueiredo JE, Gomes EA, Guimarães CT, de Paula Lana UG, Teixeira MA, Lima GV, Bressan W. Molecular analysis of endophytic bacteria from the genus Bacillus isolated from tropical maize (Zea mays L.) Braz J Microbiol. 2009;40:522–534. doi: 10.1590/S1517-83822009000300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.