Abstract
BACKGROUND
Role of vitamin A in reducing the mortality in infants more than six months of age is well known. Supplementing newborn infants with vitamin A within 48 hours of birth reduces infant mortality by almost a quarter, with the greatest benefit to those of low birth weight (LBW). Studies that could highlight deficiency states in neonates, particularly LBW babies by objective measurement of vitamin A levels would help in formulating the recommendations to supplement these babies with vitamin A.
METHODS
Cord blood plasma vitamin A levels of 154 LBW babies with birth weight in the range of 1505–2455 were analysed for plasma vitamin A (retinol) levels by HPLC method. Samples of 55 babies with normal birth weight were also analysed. LBW babies were divided into two subgroups of preterm LBW and LBW-term small for gestational age (SGA).
RESULTS
Of the 154 babies with LBW, 92 were preterm LBW and 52 were LBW-term SGA. Mean cord blood plasma vitamin A levels were significantly lower in the preterm LBW group (n = 92) compared to levels observed in babies with normal birth weight (n = 55) and LBW-term SGA subgroups (n = 62). There was no significant difference in the mean vitamin A values between the normal birth weight babies and LBW-term SGA group. There was significant positive correlation of cord blood vitamin A levels with birth weight in the entire set of (n = 154) LBW babies (r=0.37, P < 0.0001).
CONCLUSION
This study revealed significantly lower cord blood vitamin A levels in the preterm LBW babies. The level of vitamin A in LBW babies also correlated with their birth weight. There are enough evidence to support causative association between vitamin A deficiency state and neonatal morbidity. Simple interventions like vitamin A supplementation during a crucial stage of an infant's life may be beneficial in the long run. There is a need to establish norms for vitamin A levels and seriously examine the role of vitamin A supplementation for LBW babies during the immediate postnatal period.
Key Words: cord blood, low birth weight, vitamin A
INTRODUCTION
Childhood is a period that is characterised by rapid growth and development. Many factors, some modifiable and few nonmodifiable regulate this growth and development with consequent effect on long-term outcome. Along with major nutrients like protein, fat, and carbohydrates, a host of micronutrients also play a significant role in this vital process of growth. Vitamin A is one of the most important micronutrient affecting the growth of children all across the globe. It has been recognised for almost a century now that vitamin A is an essential dietary constituent and plays major role in orderly growth and differentiation of tissues.1, 2
In recent years, attention has turned to reaching newborns with safe, efficacious interventions to improve survival.3, 4 Role of vitamin A in reducing the mortality in infants more than six months of age is well known.5 Supplementing newborn infants with vitamin A within 48 hours of birth reduces infant mortality by almost a quarter, with the greatest benefit to those of low birth weight (LBW).6
World Health Organisation has a time-tested programme for supplementing the children after nine months of age with vitamin A. Sound recommendations with reasonable agreement between paediatricians and neonatologists exist for supplementation of extremely low birth weight (ELBW) babies.7 Studies that could highlight deficiency states in neonates, particularly LBW babies by objective measurement of vitamin A levels would help in formulating the recommendations to supplement these babies with vitamin A.
MATERIALS AND METHODS
During the study period from September 2006 to May 2008, neonates with a birth weight of 1505 and 2445 g were included for estimation of umbilical cord blood plasma vitamin A levels. Cord blood was collected for all the babies who were likely to be LBW and sample was discarded if the weight criterion was not fulfilled on verification of weight. One random sample per day from a baby with normal birth weight (term, birth weight more than 2500 g) was also taken as it was intended to analyse 60 samples in normal weight babies also. Samples of ELBW and very LBW babies were excluded, as it was not part of the study.
Cord blood was collected from the maternal (placental) end of the cord using a syringe from the umbilical vein. Wherever free flow of blood through the cord was available, sample was collected from free flow. Sample was allowed to flow into a large mouthed test tube or was aspirated into a sterile syringe of 5 mL. Sample was centrifuged using the equipment positioned at NICU and supernatant was drained into sterile, light-protected container.
At the laboratory, the HPLC system (LaChrome) and the compatible column and the kits used were Clinirep (Recipe) ver 3 2007 manufactured by the Laboratory Technic, Munich, Germany. To 200 μL of plasma, 20 μL of internal standard, and 25 μL of precipitation reagent I was added. Mixing was done for 30 seconds. To this 400 μL of precipitation reagent II was added. Another 30 seconds were allowed for mixing. The entire sample now was centrifuged at 10000 rpm for 10 minutes. Fifty microlitres of the supernatant was used for the HPLC analysis. The HPLC system and column used was an isocratic HPLC system with UV detector. Injection volume was 50 μL with a flow rate of 1.5 mL/minute. Wavelength used was 325 nm and after 3.5 min the wavelength was switched to 295 nm. Column temperature was maintained at 30°C during the entire runtime. Chromatogram obtained was analysed, checked for accuracy, and value entered into the master list.
Low birth weight babies were divided into two subgroups as LBW-preterm (that included preterm appropriate for gestational age-preterm AGA and preterm small for gestational age-preterm SGA) and LBW-term SGA category. New Ballard scoring system was used to assess the gestational age, supplemented by clinical and ultrasonogram criteria wherever available. Statistical analysis to look for the difference between means, correlation between variables and other appropriate tests were done using the statistical software exclusively designed for health care related studies, Medical version 9.3.0.0.
RESULTS
During the study period, a total of 224 babies were included of which 154 were LBW and 55 babies were in the normal birth weight group. Fifteen babies were excluded in view of haemolysed samples. The study group flow chart is given in the Figure 1.
Figure 1.

Flow chart depicting the study group characteristics.
The mean birth weight and cord blood vitamin A level of babies are given in the Table 1. On statistical analysis using the Z test, the mean cord blood plasma vitamin A level was significantly higher in the normal birth weight group (mean = 19.16 μg/dL) than the LBW-preterm group (mean=16.41 μg/dL) (P < 0.001). Among the LBW subgroup, babies who were LBW-preterm had significantly lower value of cord blood vitamin A (mean = 16.41 μg/dL) than the LBW-term SGA group (mean = 19.12 μg/dL) (P < 0.001). However, There was no significant difference in the mean vitamin A value between normal birth weight babies (mean = 19.16 μg/dL) and LBW-term SGA babies (mean = 19.12 μg/dL).
Table 1.
Summary statistics of birth weight and cord blood vitamin A levels.
| N | Mean | SD | Range |
||
|---|---|---|---|---|---|
| Min | Max | ||||
| Birth weight (g) | |||||
| Normal birth weight group | 55 | 2866.38 | 206.13 | 2530 | 3620 |
| LBW-preterm group | 92 | 1787.19 | 211.56 | 1505 | 2480 |
| LBW-term SGA group | 62 | 2247.82 | 227.22 | 1530 | 2490 |
| Vitamin A levels (µg/dL) | |||||
| Normal birth weight group | 55 | 19.16 95% CI: 17.80–20.51 | 5.01 | 12.00 | 34.00 |
| LBW-preterm group | 92 | 16.41 95% CI: 15.76–17.06 | 3.16 | 4.50 | 24.10 |
| LBW-term SGA group | 62 | 19.12 95% CI: 18.06–20.17 | 4.16 | 8.40 | 31.30 |
The Figure 2, Figure 3 depict the scatter diagram showing the correlation of cord blood plasma vitamin A levels with the birth weight in normal birth weight and LBW babies, respectively.
Figure 2.
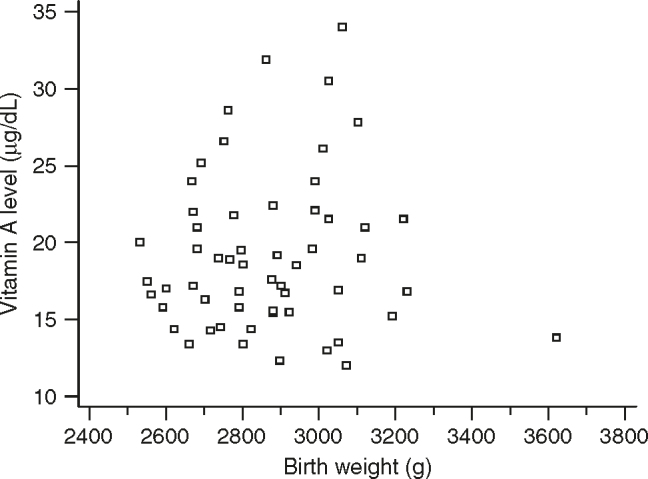
Scatter diagram showing the correlation of vitamin A level with the birth weight in normal birth weight babies.
Figure 3.
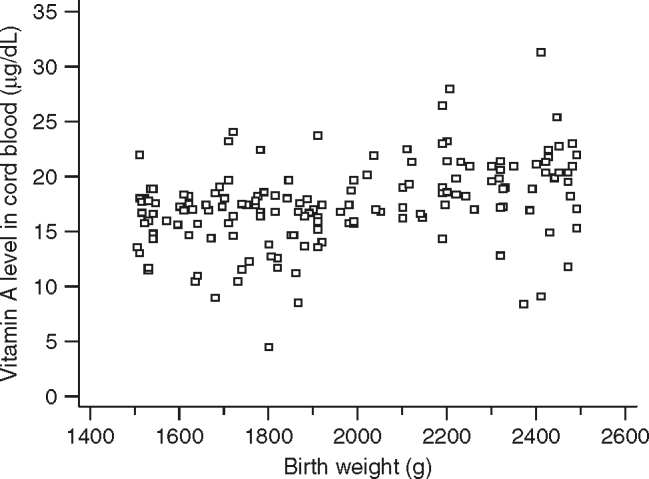
Scatter diagram showing the correlation of vitamin A level with weight in low birth weight babies.
There was significant positive correlation of cord blood vitamin A levels with birth weight in the entire set of (n = 154) LBW babies (r = 0.37, P < 0.0001). Though the correlation coefficient is 0.37, this observation is highly significant. There was no significant correlation of cord blood vitamin A levels with birth weight in term normal birth weight babies (r = 0.0449, P = 0.7427).
DISCUSSION
A well-established supplementation programme for children of more than nine months of age already exist in many nations, and recommendations to supplement ELBW babies are also reasonably clear. We planned this study to measure the cord blood plasma vitamin A levels of LBW babies to look for quantitative deficiency of vitamin A, if any.
We observed a wide range of vitamin A values in cord blood plasma from the lowest of 4.5 μg/dL in LBW-preterm group to the highest level of 34 μg/dL in the normal weight group. This observation is consistent with few other studies where in plasma vitamin A levels were noted to be in a wide range among the babies.8, 9, 10, 11, 12 There is also considerable debate on exact levels of vitamin A below which to label a baby as vitamin A deficient. Vitamin A sufficiency in older children and adults is defined as plasma concentrations in the range 0.7–2.8 μmol/L (20–80 μg/dL). Plasma concentrations of retinol less than 0.35 μmol/L (10 μg/dL) are associated with reduced hepatic stores and clinical signs of vitamin A deficiency and are considered to indicate severe deficiency.13, 14, 15 It was initially planned to analyse the subgroup of babies with plasma vitamin A level cut-off at 10 μg/dL. As there were only five babies with levels less than this cut-off in LBW and normal birth weight group combined, we did not resort to this subgroup analysis.
The mean value obtained in the LBW-preterm group of babies for vitamin A was significantly lower than the value noted in term SGA and normal birth weight groups. The difference noted between the normal birth weight babies and term SGA babies was however not significant. This observation indicates likely effect of factors related to placental transfer of nutrients from mother to the baby. Shirali et al16 in their study found correlation of maternal vitamin A levels with cord blood vitamin A levels, with a logarithmic relationship, suggesting saturable transplacental transport of vitamin A. Lower gestational age may limit this transport across the placenta and SGA babies may suffer deficiency due to reduced transfer of micronutrients due to placental disease. As most of the mothers who deliver LBW babies are also malnourished, reduced transplacental transfer of vitamin A to the babies may reflect the deficiency state among the mothers themselves. It has also been observed that mothers with reduced vitamin A levels may in turn deliver babies with LBW.17 Several variables like lack of ANC, lower maternal haemoglobin levels and reduced placental weight further affect vitamin A status of the newborn rendering them highly susceptible to vitamin A deficiency.18 All these factors could be the likely reason for the lower cord blood vitamin A observed in babies with low birth weight in comparison to normal birth weight babies in our study.
Vitamin A levels were significantly lower in LBW-preterm group than the LBW-term SGA subgroup of LBW babies. It is postulated that reduced retinol binding protein-II (RBPII) noted in preterm babies may be one of the reason for the reduced vitamin A levels observed in plasma of preterms.19, 20 Many other researchers have also inferred that plasma retinal may reflect the availability of its carrier protein, RBP, which is deficient in preterms.21, 22
We did not find any correlation between birth weight and plasma vitamin A levels in term normal birth weight babies. However, there was a significant correlation (P < 0.001) between birth weight and vitamin A levels in the whole group (n = 154) of LBW babies. Even though the correlation coefficient r = 0.37 only, this observation is noteworthy as the correlation observed is highly significant. Shah et al12 have demonstrated a significant correlation of vitamin A levels with the growth status of the baby. Ghebremeskel et al23 also concluded that there was significant correlation of vitamin A values with birth weight, length, and head circumference. In an Indian study, Agarwal et al18 demonstrated that increase in the weight of the baby predicted increased vitamin A values and it also correlated with gestational age and nutritional status of the mother. It is reasonable to assume that, lower the birth weight of the baby, the odds of such babies having lower levels of plasma vitamin A are more likely.
We adopted the measurement of cord blood plasma levels as the criteria for assessing the vitamin A status of the babies. Although retinol is bound to RBP in blood and plasma levels always do not depict correctly the deficiency state, it was the most feasible and cost-effective strategy in our study. We did not determine tissue levels of vitamin A, as it is resource intensive and complex. Subgroup analysis of babies whose mothers were given antenatal steroids and vitamin supplements could have thrown more light on impact of these factors on analyses. Simultaneous measurement of maternal values for vitamin A could have further strengthened the study findings.
In conclusion, this study revealed significantly lower cord blood vitamin A levels in the preterm LBW babies. The level of vitamin A in LBW babies correlated significantly with their birth weight. There are important implications from this observation. There are enough literature evidence to support causative association between vitamin A deficiency state and neonatal morbidity. Encouraging results have emerged with improved outcome among babies and mothers who were supplemented with vitamin A or other micronutrient combinations.22, 23, 24, 25, 26, 27 Simple interventions like vitamin A supplementation during a crucial stage of an infant's life may be beneficial in the long run. Hence, there is a need to seriously examine the role of vitamin A supplementation for LBW babies during the immediate postnatal period. Larger multicentric trials are recommended to define norms for vitamin A levels in blood in LBW babies. Such norms would bring in more objectivity for the recommendations to supplement vitamin A and help in formulating interventional strategies backed by evidence-based guidelines.
Intellectual Contributions of Authors
Study concept: Surg Captain KM Adhikari, Dr BL Somani
Statistical analysis: Surg Captain KM Adhikari, Surg Capt Sheila Mathai
Manuscript revision: Surg Captain KM Adhikari, Surg Capt Sheila Mathai
Study supervision: Maj Suprita Kalra, Brig MM Arora
Technical help: Maj Suprita Kalra, Dr BL Somani
CONFLICTS OF INTEREST
The study was sponsored/funded by the office of the DGAFMS through grant sanctioned for AFMRC project.
REFERENCES
- 1.Hopkins FG. Feeding experiments illustrating the importance of accessory factors in normal dietaries. J Physiol. 1912;44:425–460. doi: 10.1113/jphysiol.1912.sp001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCollum EV, Davis M. The influence of certain vegetable fats on growth. J Biol Chem. 1915;21:179–182. [Google Scholar]
- 3.Martines J, Paul VK, Bhutta ZA. Neonatal survival: a call for action. Lancet. 2005;365:1189–1197. doi: 10.1016/S0140-6736(05)71882-1. [DOI] [PubMed] [Google Scholar]
- 4.Darmstadt GL, Bhutta ZA, Cousens S. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365:977–988. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 5.Humphrey JH, Agoestina T, Wu L. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J Pediatr. 1996;128:489–496. doi: 10.1016/s0022-3476(96)70359-1. [DOI] [PubMed] [Google Scholar]
- 6.Rahmathullah L, Tielsch JM, Thulasiraj RD. Impact of supplementing newborn infants with vitamin A on early infant mortality: community based randomised trial in Southern India. BMJ. 2003;327:254–259. doi: 10.1136/bmj.327.7409.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy KA, Stoll BJ, Ehrenkranz RA. Vitamin A to prevent bronchopulmonary dysplasia in very-low-birth-weight infants: has the dose been too low? Early Hum Dev. 1997;49:19–31. doi: 10.1016/s0378-3782(97)01869-0. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Ranjan R, Basu S. Antioxidant levels in cord blood of low birth weight newborns. Indian Paediatr. 2008;45:583–585. [PubMed] [Google Scholar]
- 9.Mupanemunda RH, Lee DS, Fraher LJ. Postnatal changes in serum retinol status in very low birth weight infants. Early Hum Dev. 1994;38:45–54. doi: 10.1016/0378-3782(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 10.Navarro J, Causse MB, Desquilbet N. The vitamin status of low birth weight infants and their mothers. J Paediatr Gastroenterol Nutr. 1984;3:744–748. doi: 10.1097/00005176-198411000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Rondo PH, Abbott R, Rodrigues LC, Tomkins AM. Vitamin A, folate, and iron concentrations in cord and maternal blood of intra-uterine growth retarded and appropriate birth weight babies. Eur J Clin Nutr. 1995;49:391–399. [PubMed] [Google Scholar]
- 12.Shah RS, Rajalakshmi R. Vitamin A status of the newborn in relation to gestational age, body weight, and maternal nutritional status. Am J Clin Nutr. 1984;40:794–800. doi: 10.1093/ajcn/40.4.794. [DOI] [PubMed] [Google Scholar]
- 13.Pitt GAJ. The assessment of vitamin A status. Proc Nutr Soc. 1981;40:173–178. doi: 10.1079/pns19810026. [DOI] [PubMed] [Google Scholar]
- 14.Underwood BA. Vitamin A in animal and human nutrition. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids. Academic Press; Orlando FL: 1984. pp. 282–392. [Google Scholar]
- 15.US Department of Health, Education and Welfare Guidelines for classification and interpretation of group blood and urine data collected as part of the National Nutrition Survey. Paediatr Res. 1970;4:103. doi: 10.1203/00006450-197001000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Shirali GS, Oelberg DG, Mehta KP. Maternal-neonatal serum vitamin A concentrations. J Paediatr Gastroenterol Nutr. 1989;9:62–66. [PubMed] [Google Scholar]
- 17.Hasin A, Begum R, Khan MR, Ahmed F. Relationship between birth weight and biochemical measures of maternal nutritional status at delivery in Bangladeshi urban poors. Int J Food Sci Nutr. 1996;47:273–279. doi: 10.3109/09637489609012588. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal K, Dabke AT, Phuljhele NL, Khandwal OP. Factors affecting serum vitamin A levels in matched maternal-cord pairs. Indian J Paediatr. 2008;75:443–446. doi: 10.1007/s12098-008-0070-1. [DOI] [PubMed] [Google Scholar]
- 19.Ong DE. A novel retinol-binding protein from rat: purification and partial characterization. J Biol Chem. 1984;259:1476–1482. [PubMed] [Google Scholar]
- 20.Ong DE, Page DL. Cellular retinol-binding protein (type two) is abundant in human small intestine. J Lipid Res. 1987;28:739–745. [PubMed] [Google Scholar]
- 21.Shenai JP, Chytil F, Jhaveri A. Plasma vitamin A and retinol-binding protein in preterm and term neonates. J Pediatr. 1981;99:302–305. doi: 10.1016/s0022-3476(81)80484-2. [DOI] [PubMed] [Google Scholar]
- 22.Woodruff CW, Latham CB, James EP. Vitamin A status of preterm infants: the influence of feeding and vitamin supplements. Am J Clin Nutr. 1982;44:384–389. doi: 10.1093/ajcn/44.3.384. [DOI] [PubMed] [Google Scholar]
- 23.Ghebremeskel K, Burns L, Burden TJ. Vitamin A and related essential nutrients in cord blood: relationships with anthropometric measurements at birth. Early Hum Dev. 1994;39:177–188. doi: 10.1016/0378-3782(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 24.Ambalavanan N, Wu TJ, Tyson J. A comparison of three vitamin A dosing regimes in extremely-low-birth-weight infants. J Paediatric. 2003;142:656–661. doi: 10.1067/mpd.2003.214. [DOI] [PubMed] [Google Scholar]
- 25.Hininger I, Favier M, Arnaud J. Effects of a combined micro-nutrient supplementation on maternal biological status and newborn anthropometrics measurements: a randomized double-blind, placebo-controlled trial in apparently healthy pregnant women. Eur J Clin Nutr. 2004;58:52–59. doi: 10.1038/sj.ejcn.1601745. [DOI] [PubMed] [Google Scholar]
- 26.Gross R, Hansel H, Schultink W. Moderate zinc and vitamin A deficiency in breast milk of mothers from East-Jakarta. Eur J Clin Nutr. 1998;52:884–890. doi: 10.1038/sj.ejcn.1600660. [DOI] [PubMed] [Google Scholar]
- 27.Hendricks M, Beardsley J, Bourne L, Mzamo B, Golden B. Are opportunities for vitamin A supplementation being utilised at primary healthcare clinics in the Western Cape Province of South Africa? Public Health Nutr. 2007;10:1082–1088. doi: 10.1017/S1368980007699522. [DOI] [PubMed] [Google Scholar]


