Abstract
Perispinal injection is a novel emerging method of drug delivery to the central nervous system (CNS). Physiological barriers prevent macromolecules from efficiently penetrating into the CNS after systemic administration. Perispinal injection is designed to use the cerebrospinal venous system (CSVS) to enhance delivery of drugs to the CNS. It delivers a substance into the anatomic area posterior to the ligamentum flavum, an anatomic region drained by the external vertebral venous plexus (EVVP), a division of the CSVS. Blood within the EVVP communicates with the deeper venous plexuses of the CSVS. The anatomical basis for this method originates in the detailed studies of the CSVS published in 1819 by the French anatomist Gilbert Breschet. By the turn of the century, Breschet’s findings were nearly forgotten, until rediscovered by American anatomist Oscar Batson in 1940. Batson confirmed the unique, linear, bidirectional and retrograde flow of blood between the spinal and cerebral divisions of the CSVS, made possible by the absence of venous valves. Recently, additional supporting evidence was discovered in the publications of American neurologist Corning. Analysis suggests that Corning’s famous first use of cocaine for spinal anesthesia in 1885 was in fact based on Breschet’s anatomical findings, and accomplished by perispinal injection. The therapeutic potential of perispinal injection for CNS disorders is highlighted by the rapid neurological improvement in patients with otherwise intractable neuroinflammatory disorders that may ensue following perispinal etanercept administration. Perispinal delivery merits intense investigation as a new method of enhanced delivery of macromolecules to the CNS and related structures.
Electronic supplementary material
The online version of this article (doi:10.1007/s40263-016-0339-2) contains supplementary material, which is available to authorized users.
Key Points
| Perispinal injection is a novel method of drug delivery to the CNS. |
| Perispinal injection utilizes the cerebrospinal venous system (CSVS) to facilitate drug delivery to the CNS by retrograde venous flow. |
| Macromolecules delivered posterior to the spine are absorbed into the CSVS. |
Introduction
“It seems incredible that a great functional complex of veins would escape recognition as a system until 1940… In the first four decades of the last century, our knowledge of the vertebral veins was developed and then almost forgotten.” Batson, 1940 [1].
Physiological barriers, including the blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier (BCSFB), prevent large molecules (those with a molecular weight [MW] over 600) and many smaller molecules from efficiently penetrating into the central nervous system (CNS) after systemic administration [2–4]. Specialized methods of drug delivery are required to maximize the therapeutic potential of fusion proteins, monoclonal antibodies, and other macromolecules for CNS indications [2–5].
One such specialized method, perispinal1 administration, has been used to deliver the fusion protein etanercept (MW 150,000) to the CNS [6–16]. Perispinal administration involves delivery into the anatomic region posterior to the ligamentum flavum and the spinal canal, and is therefore less complicated than epidural or intrathecal injection [6–16]. Perispinal administration delivers a substance into the anatomic region drained by the external vertebral venous plexus (EVVP), a division of the cerebrospinal venous system (CSVS) [1, 6–20]. Blood within the EVVP communicates with the deeper, valveless, bidirectional venous plexuses comprising the remainder of the CSVS [1, 6–25]. Bidirectional and retrograde blood flow between the spinal and cerebral divisions of the CSVS is made possible by the absence of venous valves within the internal vertebral venous plexus [1, 19–31]. The anatomical basis for perispinal administration of etanercept has its origins in the detailed studies of the CSVS published in 1819 and 1829 by the French anatomist Gilbert Breschet [21, 22] (Figs. 1, 2, 3). Breschet’s findings were nearly forgotten after the turn of the century, until they were rediscovered and confirmed by American anatomist Oscar Batson in 1940 [1, 20].
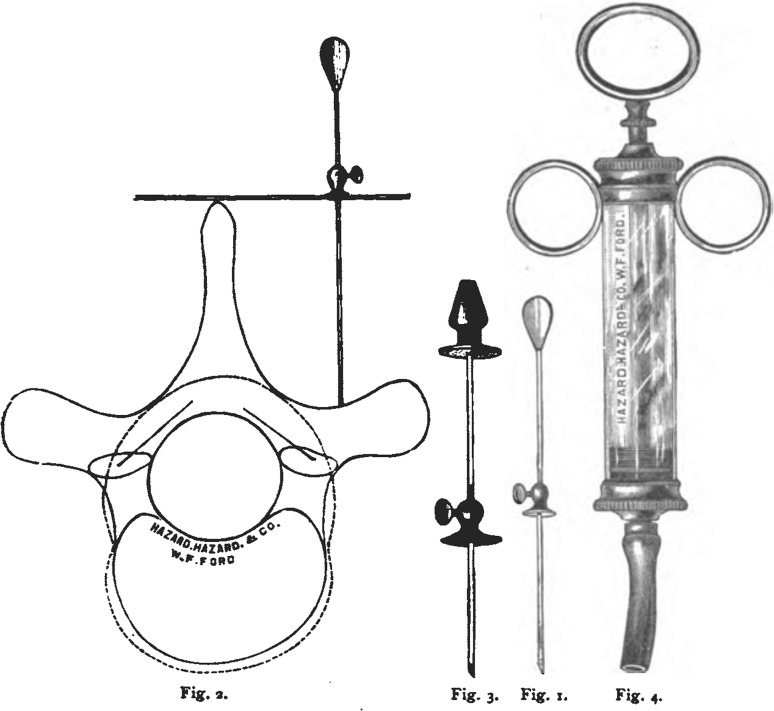
Fig. 1.
Cerebrospinal venous system. Detail of plate 5 from Breschet G, Recherches anatomiques physiologiques et pathologiques sur le systáeme veineux. Paris: Rouen fráeres; 1829. Courtesy of the Sidney Tobinick collection
Fig. 2.
Cerebrospinal venous system. Detail of plate from Breschet G, Essai sur les veines du rachis. Paris: Faculte de Medecine de Paris; 1819. Courtesy of the Sidney Tobinick collection
Fig. 3.
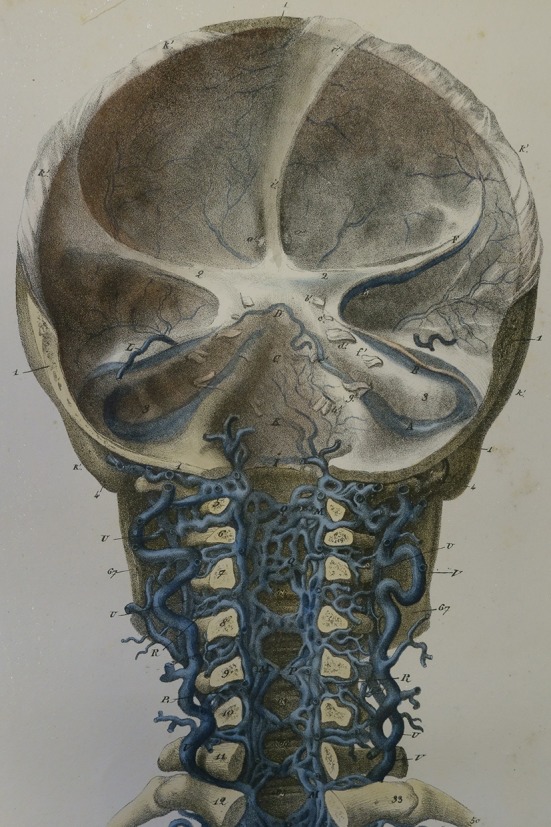
Cerebrospinal venous system. Detail of plate III from Breschet G, Recherches anatomiques physiologiques et pathologiques sur le systáeme veineux. Paris: Rouen fráeres; 1829. Courtesy of the Sidney Tobinick collection
Recently, additional supporting evidence, previously unrecognized, was discovered in the publications of American neurologist James Leonard Corning [32–36]. In 1885, the same year that Paul Ehrlich provided the first experimental evidence of the BBB, Corning reported rapid onset of spinal anesthesia after perispinal injection of cocaine ‘between the spinous processes’ (i.e. by interspinous injection), likely as a result of Corning’s familiarity with Breschet’s anatomical findings [32–36].
Breschet and the Cerebrospinal Venous System (CSVS)
“… blood is poured by the dorsi-spinal, the basi-vertebral and the spinal-medulli veins, and by the spinal plexus, depositing it to all parts along these veins….” Gilbert Breschet 1819 [21] (Fig. 2)
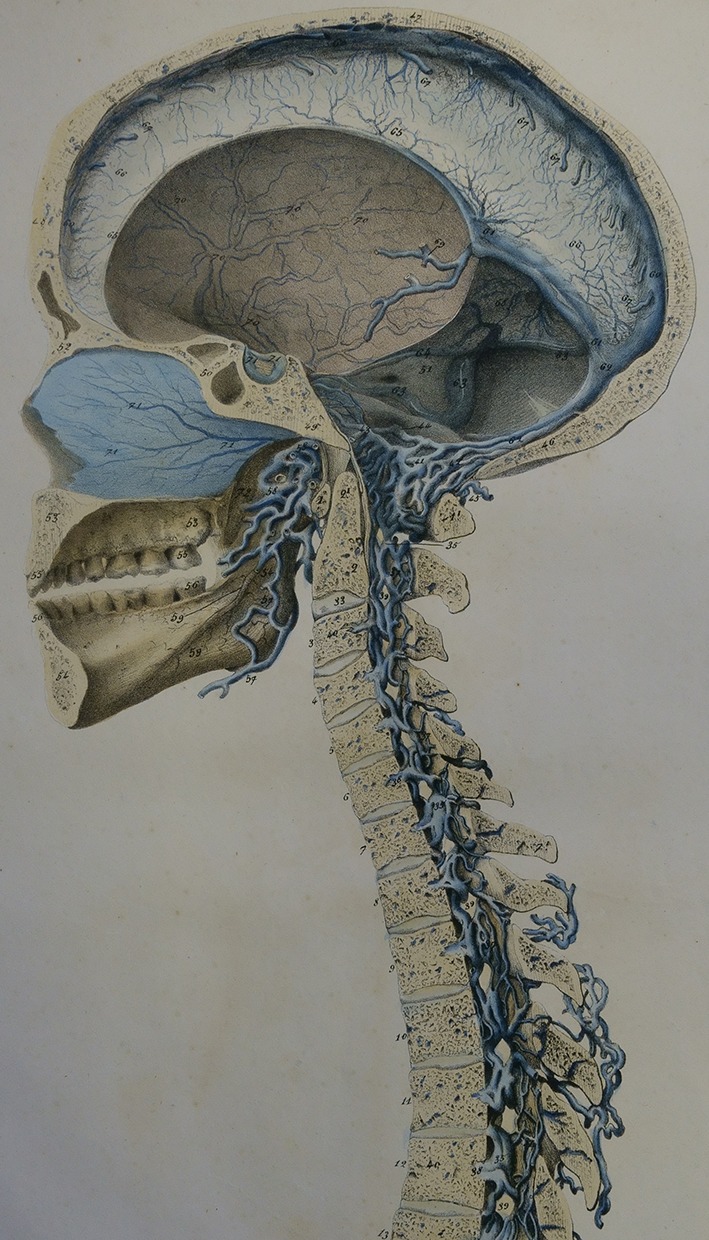
Gilbert Breschet (1783–1845), Professor of Anatomy at the University of Paris, surgeon to the Hotel Dieu in Paris, consulting surgeon to King Louis Phillipe, and member of the Royal Swedish Academy of Sciences, accurately detailed the anatomy and physiology of the spinal venous plexuses and their interconnections and drainage patterns, forming the basis for the modern conception of the CSVS [1, 17, 19–23, 25, 30] (Figs. 1, 2, 3). In the second half of the 19th century, Breschet’s findings regarding the anatomy and physiology of the spinal veins were widely known in Europe and were detailed in major anatomy texts of the time, including Curveilhier’s Anatomy (1844); volumes III (1847) and IV (1852) of Todd’s Cyclopaedia of Anatomy and Physiology (Fig. 4); the first (London 1858) and later editions of Gray’s Anatomy (Figs. 5, 6); and the 1867 and later editions of Quain’s Anatomy [37–41].
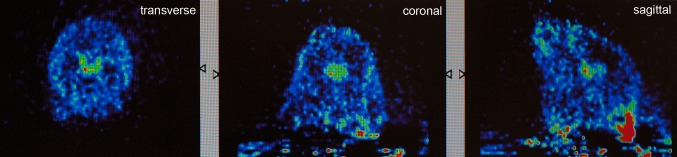
Fig. 9.
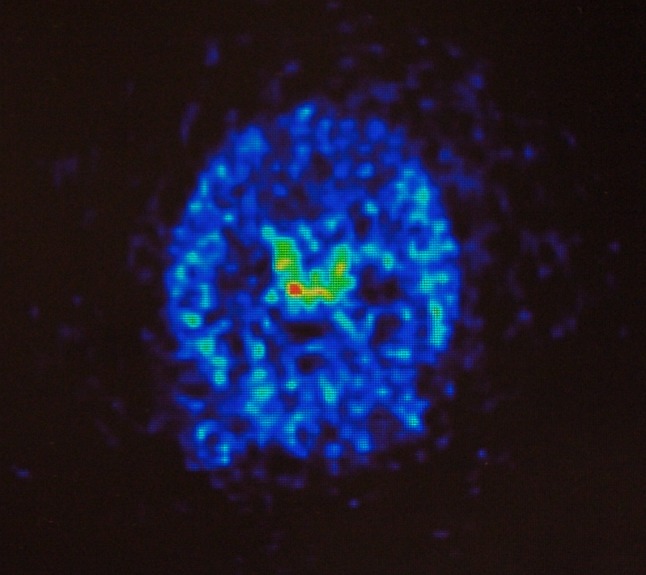
Positron emission tomography image, transverse section, of a living rat brain following perispinal extrathecal administration of 64Cu-DOTA-etanercept, imaged 5–10 min following the administration of etanercept. Note enhanced signal in the choroid plexus. Reproduced from Tobinick et al. [18]
Fig. 4.
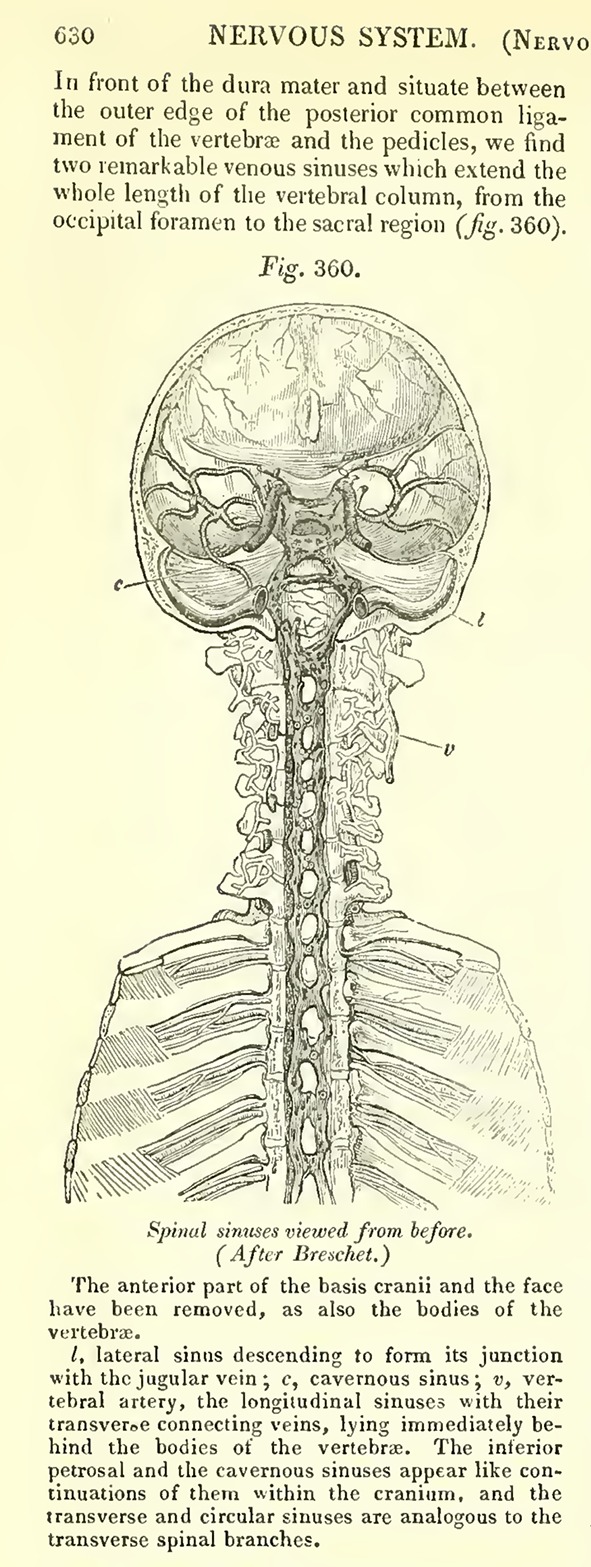
Cerebrospinal venous system. Detail from Todd RB (ed), The Cyclopaedia of Anatomy and Physiology. 1847, page 630, Fig. 360; after Breschet (1829)
Fig. 6.
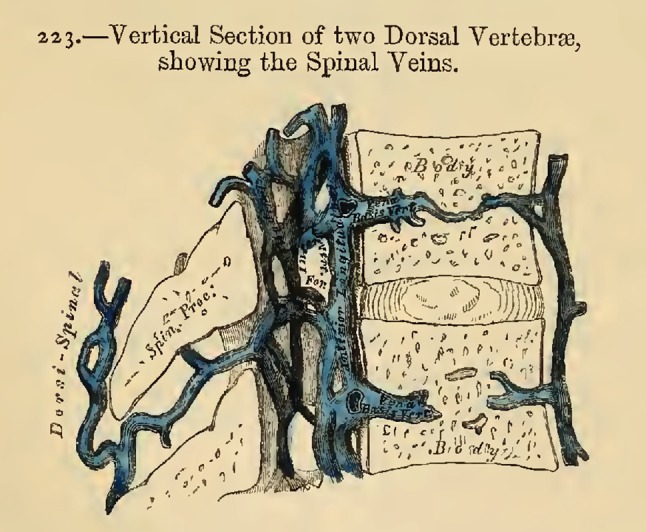
Spinal veins. From Gray, 1858. Figure 222, page 416 from Gray H and Carter HV, Anatomy, descriptive and surgical. 1st ed. London: John W. Parker and Son.; 1858; after Breschet (1829)
The first of Breschet’s major works on the spinal veins, his “Essai sur les veines du rachis [Essay on the Veins of the Spine]”, was published in 1819 [21] (Fig. 2). This work secured for Breschet the highly sought-after post of Inspector-General of Anatomy at the Faculty of Medicine in Paris [21, 38, 42]. At the Faculty of Medicine, Breschet worked with an esteemed group, including the anatomists Jean Cruveilhier and Guillaume Dupytren [20, 21, 38, 43]. The treatise is divided into nine sections derived from Breschet’s “careful study of the sources, the path, the connections and the endings of the veins of the spine … lengthy, often repeated trials and errors [of specialized injections] … [and] dissection of parts of this venous system” [21]. Sections of the treatise discuss all of the interconnected venous plexuses of the spine, specifically including sections on the EVVP (the ‘dorsi-spinal veins’), the veins of the spinal cord, and the free communication of the blood flow within and between these venous plexuses and the cerebral veins (Figs. 1, 2, 3, 4). The treatise gives details regarding the specialized injection methods that Breschet utilized, including using ichtyocolle and “wax, soft turpentine, and a body of resin, colored with iron cyanide (Prussian blue)” to meticulously map out the anatomy, venous blood flow patterns and connectivity of the veins in and around the spine, the spinal cord, and the brain [21, 22, 37, 39, 40].
Breschet found that (1) the EVVP drains the anatomic area posterior to the spine, including the skin and muscles posterior to the spine (Figs. 1, 2, 3, 4, 5, 6, 7); (2) veins comprising the EVVP perforate the ligamentum flavum to join the internal vertebral venous plexus (Figs. 1, 2, 3, 4, 5, 6, 7); and (3) all of the spinal venous plexuses, including those of the spinal cord, were interconnected and that blood within the venous plexuses, constituting the CSVS, intercommunicates [21, 22, 37, 39–41, 44–46] (Figs. 1, 2, 3, 4, 5, 6, 7). Breschet and colleagues depicted the anatomic continuity of the spinal and cerebral venous systems (Figs. 1, 2, 3, 4). As Cruveilhier wrote, cerebral venous drainage could take place via outflow through the vertebral venous plexus [17, 19–22, 37, 39, 45] (Figs. 1, 2, 3, 4).2
Fig. 5.
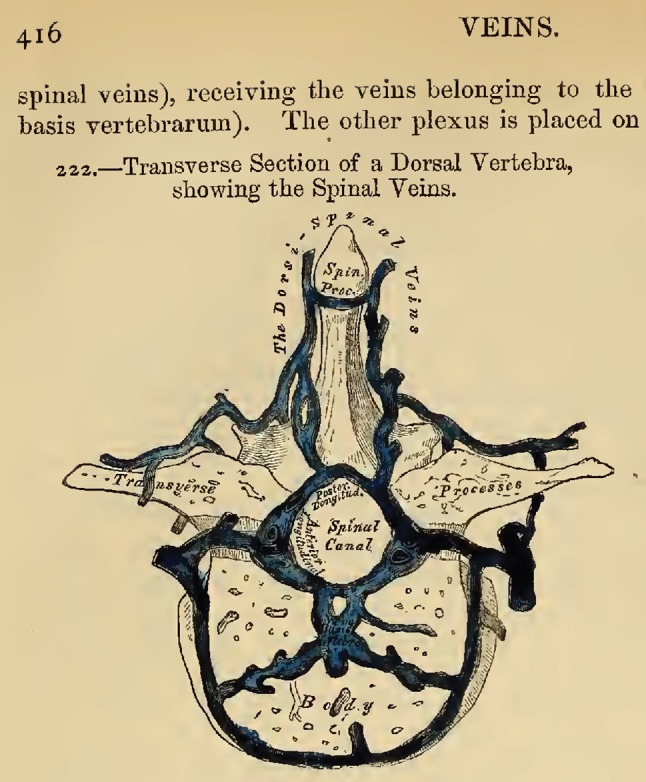
Spinal veins. From Gray, 1858. Figure 222, page 416 from Gray H and Carter HV, Anatomy, descriptive and surgical. 1st ed. London: John W. Parker and Son.; 1858; after Breschet (1829)
Fig. 7.
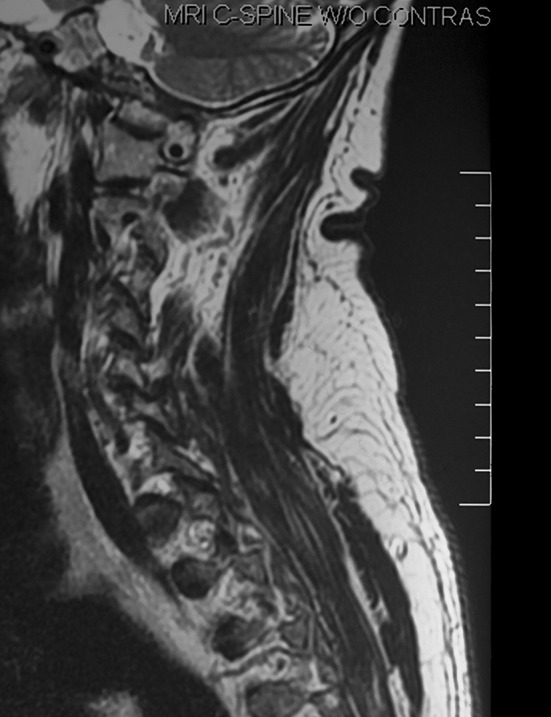
External vertebral venous plexus in the cervical subcutaneous space. Magnetic resonance image. Courtesy of the Sidney Tobinick Collection
The accuracy of Breschet’s pioneering descriptions has been confirmed by a multitude of subsequent anatomic and clinical studies [1, 17, 19–23, 25, 27, 28, 30, 31, 47, 48] (review [17]). The First Edition of Gray’s Anatomy (1858) states:
“The veins of the spine are described and illustrated from the well-known work of Breschet (Gray 1858, Preface [39])… . The Dorsi-Spinal Veins [EVVP] commence by small branches, which receive their blood from the integument of the back of the spine, and from the muscles in the vertebral grooves. They form a complicated net-work, which surrounds the spinous processes, laminae, and the transverse and articular processes of all the vertebrae. At the bases of the transverse processes, they communicate, by means of ascending and descending branches, with the veins surrounding the contiguous vertebrae, and with the veins in the interior of the spine, in the intervals between the arches of the vertebrae, perforating the ligamenta subflava [ligamentum flavum]… .” Gray 1858, p. 415 [39].
Today, we owe Breschet a great debt for his detailed and accurate description of the spinal venous plexuses, long before the availability of radiologic methods of imaging vascular pathways [20–22, 37]. Modern anatomical texts and reviews, including current editions of Gray’s Anatomy and Netter’s anatomical atlases, confirm Breschet’s findings [19, 25, 30, 48–52]. Magnetic resonance imaging of the spine clearly depicts the EVVP in sagittal images of the spine (Fig. 7).
Corning and Perispinal Injection of Cocaine
James Leonard Corning (1855–1923), although born in the US, received his Doctor of Medicine degree from the University of Wurzburg in Bavaria, Germany, in 1878, prior to returning to the US to practice neurology in New York City [53, 54]. A year after Sigmund Freud and Carl Kollar published their 1884 papers noting the local anesthetic properties of cocaine, Corning began experimenting with neurological applications of cocaine [33, 55, 56]. He developed novel methods enabling the effective use of cocaine as a local anesthetic in lower concentration, thereby limiting its systemic toxicity [33, 57].
By 1885, Corning’s clinical experience with cocaine and knowledge of spinal venous anatomy led to the first demonstration of spinal anesthesia [32–36]. In view of the place and details of his education, as well as his publications, Corning’s famous first use of cocaine for spinal anesthesia in 1885 was in fact likely based on Breschet’s findings regarding spinal venous anatomy and accomplished by perispinal injection [38, 42, 53, 54].3, 4 Corning explained the scientific rationale in his famous 1885 paper [32]:
“… in order to obtain the most immediate, direct, and powerful effects upon the cord with a minimum quantity of a medicinal substance, it is by no means necessary to bring the substance into direct contact with the cord; it is not necessary to inject the same beneath the membranes, as in the case of the frog, since the effects are entirely due to the absorption of the fluid by the minute vessels. On the other hand, in order to obtain these local effects, it is first necessary to inject the solution in the vicinity of the cord, and secondly, to select such a spot as will insure the most direct possible entry of the fluid into the circulation about the cord … .
Protocol of Experiments … As the introduction of a hypodermic needle beneath the membranes of the medulla spinalis is not practicable without removal of the arches of the vertebrae (on account of the danger of wounding the cord), I decided to inject the anesthetic between the spinous processes of the lower dorsal vertebrae. I was led to resort to this expedient from a knowledge of the fact that in the human subject numerous small veins (venae spinosae) run down between the spinous processes of the vertebrae, and, entering the spinal canal, join the more considerable vessels of the plexus spinalis interna. From these theoretical considerations I reasoned that it was highly probable that, if the anaesthetic was placed between the spinous processes of the vertebrae, it (the anaesthetic) would be rapidly absorbed by the minute ramifications of the veins referred to, and, being transported by the blood to the substance of the cord, would give rise to anesthesia of the sensory and perhaps also of the motor tracts of the same… .”
Corning’s injections of cocaine, “placed between the spinous processes” using a hollow needle that he later depicted (Fig. 8), resulted in spinal anesthesia, as he detailed:
“Experiment I.—This was performed on a young dog … . I injected … cocaine into the space situated between the spinous processes of two of the inferior dorsal vertebrae. Five minutes after the injection there were evidences of marked inco-ordination in the posterior extremities … . A few minutes later there was marked evidence of weakness in the hind legs, but there were no signs whatever of feebleness in the anterior extremities. I now tested the condition of sensibility by means of a powerful faradaic battery, one of the conducting cords of which was attached to a fine wire brush. When the wire brush was applied to the hind-legs, there was no reflex action whatever … .
Experiment II.—This was performed on a man … . I injected … cocaine into the space situated between the spinous processes of the eleventh and twelfth dorsal vertebrae … [there was no effect and I repeated the injection] … . About ten minutes later the patient complained that his legs ‘felt sleepy’; and, on making a careful examination with the wire brush, I found that sensibility was greatly impaired … . The impairment of sensibility was principally limited to the lower extremities, the lumbar regions, the penis, and the scrotum … .” Corning, 1885 [32].
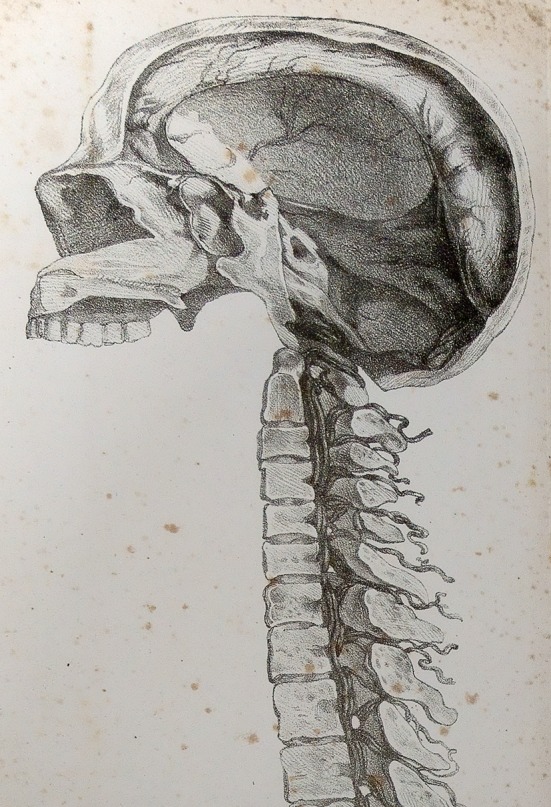
Fig. 8.
Figures from Corning’s 1888 article, Corning JL, XXI—A Further Contribution on Local Medication of the Spinal Cord, with Cases. Transactions of the Medical Society of the State of New York for the Year 1888. 1888: pages 260–269. Figure 1 depicts the solid needle, 3 inches in length, utilized in the apparatus depicted in Fig. 2. Figure 2 depicts the spinal canal, the ‘foramen vertebrae’, and the method Corning utilized to estimate the depth of the posterior border of same. Figure 3 depicts the hollow needle Corning utilized to deliver cocaine using the 6.2 ml syringe depicted in Fig. 4
The details included in his publications suggest that Corning utilized perispinal administration of cocaine delivered by interspinous injection into the interspinous space, posterior to the spinal canal i.e. superficial (posterior) to the ligamentum flavum [32–34, 36, 58] (see electronic supplementary material).
In later publications, Corning described a more invasive method of delivering cocaine, namely intrathecal injection, which required needle penetration through both the ligamentum flavum and the dura mater [35, 36]. Corning distinguished the clinical results obtained with these two methods, apparently favoring the perispinal method: “As a rule, the paraesthesia and anaesthesia are more irregularly distributed [with intrathecal injection at L 2-3] than when the posterior columns of the cord are anaesthetized in the manner first described [by perispinal injection at T10-11].” [36].
Intrathecal delivery of local anesthetics was rapidly adopted as a method of achieving spinal anesthesia, despite potential complications related to intrathecal needle delivery [59–61]. By the early 20th century Corning’s perispinal method had been misconstrued, particularly in the US [62, 63].5
Batson and the CSVS
Oscar Vivian Batson (1894–1979), Professor of Anatomy at the University of Pennsylvania, is best known for establishing the role of the vertebral venous plexuses (‘Batson’s plexus’) in the dissemination of cancer [1, 20]. Batson’s finding that the vertebral veins were valveless and communicated freely with intracranial veins provided an explanation for patterns of cerebral metastasis via the CSVS that had otherwise been unexplained [1, 20]. In fact, the extent and significance of Batson’s anatomical and physiological studies of the vertebral veins have significance well beyond Batson’s plexus as a route of cancer metastasis [9, 11, 17, 19, 64–66]. Batson re-discovered the work of Breschet and produced further evidence of the anatomic continuity of the spinal and cerebral venous plexuses by injection and radiologic experiments [1, 20, 27]. Batson showed that radio-opaque dye injected in peripheral regions drained by the vertebral veins could produce visible delivery of dye into the cerebral veins of human cadavers via the vertebral venous plexus (see Figs. 5, 6, 7 in Batson [1]). Subsequent animal and human experiments by Batson and Anderson confirmed these findings of retrograde venous flow cephalad through the vertebral venous plexuses into the cerebral venous sinuses [1, 20, 27]. Batson’s experiments validated Breschet’s findings and established the unique, linear, bidirectional nature of blood flow within the CSVS [1, 17, 20–22, 26–28]. The linear, bidirectional blood flow between the valveless cerebral and spinal divisions of the CSVS contrasts with the circular nature of blood flow within the systemic circulation, as established by Harvey centuries earlier [1, 17, 19, 20, 26].
Batson’s finding that dye injected into a peripheral catchment area of Batson’s plexus could reach the cerebral veins is supported by clinical and pathological findings [19, 31, 52, 67, 68], including observations of bilateral blurred vision occurring within 1 min of injection of a 3 mL test dose of lidocaine meant for epidural analgesia [31]6 :
“We believe her blurred vision was a result of direct test dose intracranial venous system dissemination via Batson’s vertebral venous plexus … . Batson’s vertebral venous plexus … communicates directly with … the intracranial venous system.” Vallejo et al. [31].
Although Batson made no mention of the use of the vertebral venous plexus for drug delivery, nor of Corning, carriage of cocaine through Batson’s plexus to the spinal cord, after perispinal interspinous injection, provides an anatomic explanation for the rapid spinal analgesic and anesthetic effects of cocaine reported by Corning after perispinal injection [32–34, 69].
Perispinal Administration of Etanercept
Clinical experience with perispinal administration of etanercept began with its use for spinal disorders, first reported in 2001 [6, 12, 70–72]. The therapeutic potential of etanercept for the treatment of spinal disorders is today supported by independent studies, including multiple randomized clinical studies7 [73–76]. After CNS improvements were noted in multiple patients treated for intractable intervertebral disc-related pain using perispinal etanercept (PSE; 25 mg) [6, 12, 70], an institutional review board-approved clinical trial of PSE 25–50 mg weekly administered open-label in 15 subjects with mild-to-severe Alzheimer’s disease over a period of 6 months was performed [13].8 Additional clinical experience suggests the therapeutic potential of PSE for additional forms of dementia [15, 77]. More recently, PSE has been successfully utilized in more than 1000 patients for treatment of chronic intractable neurological dysfunction after stroke or brain injury [10, 11, 16, 78]. The scientific rationale supporting the use of etanercept for stroke or brain injury includes multiple, independent studies and reviews [79–96]. Rapid neurological improvement, beginning within minutes of perispinal injection, is characteristically seen following PSE injection, suggesting novel patterns of etanercept distribution to the CNS after perispinal administration [6, 7, 10–16, 65, 70, 77, 78, 97].
For the treatment of brain disorders, Trendelenburg positioning for several minutes is used immediately after PSE is administered [10, 11, 13–15]. This head-down tilt positioning is used to attempt to facilitate delivery of etanercept into the choroid plexus and cerebrospinal fluid (CSF) after its absorption into the CSVS [9, 11, 65, 98] as it has been demonstrated in basic science models that head-down tilt can increase intracerebral venous pressure and facilitate passage of plasma proteins into the CSF [18, 99, 100].
In vivo drug distribution after perispinal administration has been investigated by independent academic scientists in collaboration with this author [9, 18]. In 2007, enhanced delivery of radiolabeled diethylene triamine pentaacetic acid (DTPA) into the cerebral venous system after perispinal (compared with antecubital) injection followed by Trendelenburg positioning was observed in a human subject [9]. Following this human result, in collaboration with scientists at Stanford, the in vivo distribution of radiolabeled etanercept after perispinal administration and head-down tilt in a rat was investigated [18]. Positron emission tomography (PET) imaging suggested rapid penetration of radiolabeled etanercept into the CSF within the cerebral ventricles, with accentuation of signal within the choroid plexus within the ventricles [9, 18] (Fig. 9, transverse image). Coronal and sagittal PET images acquired at the same time as the transverse image (Fig. 10) confirm the pattern of delivery suggested by the transverse image.9
Fig. 10.
Positron emission tomography image, transverse, coronal and sagittal sections of a living rat brain following perispinal extrathecal administration of 64Cu-DOTA-etanercept, imaged 5–10 min following the administration of etanercept. Reproduced in part from Tobinick et al. [18]
In 2014–2015, six basic science studies were published providing independent support for the therapeutic potential of etanercept in stroke models [79, 84, 86, 93, 94, 101]. These studies join the increasing evidence supporting the therapeutic potential of etanercept for multiple brain and spinal cord disorders [6–16, 18, 24, 64–66, 70–76, 78–81, 83–94, 97, 102–116]. Emerging findings regarding movement of molecules through the spinal veins, CSF, brain interstitial fluid, lymphatics and transport of macromolecules through physiological CNS barriers suggest the existence of previously unappreciated anatomic pathways that may facilitate delivery of large molecules into the CSF and the brain after perispinal delivery [4, 25, 50, 52, 98, 117, 118–123]. These new findings and accumulating evidence of the central involvement of neuroinflammatory mechanisms in brain disorders challenge existing dogma regarding brain physiology, support the rapid neurological effects seen following PSE administration, and underscore the importance of further research exploring CNS delivery of etanercept and other large molecules after perispinal injection [6, 7, 9–16, 24, 25, 50, 52, 70, 77–85, 87–97, 106, 114, 117, 119–123].
Therapeutic Implications, Potential Advantages and Limitations of Perispinal Delivery
As Corning first demonstrated in 1885, the ability to selectively deliver a drug to the CNS by perispinal injection can have significant therapeutic implications [32, 124]. Corning’s first perispinal injection of cocaine produced spinal anesthesia without the risk of dural puncture [32].10 A century later, emerging evidence suggests that perispinal delivery has the potential to enable treatment of intractable CNS disorders with drugs that, if administered systemically, would have difficulty reaching the CNS in a therapeutic concentration [6, 7, 9–16, 70, 77]; cf. epidural or intrathecal delivery11 [12, 73–75, 125–127].
Further research is needed, but current evidence suggests the potential advantages of perispinal delivery include the following:
Less invasive and less complex administration compared with neuraxial (epidural or intrathecal) delivery.
Elimination of two of the procedural risks of neuraxial delivery: post-dural puncture headache and needle injury to the spinal cord.
Suitability for use by the primary care physician in the primary care setting, without the requirement for specialized imaging, such as fluoroscopy, to facilitate needle placement.
Rapid and sustained brain effects, suggesting a different and more rapid pattern of brain delivery than that produced by spinal epidural or intrathecal delivery.
Delivery of CNS-active drugs, including macromolecules, to the CSF and CNS in therapeutically effective quantity, which drugs would otherwise have difficulty penetrating the BBB and/or the BCSFB in an optimal therapeutic amount.
The limitations of perispinal delivery, at present, include the following:
The need for basic science and clinical investigation to provide additional data regarding drug distribution, pharmacodynamics, pharmacokinetics, and safety and efficacy data for each drug candidate delivered by perispinal administration.
The need for funding and completion of randomized, double-blind, controlled clinical trials (RCT) to provide the additional data regarding safety and efficacy necessary for regulatory approval of drugs delivered by perispinal administration.
Limited knowledge of the functionality of the BBB and the BCSFB, as well as the distribution, pharmacokinetics, and pharmacodynamics of drugs after perispinal delivery and limited knowledge of the extent or function of drug efflux transporters in the CSVS vasculature.
Limited familiarity of the scientific and medical communities with cerebrospinal venous anatomy and physiology.
Further research addressing all of the limitations enumerated above is needed. At present, RCT data for perispinal delivery for the treatment of brain disorders is not yet available, although a randomized, double-blind clinical trial of PSE for the treatment of Alzheimer’s disease is currently underway at Griffith University in Australia (Australian New Zealand Clinical Trials Registry ID ACTRN12612000876897), with an RCT of PSE for post-stroke neurological dysfunction having received Institutional Review Board approval and scheduled to begin in 2016 (ACTRN12615001377527).
Conclusions
Perispinal injection is a novel emerging method of drug delivery to the CNS. Corning utilized knowledge of this anatomical pathway to successfully achieve spinal anesthesia by perispinal injection of cocaine in 1885. More than a century later, the therapeutic potential of perispinal injection for CNS disorders is highlighted by the rapid neurological improvement in patients with otherwise intractable neuroinflammatory disorders that may ensue following the administration of PSE. Double-blind, placebo-controlled clinical trials are necessary to fully characterize the efficacy of perispinal injection for drug delivery to the CNS and to obtain regulatory approval. More studies are needed in order to standardize methods of perispinal delivery. Perispinal delivery merits intense basic science and clinical investigation as a new method for enhancing delivery of macromolecules to the CNS and related structures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This article is dedicated to the memory of Sidney Tobinick, loving father and husband.
Compliance with Ethical Standards
Funding
No outside funding was received in support of this publication, and the author did not receive funding for writing this paper.
Conflict of interest
Edward Tobinick has multiple issued and pending US and foreign patents, assigned to TACT IP, LLC, which claim perispinal methods of use of etanercept and other drugs for the treatment of neurological disorders, including, but not limited to, US patents 6419944, 6537549, 6982089, 7214658, 7629311, 8119127, 8236306, 8349323, and 8900583, and Australian patents 758523 and 2011323616 B2. Dr. Tobinick is the Chief Executive Officer of TACT IP, LLC, and founder of the Institute of Neurological Recovery, a group of medical practices that utilize perispinal etanercept as a therapeutic modality, and that also train physicians.
Footnotes
‘Perispinal’ is used herein to refer to drug delivery into the anatomic area drained by the EVVP posterior (superficial) to the ligamentum flavum and the spinal canal, distinguished from epidural and intrathecal drug delivery.
“If all the jugular veins were obliterated, the venous circulation in the head would still continue, and would be carried on through the spinal veins. I have tied the two external jugular veins in a dog. The animal showed no sign of cerebral congestion … . In this case, the circulation was evidently carried on by means of the spinal veins.” [37, p. 611].
Prior to beginning to practice neurology in New York City, Corning visited and studied in hospitals and other medical institutions in Vienna, Paris and London; he did so “… for the purpose of familiarizing himself with the scientific and clinical methods prevalent in the various capitals of Europe”. [53].
Breschet was a member of the German medical societies of Bonn, Erlangen and Heidelberg, understood the German language, and translated German medical texts into French. His work was well known in Germany during the second half of the 19th century [42].
Medical historians often give credit to Bier [60] for the first publication of spinal anesthesia by intrathecal injection. It is unclear if they are aware that Corning gave an unequivocal description of intrathecal injection of cocaine in 1894 [35] (pp. 248–254), as well as in 1897 [36].
Parkinson earlier recognized that “blood may run freely in either direction between the orbit and coccyx” through Batson’s and Breschet’s veins [29].
The multiple randomized clinical studies of etanercept for the treatment of disc-related pain and/or radiculopathy include three clinical trials of epidural etanercept (all with 80 subjects or less; two 0.5–12.5 mg doses 2 weeks apart [73, 74], or one 10 mg dose [75]) and one using a single 10 mg dose of intradiscal etanercept [76].
There was significant improvement with treatment, as measured by all of the primary efficacy variables, including the Mini-Mental State Examination, the Alzheimer’s Disease Assessment Scale-Cognitive subscale, and Severe Impairment Battery [13].
Additionally, the coronal and sagittal PET images reveal high signal in the area of perispinal injection in the posterior neck, separate from and discontinuous with the area of high signal in the choroid plexus. This signal pattern, with accentuation in the choroid plexus, suggests cerebrospinal venous delivery of radiolabeled etanercept to the choroid plexus as the primary event, with secondary delivery to the CSF via the choroid plexus. These observations merit further study. Study of primates or large animals utilizing PET imaging is the preferred model for further investigation, for multiple reasons: it is exceedingly difficult to deliver etanercept into the interspinous space in small animals without tearing the small branches of the EVVP present therein, reducing the reliability and repeatability of this method in small animals; hydrostatic pressure with Trendelenburg positioning may be greater in larger animals, resulting in enhanced delivery into the CSF; and PET imaging has higher resolution than single-photon emission computed tomography (SPECT) imaging, thereby it is capable of producing finer anatomical detail.
Corning subsequently utilized perispinal injections of cocaine, from six to 12 or more per patient, for the treatment of spinal pain [124].
Little is known regarding the extent of drug efflux transporters in the CSVS vasculature. However, it has been recognized for decades that epidural delivery is capable of delivering drugs across the thecal membranes into the CSF in therapeutic concentration without needle penetration of the dura. Indeed, this selective enhancement of drug concentration in the CSF surrounding the lumbar cord (with limited craniad distribution) is the basis for epidural and spinal (intrathecal) anesthesia [125, 126]. For example, epidural injection of opioids may produce CSF levels hundreds of times greater than that achieved in plasma [126]. However, intrathecal delivery into the lumbar CSF will not necessarily lead to rapid distribution into the brain [127].
References
- 1.Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg. 1940;112(1):138–149. doi: 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greig NH, Brossi A, Pei X-F, Ingram DK, Soncrant TT. Designing drugs for optimal nervous system activity. In: Greenwood J, editor. New concepts of a blood–brain barrier. New York: Plenum Press; 1995. pp. 251–264. [Google Scholar]
- 3.Johanson CE, Duncan JA, Stopa EG, Baird A. Enhanced prospects for drug delivery and brain targeting by the choroid plexus-CSF route. Pharm Res. 2005;22(7):1011–1037. doi: 10.1007/s11095-005-6039-0. [DOI] [PubMed] [Google Scholar]
- 4.Banks WA. From blood–brain barrier to blood–brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15:275–292. doi: 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- 5.Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101(2):466–468. doi: 10.1182/blood-2002-06-1636. [DOI] [PubMed] [Google Scholar]
- 6.Tobinick E, Davoodifar S. Efficacy of etanercept delivered by perispinal administration for chronic back and/or neck disc-related pain: a study of clinical observations in 143 patients. Curr Med Res Opin. 2004;20(7):1075–1085. doi: 10.1185/030079903125004286. [DOI] [PubMed] [Google Scholar]
- 7.Tobinick E. Perispinal etanercept for treatment of Alzheimer’s disease. Curr Alzheimer Res. 2007;4(5):550–552. doi: 10.2174/156720507783018217. [DOI] [PubMed] [Google Scholar]
- 8.Perispinal administration of anti-TNF agent results in rapid cognitive improvement in AD. Nat Clin Pract Neurol. 2008;4(4):181.
- 9.Tobinick E. Perispinal etanercept: a new therapeutic paradigm in neurology. Expert Rev Neurother. 2010;10(6):985–1002. doi: 10.1586/ern.10.52. [DOI] [PubMed] [Google Scholar]
- 10.Tobinick E. Rapid improvement of chronic stroke deficits after perispinal etanercept: three consecutive cases. CNS Drugs. 2011;25(2):145–155. doi: 10.2165/11588400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Tobinick E, Kim NM, Reyzin G, Rodriguez-Romanacce H, Depuy V. Selective TNF inhibition for chronic stroke and traumatic brain injury: an observational study involving 629 consecutive patients treated with perispinal etanercept. CNS Drugs. 2012;26(12):1051–1070. doi: 10.1007/s40263-012-0013-2. [DOI] [PubMed] [Google Scholar]
- 12.Tobinick EL, Britschgi-Davoodifar S. Perispinal TNF-alpha inhibition for discogenic pain. Swiss Med Wkly. 2003;133(11–12):170–177. doi: 10.4414/smw.2003.10163. [DOI] [PubMed] [Google Scholar]
- 13.Tobinick E, Gross H, Weinberger A, Cohen H. TNF-alpha modulation for treatment of Alzheimer’s disease: a 6-month pilot study. MedGenMed. 2006;8(2):25. [PMC free article] [PubMed] [Google Scholar]
- 14.Tobinick EL, Gross H. Rapid cognitive improvement in Alzheimer’s disease following perispinal etanercept administration. J Neuroinflamm. 2008;5:2. doi: 10.1186/1742-2094-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobinick EL, Gross H. Rapid improvement in verbal fluency and aphasia following perispinal etanercept in Alzheimer’s disease. BMC Neurol. 2008;8:27. doi: 10.1186/1471-2377-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tobinick E, Rodriguez-Romanacce H, Levine A, Ignatowski TA, Spengler RN. Immediate neurological recovery following perispinal etanercept years after brain injury. Clin Drug Investig. 2014;34(5):361–366. doi: 10.1007/s40261-014-0186-1. [DOI] [PubMed] [Google Scholar]
- 17.Tobinick E. The cerebrospinal venous system: anatomy, physiology, and clinical implications. MedGenMed. 2006;8(1):53. [PubMed] [Google Scholar]
- 18.Tobinick EL, Chen K, Chen X. Rapid intracerebroventricular delivery of Cu-DOTA-etanercept after peripheral administration demonstrated by PET imaging. BMC Res Notes. 2009;2:28. doi: 10.1186/1756-0500-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathoo N, Caris EC, Wiener JA, Mendel E. History of the vertebral venous plexus and the significant contributions of Breschet and Batson. Neurosurgery. 2011;69(5):1007–1014. doi: 10.1227/NEU.0b013e3182274865. [DOI] [PubMed] [Google Scholar]
- 20.Batson OV. The vertebral vein system. Caldwell lecture, 1956. Am J Roentgenol Radium Ther Nucl Med. 1957;78(2):195–212. [PubMed] [Google Scholar]
- 21.Breschet G. Essai sur les veines du rachis [Theses presentees et soutenues publiq. devant les juges concours le 28. Avril 1819] Paris: Faculte de Medecine de Paris; 1819. [Google Scholar]
- 22.Breschet G. Recherches anatomiques physiologiques et pathologiques sur le systáeme veineux. Paris: Rouen fráeres; 1829. [Google Scholar]
- 23.Stringer MD, Restieaux M, Fisher AL, Crosado B. The vertebral venous plexuses: the internal veins are muscular and external veins have valves. Clin Anat. 2012;25(5):609–618. doi: 10.1002/ca.21281. [DOI] [PubMed] [Google Scholar]
- 24.Ignatowski TA, Spengler RN, Dhandapani KM, Folkersma H, Butterworth RF, Tobinick E. Perispinal etanercept for post-stroke neurological and cognitive dysfunction: scientific rationale and current evidence. CNS Drugs. 2014;28(8):679–697. doi: 10.1007/s40263-014-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griessenauer CJ, Raborn J, Foreman P, Shoja MM, Loukas M, Tubbs RS. Venous drainage of the spine and spinal cord: a comprehensive review of its history, embryology, anatomy, physiology, and pathology. Clin Anat. 2015;28(1):75–87. doi: 10.1002/ca.22354. [DOI] [PubMed] [Google Scholar]
- 26.Herlihy WF. Revision of the venous system; the role of the vertebral veins. Med J Aust. 1947;1(22):661–672. [PubMed] [Google Scholar]
- 27.Anderson R. Diodrast studies of the vertebral and cranial venous systems to show their probable role in cerebral metastases. J Neurosurg. 1951;8(4):411–422. doi: 10.3171/jns.1951.8.4.0411. [DOI] [PubMed] [Google Scholar]
- 28.Epstein HM, Linde HW, Crampton AR, Ciric IS, Eckenhoff JE. The vertebral venous plexus as a major cerebral venous outflow tract. Anesthesiology. 1970;32(4):332–337. doi: 10.1097/00000542-197004000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Parkinson D. Extradural neural axis compartment. Neurosurg Focus. 2000;8(2). [DOI] [PubMed]
- 30.Tubbs RS, Hansasuta A, Loukas M, Louis RG, Jr, Shoja MM, Salter EG, et al. The basilar venous plexus. Clin Anat. 2007;20(7):755–759. doi: 10.1002/ca.20494. [DOI] [PubMed] [Google Scholar]
- 31.Vallejo MC, Beaman ST, Ramanathan S. Blurred vision as the only symptom of a positive epidural test dose. Anesth Analg. 2006;102(3):973–974. doi: 10.1213/01.ANE.0000199174.30944.B4. [DOI] [PubMed] [Google Scholar]
- 32.Corning JL. Spinal anaesthesia and local medication of the cord. N Y Med J. 1885;42:483–485. [Google Scholar]
- 33.Corning JL. Local anaesthesia in general medicine and surgery. New York: D. Appleton and Company; 1886. [Google Scholar]
- 34.Corning JL. A further contribution on local medication of the spinal cord, with cases. Trans Med Soc State N Y. 1888;260–9.
- 35.Corning JL. Pain in its neuro-pathological, diagnostic, medico-legal, and neuro-therapeutic relations. Philadelphia: J. B. Lippincott Company; 1894. [Google Scholar]
- 36.Corning JL. Cocaine: local medication of the spinal cord. In: Foster FP, editor. Reference book of practical therapeutics. New York: D. Appleton and Company; 1897. [Google Scholar]
- 37.Cruveilhier J. The anatomy of the human body, the first American, from the last Paris edition. New York: Harper and Brothers; 1844. [Google Scholar]
- 38.Breschet M. Obituary. Lancet. 1845;2(2):188–189. [Google Scholar]
- 39.Gray H, Carter HV. Anatomy, descriptive and surgical. 1. London: John W. Parker and Son; 1858. [Google Scholar]
- 40.Quain J. The elements of anatomy, seventh edition. 7. London: James Walton; 1867. [Google Scholar]
- 41.Quain J, Sharpey-Schäfer EA, Thomson A. Quain’s elements of anatomy. 8. New York: William Wood and Co.; 1878. [Google Scholar]
- 42.Huard P, Breschet G. Complete dictionary of scientific biography. 2008. http://www.encyclopedia.com/doc/1G2-2830900619.html. Accessed 27 Dec 2015.
- 43.Andral M, Cruveilhier M, Ferrus M, Demarquay M. Discours prononcés aux obsèques de M. Breschet [Speeches delivered at the funeral of Mr Breschet]. Paris, 1845. http://www.biusante.parisdescartes.fr/histmed/medica/cote?90945x13x13. Accessed 27 Dec 2015.
- 44.Gray H. Anatomy, descriptive and surgical. Philadelphia: Henry C. Lea; 1878. [Google Scholar]
- 45.Todd RB. Nervous system: nervous centres, the meninges. In: Todd RB (ed). The cyclopaedia of anatomy and physiology. London: Longman, Brown, Green, Longmans and Roberts; 1847.
- 46.Todd RB. Veins of the spine (Rachidian veins: Breschet). In: Todd RB (ed). The cyclopedia of anatomy and physiology. London: Longman, Brown, Green, Longmans, and Roberts; 1852.
- 47.Parkinson D. History of the extradural neural axis compartment. Surg Neurol. 2000;54(6):422–431. doi: 10.1016/S0090-3019(00)00342-6. [DOI] [PubMed] [Google Scholar]
- 48.Standring S. Gray’s anatomy: the anatomical basis of clinical practice. 41. New York: Elsevier Limited; 2016. [Google Scholar]
- 49.Netter FH. Plate 2-5: veins of spinal cord, nerve roots, and vertebrae. In: Jones HR, Burns TM, Aminoff MJ, editors. The netter collection of medical illustrations. 7. 2. Philadelphia: Elsevier; 2013. p. 54. [Google Scholar]
- 50.Barami K, Sood S. The cerebral venous system and the postural regulation of intracranial pressure: implications in the management of patients with cerebrospinal fluid diversion. Childs Nerv Syst. 2016;32(4):599–607. doi: 10.1007/s00381-015-3010-1. [DOI] [PubMed] [Google Scholar]
- 51.Moes P, Maillot C. Superficial veins of the human spinal cord. An attempt at classification [in French] Arch Anat Histol Embryol. 1981;64:5–110. [PubMed] [Google Scholar]
- 52.Tsutsumi S, Ogino I, Miyajima M, Ito M, Arai H, Yasumoto Y. Cerebrospinal fluid drainage through the diploic and spinal epidural veins. J Anat. 2015;227(3):297–301. doi: 10.1111/joa.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anonymous. Biographical sketch of Doctor James Leonard Corning, of New York City, and his recent remarkable discoveries in local anesthesia. VA Med Month. 1886;12:713–9.
- 54.Corning JL., Sr . Recollections of a life. New York: The Knickerbocker Press; 1898. [Google Scholar]
- 55.Freud S. Uber coca. Centralblatt fur die gesamte Therapie. 1884;2:289–314. [Google Scholar]
- 56.Koller C. On the use of cocaine for producing anesthesia on the eye. Lancet. 1884;2:990–992. doi: 10.1016/S0140-6736(02)28859-5. [DOI] [Google Scholar]
- 57.Corning JL. On the prolongation of the anesthetic effects of the hydrochlorate of cocaine when subcutaneously injected. An experimental study. N Y Med J. 1885;42:317. [Google Scholar]
- 58.Corning JL. A further contribution on local medication of the spinal cord, with cases. In: Shrady GF (ed). The Medical Record. 33. New York: William Wood and Company; 1888. p. 291–3.
- 59.Brown DL, Fink BR. The history of neural blockade and pain management. In: Cousins MJ, Bridenbaugh PO, editors. Neural blockade in clinical anesthesia and management of pain. 3. Philadelphia: Lippincott-Raven Publishers; 1998. pp. 3–27. [Google Scholar]
- 60.Bier A. Versuche uber Cocainisirung des Ruckenmarkes. Dtsch Ztschr Chir. 1899;51:361–369. doi: 10.1007/BF02792160. [DOI] [Google Scholar]
- 61.Wulf HF. The centennial of spinal anesthesia. Anesthesiology. 1998;89(2):500–506. doi: 10.1097/00000542-199808000-00028. [DOI] [PubMed] [Google Scholar]
- 62.Marcus L. Medullary narcosis (Corning’s method): its history and development. Med Rec. 1900;58(15):561–563. [Google Scholar]
- 63.Wyeth JA. Comments on some new surgical methods. N Y State J Med. 1902;2(1):17–21. [Google Scholar]
- 64.Tobinick E. Perispinal etanercept for neuroinflammatory disorders. Drug Discov Today. 2009;14(3–4):168–177. doi: 10.1016/j.drudis.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 65.Tobinick E. Deciphering the physiology underlying the rapid clinical effects of perispinal etanercept in Alzheimer’s disease. Curr Alzheimer Res. 2012;9(1):99–109. doi: 10.2174/156720512799015073. [DOI] [PubMed] [Google Scholar]
- 66.Tobinick E. Tumour necrosis factor modulation for treatment of Alzheimer’s disease: rationale and current evidence. CNS Drugs. 2009;23(9):713–725. doi: 10.2165/11310810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 67.Puri AS, Telischak NA, Vissapragada R, Thomas AJ. Analysis of venous drainage in three patients with extradural spinal arteriovenous fistulae at the craniovertebral junction with potentially benign implication. J Neurointerv Surg. 2013. [DOI] [PubMed]
- 68.Strong C, Yanamadala V, Khanna A, Walcott BP, Nahed BV, Borges LF, et al. Surgical treatment options and management strategies of metastatic renal cell carcinoma to the lumbar spinal nerve roots. J Clin Neurosci. 2013;20(11):1546–1549. doi: 10.1016/j.jocn.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallace M, Yaksh TL. Long-term spinal analgesic delivery: a review of the preclinical and clinical literature. Reg Anesth Pain Med. 2000;25(2):117–157. doi: 10.1053/rapm.2000.0250117. [DOI] [PubMed] [Google Scholar]
- 70.Tobinick EL. Targeted etanercept for discogenic neck pain: uncontrolled, open-label results in two adults. Clin Ther. 2003;25(4):1211–1218. doi: 10.1016/S0149-2918(03)80077-2. [DOI] [PubMed] [Google Scholar]
- 71.Tobinick EL. Targeted etanercept for treatment-refractory pain due to bone metastasis: two case reports. Clin Ther. 2003;25(8):2279–2288. doi: 10.1016/S0149-2918(03)80219-9. [DOI] [PubMed] [Google Scholar]
- 72.Tobinick E, inventor. TACT IP, LLC, assignee. US patent 6,419,944. Cytokine antagonists for the treatment of localized disorders. Filed 5 Apr 2001. USA 2001, 16 Jul 2002.
- 73.Cohen SP, Bogduk N, Dragovich A, Buckenmaier CC, 3rd, Griffith S, Kurihara C, et al. Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology. 2009;110(5):1116–1126. doi: 10.1097/ALN.0b013e3181a05aa0. [DOI] [PubMed] [Google Scholar]
- 74.Freeman BJ, Ludbrook GL, Hall S, Cousins M, Mitchell B, Jaros M, et al. Randomized, double-blind, placebo-controlled, trial of transforaminal epidural etanercept for the treatment of symptomatic lumbar disc herniation. Spine. 2013;38(23):1986–1994. doi: 10.1097/01.brs.0000435140.61593.4c. [DOI] [PubMed] [Google Scholar]
- 75.Ohtori S, Miyagi M, Eguchi Y, Inoue G, Orita S, Ochiai N, et al. Epidural administration of spinal nerves with the tumor necrosis factor-alpha inhibitor, etanercept, compared with dexamethasone for treatment of sciatica in patients with lumbar spinal stenosis: a prospective randomized study. Spine. 2012;37(6):439–444. doi: 10.1097/BRS.0b013e318238af83. [DOI] [PubMed] [Google Scholar]
- 76.Sainoh T, Orita S, Miyagi M, Inoue G, Kamoda H, Ishikawa T, et al. Single intradiscal administration of the tumor necrosis factor-alpha inhibitor, etanercept, for patients with discogenic low back pain. Pain Med. 2016;17:40–45. doi: 10.1111/pme.12892. [DOI] [PubMed] [Google Scholar]
- 77.Tobinick E. Perispinal etanercept produces rapid improvement in primary progressive aphasia: identification of a novel, rapidly reversible TNF-mediated pathophysiologic mechanism. Medscape J Med. 2008;10(6):135. [PMC free article] [PubMed] [Google Scholar]
- 78.Ignatowski TA, Spengler RN, Tobinick E. Authors’ reply to Whitlock: perispinal etanercept for post-stroke neurological and cognitive dysfunction: scientific rationale and current evidence. CNS Drugs. 2014;28(12):1207–1213. doi: 10.1007/s40263-014-0212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arango-Davila CA, Vera A, Londono AC, Echeverri AF, Canas F, Cardozo CF, et al. Soluble or soluble/membrane TNF-alpha inhibitors protect the brain from focal ischemic injury in rats. Int J Neurosci. 2015;125(12):936–940. doi: 10.3109/00207454.2014.980906. [DOI] [PubMed] [Google Scholar]
- 80.Bergold PJ. Treatment of traumatic brain injury with anti-inflammatory drugs. Exp Neurol. 2016;275(Pt 3):367–380. doi: 10.1016/j.expneurol.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chio CC, Lin JW, Chang MW, Wang CC, Kuo JR, Yang CZ, et al. Therapeutic evaluation of etanercept in a model of traumatic brain injury. J Neurochem. 2010;115(4):921–929. doi: 10.1111/j.1471-4159.2010.06969.x. [DOI] [PubMed] [Google Scholar]
- 82.Clark I. New hope for survivors of stroke and traumatic brain injury. CNS Drugs. 2012;26(12):1071–1072. doi: 10.1007/s40263-012-0014-1. [DOI] [PubMed] [Google Scholar]
- 83.Clark IA, Vissel B. A neurologist’s guide to TNF biology and to the principles behind the therapeutic removal of excess TNF in disease. Neural Plast. 2015;2015:358263. doi: 10.1155/2015/358263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clausen B, Degn M, Martin N, Couch Y, Karimi L, Ormhoj M, et al. Systemically administered anti-TNF therapy ameliorates functional outcomes after focal cerebral ischemia. J Neuroinflamm. 2014;11(1):203. doi: 10.1186/s12974-014-0203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hellewell S, Semple BD, Morganti-Kossmann MC. Therapies negating neuroinflammation after brain trauma. Brain Res. 2015 doi: 10.1016/j.brainres.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 86.Iwata N, Takayama H, Xuan M, Kamiuchi S, Matsuzaki H, Okazaki M, et al. Effects of etanercept against transient cerebral ischemia in diabetic rats. Biomed Res Int. 2015;2015:189292. doi: 10.1155/2015/189292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lei B, Dawson HN, Roulhac-Wilson B, Wang H, Laskowitz DT, James ML. Tumor necrosis factor alpha antagonism improves neurological recovery in murine intracerebral hemorrhage. J Neuroinflamm. 2013;10(1):103. doi: 10.1186/1742-2094-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muccigrosso MM, Ford J, Benner B, Moussa D, Burnsides C, Fenn AM, et al. Cognitive deficits develop 1 month after diffuse brain injury and are exaggerated by microglia-associated reactivity to peripheral immune challenge. Brain Behav Immun. 2016;54:95–109. doi: 10.1016/j.bbi.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez-Polo JR, Rea HC, Johnson KM, Parsley MA, Unabia GC, Xu GY, et al. Inflammatory cytokine receptor blockade in a rodent model of mild traumatic brain injury. J Neurosci Res. 2016;94(1):27–38. doi: 10.1002/jnr.23617. [DOI] [PubMed] [Google Scholar]
- 90.Siniscalchi A, Gallelli L, Malferrari G, Pirritano D, Serra R, Santangelo E, et al. Cerebral stroke injury: the role of cytokines and brain inflammation. J Basic Clin Physiol Pharmacol. 2014;25(2):131–137. doi: 10.1515/jbcpp-2013-0121. [DOI] [PubMed] [Google Scholar]
- 91.Siniscalchi A, Iannacchero R, Anticoli S, Pezzella FR, De Sarro G, Gallelli L. Anti-inflammatory strategies in stroke: a potential therapeutic target. Curr Vasc Pharmacol. 2016;14(1):98–105. doi: 10.2174/1570161113666150923111329. [DOI] [PubMed] [Google Scholar]
- 92.Tuttolomondo A, Pecoraro R, Pinto A. Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: a review of evidence to date. Drug Des Devel Ther. 2014;8:2221–2239. doi: 10.2147/DDDT.S67655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yagi K, Lidington D, Wan H, Fares JC, Meissner A, Sumiyoshi M, et al. Therapeutically targeting tumor necrosis factor-alpha/sphingosine-1-phosphate signaling corrects myogenic reactivity in subarachnoid hemorrhage. Stroke. 2015;46(8):2260–2270. doi: 10.1161/STROKEAHA.114.006365. [DOI] [PubMed] [Google Scholar]
- 94.Zhang BF, Song JN, Ma XD, Zhao YL, Liu ZW, Li Y, et al. Etanercept alleviates early brain injury following experimental subarachnoid hemorrhage and the possible role of tumor necrosis factor-alpha and c-Jun N-terminal kinase pathway. Neurochem Res. 2015;40(3):591–599. doi: 10.1007/s11064-014-1506-9. [DOI] [PubMed] [Google Scholar]
- 95.Esposito E, Cuzzocrea S. Anti-TNF therapy in the injured spinal cord. Trends Pharmacol Sci. 2011;32(2):107–115. doi: 10.1016/j.tips.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 96.Feng P, Jyotaki M, Kim A, Chai J, Simon N, Zhou M, et al. Regulation of bitter taste responses by tumor necrosis factor. Brain Behav Immun. 2015;49:32–42. doi: 10.1016/j.bbi.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Griffin WS. Perispinal etanercept: potential as an Alzheimer therapeutic. J Neuroinflammation. 2008;5:3. doi: 10.1186/1742-2094-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol. 1993;120(2):245–263. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- 99.Kotani J, Adachi R, Fujita N, Sugioka S, Ueda Y. Effect of cerebral venous congestion on the pressure-volume index in the evaluation of intracranial pressure dynamics. J Neurosurg Anesthesiol. 1993;5(2):121–126. doi: 10.1097/00008506-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 100.Wen TS, Randall DC, Zolman JF. Protein accumulation in cerebrospinal fluid during -90 degrees head-down tilt in rabbit. J Appl Physiol. 1994;77(3):1081–1086. doi: 10.1152/jappl.1994.77.3.1081. [DOI] [PubMed] [Google Scholar]
- 101.Wu MH, Huang CC, Chio CC, Tsai KJ, Chang CP, Lin NK, et al. Inhibition of peripheral TNF-alpha and downregulation of microglial activation by alpha-lipoic acid and etanercept protect rat brain against ischemic stroke. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9418-5. [DOI] [PubMed] [Google Scholar]
- 102.Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Bramanti P, et al. Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. J Pharmacol Exp Ther. 2006;316(3):1006–1016. doi: 10.1124/jpet.105.097188. [DOI] [PubMed] [Google Scholar]
- 103.Marchand F, Tsantoulas C, Singh D, Grist J, Clark AK, Bradbury EJ, et al. Effects of etanercept and minocycline in a rat model of spinal cord injury. Eur J Pain. 2009;13(7):673–681. doi: 10.1016/j.ejpain.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 104.Bassi E, De Filippi C. Beneficial neurological effects observed in a patient with psoriasis treated with etanercept. Am J Clin Dermatol. 2010;11(Suppl 1):44–45. doi: 10.1007/BF03257173. [DOI] [PubMed] [Google Scholar]
- 105.Clark IA, Alleva LM, Vissel B. The roles of TNF in brain dysfunction and disease. Pharmacol Ther. 2010;128(3):519–548. doi: 10.1016/j.pharmthera.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 106.Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-alpha to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord: Drug Targets. 2011;10(3):391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shen CH, Tsai RY, Shih MS, Lin SL, Tai YH, Chien CC, et al. Etanercept restores the antinociceptive effect of morphine and suppresses spinal neuroinflammation in morphine-tolerant rats. Anesth Analg. 2011;112(2):454–459. doi: 10.1213/ANE.0b013e3182025b15. [DOI] [PubMed] [Google Scholar]
- 108.Watanabe K, Yabuki S, Sekiguchi M, Kikuchi S, Konno S. Etanercept attenuates pain-related behavior following compression of the dorsal root ganglion in the rat. Eur Spine J. 2011;20(11):1877–1884. doi: 10.1007/s00586-011-1854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roh M, Zhang Y, Murakami Y, Thanos A, Lee SC, Vavvas DG, et al. Etanercept, a widely used inhibitor of tumor necrosis factor-alpha (TNF-alpha), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS One. 2012;7(7):e40065. doi: 10.1371/journal.pone.0040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boivin N, Menasria R, Piret J, Rivest S, Boivin G. The combination of valacyclovir with an anti-TNF alpha antibody [etanercept] increases survival rate compared to antiviral therapy alone in a murine model of herpes simplex virus encephalitis. Antiviral Res. 2013;100(3):649–653. doi: 10.1016/j.antiviral.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 111.Cheong CU, Chang CP, Chao CM, Cheng BC, Yang CZ, Chio CC. Etanercept attenuates traumatic brain injury in rats by reducing brain TNF- alpha contents and by stimulating newly formed neurogenesis. Mediators Inflamm. 2013;2013:620837. doi: 10.1155/2013/620837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chio CC, Chang CH, Wang CC, Cheong CU, Chao CM, Cheng BC, et al. Etanercept attenuates traumatic brain injury in rats by reducing early microglial expression of tumor necrosis factor-alpha. BMC Neurosci. 2013;14:33. doi: 10.1186/1471-2202-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shi X, Zhou W, Huang H, Zhu H, Zhou P, Zhu H, et al. Inhibition of the inflammatory cytokine tumor necrosis factor-alpha with etanercept provides protection against lethal H1N1 influenza infection in mice. Crit Care. 2013;17(6):R301. doi: 10.1186/cc13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng X, Shen Y, Li R. Targeting TNF: a therapeutic strategy for Alzheimer’s disease. Drug Discov Today. 2014;19(11):1822–1827. doi: 10.1016/j.drudis.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 115.Winkelstein BA, Allen KD, Setton LA. Intervertebral Disc herniation: pathophysiology and emerging therapies. In: Shapiro IM, Risbud MV, editors. The intervertebral disc. Wien: Springer-Verlag; 2014. [Google Scholar]
- 116.Ye J, Jiang R, Cui M, Zhu B, Sun L, Wang Y, et al. Etanercept reduces neuroinflammation and lethality in mouse model of Japanese encephalitis. J Infect Dis. 2014;210(6):875–889. doi: 10.1093/infdis/jiu179. [DOI] [PubMed] [Google Scholar]
- 117.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Byrod G, Rydevik B, Johansson BR, Olmarker K. Transport of epidurally applied horseradish peroxidase to the endoneurial space of dorsal root ganglia: a light and electron microscopic study. J Peripher Nerv Syst. 2000;5(4):218–226. doi: 10.1046/j.1529-8027.2000.00023.x. [DOI] [PubMed] [Google Scholar]
- 119.Biceroglu H, Albayram S, Ogullar S, Hasiloglu ZI, Selcuk H, Yuksel O, et al. Direct venous spinal reabsorption of cerebrospinal fluid: a new concept with serial magnetic resonance cisternography in rabbits. J Neurosurg Spine. 2012;16(4):394–401. doi: 10.3171/2011.12.SPINE11108. [DOI] [PubMed] [Google Scholar]
- 120.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra11. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res. 2015;40(12):2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Luo C, Yao X, Li J, He B, Liu Q, Ren H, et al. Paravascular pathways contribute to vasculitis and neuroinflammation after subarachnoid hemorrhage independently of glymphatic control. Cell Death Dis. 2016;7:e2160. doi: 10.1038/cddis.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Corning JL. Spinal irritation, its symptoms, pathology and treatment. Boston Med Surg J (later, N Engl J Med) 1886;115(23):541–543. doi: 10.1056/NEJM188612091152302. [DOI] [Google Scholar]
- 125.Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61(3):276–310. doi: 10.1097/00000542-198409000-00008. [DOI] [PubMed] [Google Scholar]
- 126.Max MB, Inturrisi CE, Kaiko RF, Grabinski PY, Li CH, Foley KM. Epidural and intrathecal opiates: cerebrospinal fluid and plasma profiles in patients with chronic cancer pain. Clin Pharmacol Ther. 1985;38(6):631–641. doi: 10.1038/clpt.1985.237. [DOI] [PubMed] [Google Scholar]
- 127.Rieselbach RE, Di Chiro G, Freireich EJ, Rall DP. Subarachnoid distribution of drugs after lumbar injection. N Engl J Med. 1962;267:1273–1278. doi: 10.1056/NEJM196212202672502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.