Abstract
Background
The oral cavity is an unique environment which provides an ideal medium for bacterial growth. As a result of repeated exposure to the microorganisms present in blood and saliva, the dental health professionals and the patients are at a higher risk for developing many infectious diseases. A pilot study was carried out in the Dept of Dental Surgery, Armed Forces Medical College to assess the risk of cross infection in dental clinics.
Methods
Samples were collected from different dental surgeries of the Dept of Dental Surgery, Armed Forces Medical College and sent for microbiological culture and identification to the Dept of Microbiology, Armed Forces Medical College. The sampling was carried out in two stages, before and after implementing a set protocol.
Result
All dental unit waterlines were coated with a well established biofilm made up of filamentous and bacillus-like microorganisms in first stage of study. There was marked reduction in the number of colonies from the samples collected during second stage. Same findings were observed in the samples of aerosol produced by ultrasonic scalers.
Conclusion
The present study concluded that the new set protocol followed is significantly effective in reducing the microbial load in the water tubing, container and aerosol production. It is an effective measure for reducing the chances of cross infection in the dental surgery
Key Words: Aerosol, Biofilm, Cross infection, Colony forming units
Introduction
The oral cavity is a unique environment which provides an ideal medium for bacterial growth. Microorganisms present in the oral cavity may be transmitted from person to person through aerosol, water contamination or surface contact. Aerosol produced during use of scaler or airotor of dental chair contains droplet nuclei particles which remain in the environment for long periods of time, and is a source of infection for the patient as well as the health care provider [1]. The contaminated water in dental chair waterlines is yet another source for transmission of infection [2]. In recent years attention has focused on biofilm which form in dental chair waterlines as the potential primary source of contamination [3]. The study was undertaken to evaluate environmental bacterial contamination and to determine effectiveness of methods to control cross infection in dental practice.
Material and Methods
In this study samples were collected from different dental surgeries of the Dept of Dental Surgery, Armed Forces Medical College(AFMC), Pune. The collection of samples was done in two stages i.e., at the beginning of study and after implementing methods to control cross infection. The samples included the water from the waterline, water storage container of the dental chair, aerosol samples from ultrasonic scaler and airotor of dental chair. These samples were sent for microbiological culture and identification of microorganisms to the Dept of Microbiology, AFMC Pune.
The first stage consisted of collection of samples as baseline after following routine sterilization procedures followed in the dental surgery. In the second stage, sample collection was done 15 days after implementing methods to control cross infection for reducing contamination. The samples were collected from five different dental chairs of different dental surgeries of the Department of Dental Surgery, AFMC, Pune.
Blood agar plate was used to collect the aerosol sample during the experimental procedure. It was chosen because it is a general purpose, non selective and enriched medium that promotes the growth of microorganisms such as those sampled from air. The sample plates were placed on the tray of dental chair 6 inches away from the subject's mouth. Two plates were used on either side of the chair where the patient was seated. The trays were adjusted so that the base of the support board was at 50° angle to get maximum aerosol during the use of airotor or scaler of dental chair. To prevent air turbulence that could cause the dispersion of aerosol particles from the agar plate, both investigator and subject remained stationary for ten minutes after the treatment. The plates were covered and sent to the Department of Microbiology for culture and microbiological identification tests (Fig. 1).
Fig. 1.
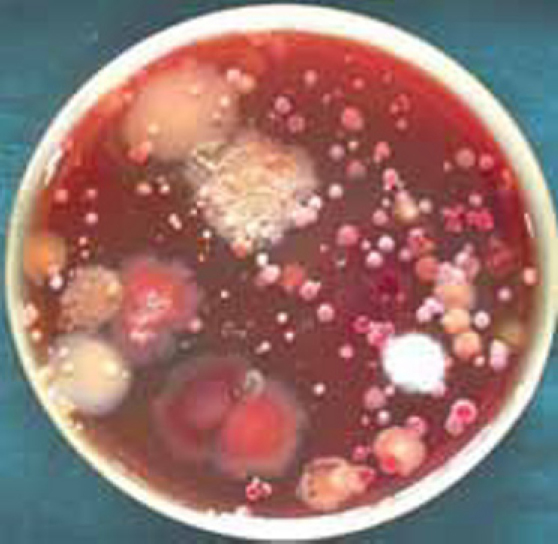
Blood agar plate in stage 1 showing large number and different types of colony forming units.
Water samples were collected from the waterline of dental chair into individual sterile tubes for culture. These water samples were transferred to a test tube containing brain heart broth media for incubation at 37° C (Fig. 2). The test tubes were examined every day and the changes in turbidity were recorded at the end of 48 hours (Fig. 3). Microbiological identification tests and techniques were performed to identify the isolates.
Fig. 2.

Water sample (stage 1) in Brain Heart Infusion Broth on day 0.
Fig. 3.

Water sample (stage 1) in Brain Heart Infusion Broth after 48 hours showing turbidity.
In the second stage of the study, samples were collected from the same dental surgeries after implementing methods to control cross infection. The protocol followed was:
-
a)
Using a high volume suction apparatus tube, kept as close as possible to the tip of ultrasonic scaler of dental chair, to prevent aerosol formation.
-
b)
Using sterile water in the water storage container of dental chair which was changed after every patient.
-
c)
Flushing of the entire tubing of dental chair waterline with distilled water for ten minutes every day.
-
d)
Weekly 0.5 percent sodium hypochlorite solution was used for flushing the tubing of dental chair waterline for a period of 5 minutes [3]. The same solution was allowed to stay in the tubing for ten minutes, followed by flushing with sterile water.
The samples were transported immediately to the Department of Microbiology, AFMC, Pune and colonies were examined by the same way as mentioned in the first stage of study (Fig. 4, Fig. 5, Fig. 6). The same investigator performed the identification of the microorganisms.
Fig. 4.
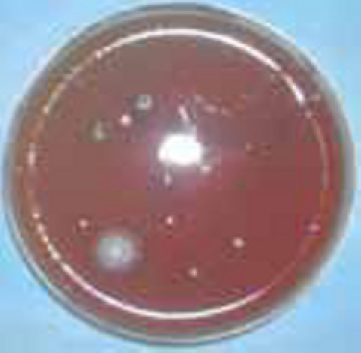
Blood agar plate in stage 2 showing reduction in number and types of colony forming units.
Fig. 5.

Water sample (stage 2) in Brain Heart Infusion Broth on day 0.
Fig. 6.

Water sample (stage 2) in Brain Heart Infusion Broth after 48 hours showing less amount of turbidity.
The numbers of colonies were counted and the same culture, identification methods and biochemical tests were performed. A comparative analysis was carried out between the first and second stages of study.
Results
The findings after the first stage were:
-
1
All water samples in stage 1 showed turbidity after 24 hours which kept on increasing with time.
-
2
There were thick sediments at the bottom of the test tube of water samples in stage 1 after 48 hours of culture.
-
3
The aerosol collection samples showed large numbers and different types of colonies in the plate.
-
4
The predominant organisms identified were pseudomonas, proteus, gram positive cocci and bacilli, aerobic spore forming bacilli and fungi (Table 1).
Table 1.
Stage 1: Microbial analysis
| Dental chair from where the samples were collected | Culture media | Microorganism found |
|---|---|---|
| Periodontology clinic (sample-1) | Blood agar | Mixed group of microbes predominantly gram positive cocci |
| Staff Surgery (sample-2) | Blood agar | Mixed group of microbes predominantly aerobic spore forming bacilli |
| Oral and Maxillofacial surgery (sample-3) | Blood agar | Mixed group of microbes predominantly pseudomonas, spore forming bacilli |
| Central OPD (sample-4) | Blood agar | Mixed group of microbes predominantly proteus, gram positive cocci |
| Central OPD (sample-5) | Blood agar | Mixed group of microbes predominantly aerobic spore forming bacilli fungi and pseudomonas |
The findings after the second stage were:
-
1
There was marked reduction in the number of colonies from the samples collected during the second stage.
-
2
There was marked reduction in the turbidity of the water sample collected in stage 2 after 48 hours of incubation.
-
3
The sub culture also showed less number of colonies. The culture shows predominant aerobic spore forming bacilli and cocci (Table 2).
Table 2.
Stage 2: Microbial analysis
| Dental chair from where the samples were collected | Culture media | Microorganism found |
|---|---|---|
| Periodontology clinic (sample-1) | Blood agar | Predominantly aerobic spore forming bacilli and cocci, with marked reduction in number of colonies from stage 1. |
| Staff surgery (sample-2) | Blood agar | −do- |
| Oral and Maxillofacial surgery (sample-3) | Blood agar | −do- |
| Central OPD (sample-4) | Blood agar | −do- |
| Central OPD (sample-5) | Blood agar | −do- |
Discussion
The air, surface, and water samples as well as instruments of dental surgeries have been studied concurrently for bacterial contamination. The contamination of air was fairly high, mainly with alpha haemolytic Streptococci, Staphylococci and fungi. This could be due to the infrequent use of devices for reducing airborne microbial contamination—such as high speed vacuums, dams or oral disinfectants [5]. Air contamination was also responsible for surface contamination by bacteria Streptococci and Staphylococci found in trolleys placed next to dental units. Dental unit water samples showed high levels of microbial contamination. Most dentists are probably unaware of this potentially dangerous water pollution, for which no guidelines have yet been published.
Research has shown that infective hazards are present in dental practice, because many infections can be transmitted by blood or saliva through direct or indirect contact, droplets, aerosols or contaminated instruments and equipment. All dental personnel, including dental surgeons, nurses and dental hygienists, while being at risk may also transmit infection to patients by the use of contaminated dental instruments. Water stagnation, biofilm production and lack of disinfection in dental unit water systems promote the proliferation of microorganisms [6]. Although it is well known that air, surfaces, dental materials, instruments and water in dental units could be vehicles for cross contamination with various microorganisms, detailed information on the microbial contamination of the dental surgery environment is still lacking. Infectious aerosols may be generated during dental practice, especially when high speed hand pieces or ultrasonic scalers are used without a high volume evacuator. The potential air contamination of dental surgeries by infectious aerosols has also been pointed out by the Centres for Disease Control and Prevention in Atlanta, which recommended that all sources of blood contaminated splatter and aerosols be minimized with rubber dams, high velocity evacuation and proper positioning of the patient. Previous studies have shown extensive contamination of water in dental units, not only with water saprophytes, but also with some potentially pathogenic microorganisms such as Legionella pneumophila and Pseudomonas aeruginosa. The presence of high heterotrophic bacterial counts and biofilm can be a risk for cross infection in dental surgery [7].
The results of the present study showed that the use of high volume suction apparatus kept next to the ultrasonic scaler or airotor hand piece reduced the quantum of aerosol production. The use of 0.5 percent sodium hypochlorite solution flushing for a period of five minutes and allowing it to stay in the tubing of dental waterlines for a duration of ten minutes helped reduce the formation of biofilm in the tube as well as the container. The above protocol can be used to control cross infection by the dental personnel involved in health care delivery system.
As a result of repeated exposure to these microorganisms present in blood and saliva, the dental health professionals and the patients can be placed at a higher risk for developing many infectious diseases [8]. The aim of study was not to identify the individual organisms present in the samples but to assess whether any reduction in the colony forming units (CFU) after implementing the set protocol. The present study concluded that the use of high volume suction apparatus and 0.5 percent sodium hypochlorite solution was significantly effective in reducing the microbial load. Therefore, it is an effective measure for reducing the chance of cross infection in the dental surgery.
Conflicts of Interest
None identified
Intellectual Contribution of Authors
Study Concept : Col VB Mandlik, Lt Col AK Jha, Col S Kumar
Drafting & Manuscript Revision : Maj M Kosala, Lt Col AK Jha, Sqn Ldr T Prasanth
Statistical Analysis : Maj M Kosala, Lt Col AK Jha, Sqn Ldr T Prasanth
Study Supervision : Col VB Mandlik, Lt Col AK Jha
References
- 1.Szymanska J. Dental bioaerosol as an occupational hazard in a dentist's workplace. Ann Agric Environ Med. 2007;14:203–207. [PubMed] [Google Scholar]
- 2.James TW, David JB, Allan MB, Martin RF, Michael VM, Philip M. Microbial biofilm formation and contamination of dental unit water systems in general dental practice. Appl Environ Microbiol. 2000;66:3363–3367. doi: 10.1128/aem.66.8.3363-3367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clapeau G, Decroix B, Bakayoko-Ly R, Varenne B, Dosso-Hien D, Decroix MO. Survey of methods of cleaning, decontamination, disinfection and sterilization in dental health services in tropical areas. J Clin Microbiol. 1997;7:323–329. [PubMed] [Google Scholar]
- 5.Edward EP, Amandeep SB. Dental Unit Waterline Contamination and its Possible Implications during Periodontal Surgery. J Periodontol. 2001;72:393–400. doi: 10.1902/jop.2001.72.3.393. [DOI] [PubMed] [Google Scholar]
- 6.Paolo C, Giorgio L, Maria TM, Christian N, Cesira P, Margherita B. Italian multicenter study on infection hazards during dental practice: Control of environmental microbial contamination in public dental surgeries. BMC Public Health. 2008;8:187–194. doi: 10.1186/1471-2458-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monarca S, Grottolo M, Renzi D, Paganelli C, Sapelli P, Zerbini I. Evaluation of environmental bacterial contamination and procedures to control cross infection in a sample of Italian dental surgeries. Occup Environ Med. 2000;57:721–726. doi: 10.1136/oem.57.11.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King TB, Muzzin KB, Berry CW, Anders LM. The effectiveness of an aerosol reduction device for ultrasonic scaler. J Periodontol. 1997;68:45–49. doi: 10.1902/jop.1997.68.1.45. [DOI] [PubMed] [Google Scholar]
Uncited Reference
- 4.Ayliffe GAJ, Maillard JY, Allan DR, William BH, Adam P. Disinfection, preservation and sterilization of dental surgery. 4th edition. 2004. p. 603. [Google Scholar]


