Abstract
Background
Salbutamol is the standard recommendation in bronchial asthma. However, the use of bronchodilators in wheeze associated respiratory infections including bronchiolitis continues to be controversial. The aim of this study was to compare the efficacy of nebulised salbutamol versus epinephrine in ‘first time’ wheezy children using clinical parameters and airway resistance.
Methods
Sixty children between two months to 60 months were recruited, 30 in each treatment group. Children received periodic (0, 20, 40 minutes) doses of either salbutamol (0.15mg/kg with 3ml saline subject to a minimum of 2.5mg) or laevo- epinephrine (1:1000, 0.5ml/kg subject to a max of 2.5ml with 3ml saline) via nebuliser along with oxygen. Changes in heart rate (HR), oxygen saturation (SpO2), respiratory rate (RR) and respiratory distress assessment instrument (RDAI) were assessed along with measurement of airway resistance using interrupter method (Hand held spirometer-Microloop with MicroRint module).
Result
The respiratory status was better in the epinephrine group with significant improvement in RR, RDAI score, SpO2 and fall of airway resistance. There were no significant side effects noted in either group.
Conclusion
Nebulised epinephrine is a useful and safe drug for wheezy children and is superior to salbutamol. This needs validation by large multicentric randomized blinded studies.
Key Words: Wheezy children, Nebulised bronchodilators, Epinephrine, Salbutamol
Introduction
Wheeze associated with respiratory tract infection (WARI) is an extremely common problem in children less than five years of age with reported attack rates in the western literature being as high as 11.4 per 100 children in the first year and 6 per 100 children in the second year of life. Viral respiratory infection in young children is often associated with small airway obstruction, secondary to an inflammatory process and/or spasm of the bronchial musculature. Bronchiolitis, wheezy bronchitis, infantile asthma and WARI are common diagnoses in such infants [1]. Acute viral bronchiolitis is one of the commonest causes in this cluster especially in children less than two years with the majority occurring in infancy. In the west, around 1% of healthy infants are hospitalized with bronchiolitis annually [2, 3]. The treatment of infants with bronchiolitis has been largely supportive, with minimal handling of infant, supplemental oxygen and the use of intravenous fluids or ventilatory support where necessary. Despite the proven role of bronchodilators in children below two years [4], its role in bronchiolitis is still controversial [5]. As mucosal edema is an important component of airway obstruction in infants with bronchiolitis and WARI, use of a combined α–adrenergic and β-adrenergic agonist, such as epinephrine was postulated to offer better benefit with its effects of reducing the mucosal edema and achieving satisfactory bronchodilation [3]. There have been several trials of bronchodilators in bronchiolitis and few in WARI including one from India, with varied results and conclusion [5]. Many studies used racemic epinephrine (which is not available in our country) reported some improvement in short-term outcomes, although the condition of a few patients worsened, as measured by clinical scores, pulmonary mechanics or oximetric findings after they received epinephrine [6, 7, 8, 9, 10].
Use of lung functions would be a good objective evidence of response to bronchodilators in children. However, the conventional lung function tests cannot be used for children less than five years. Researchers have tried measurement of airway resistance using the interrupter method. The interrupter technique is a noninvasive technique for estimating flow resistance, an important determinant of lung function, especially in children too young to accomplish forced respiratory maneuvers in a reproducible manner. The interrupter technique is easy to use in young children. Several recent studies used interrupter resistance (Rint) measurements in wheezy and/or asthmatic young children, particularly for testing bronchoreactivity [11, 12]. There are no randomised studies available regarding use of airway resistance to assess therapeutic response in wheezy children.
We conducted a randomized study to examine the effect of nebulized salbutamol versus readily available laevorotatory form of epinephrine in children with wheeze associated respiratory conditions using clinical parameters and airway resistance.
Material and Methods
The study was conducted in ‘first time’ wheezy children reporting to the paediatric out patient department of a tertiary hospital between May 2006 to May 2008. Children chosen (consecutive sample) were between 2 to 60 months of age and were diagnosed as bronchiolitis or WARI based on the typical clinical profile with history of coryza and/or fever followed by respiratory distress. Other common differential conditions were excluded following a hemogram and chest radiograph. These children underwent blood tests for urea, creatinine and electrolytes when they were sick enough to be on IV fluids. Blood culture was done in cases with high fever >38.5°C. Children with history of any episode of respiratory distress in the past; family history of atopy/asthma; history of prolonged respiratory distress in newborn period; those with any chronic cardiac/pulmonary illness; those having received corticosteroids in any form in the preceeding 72 hours and children in respiratory failure were excluded.
A total sample size of 60 was considered adequate based on ability to detect a difference of at least 5% SpO2 because of the intervention with a standard deviation of 1.5 based on an earlier study [1], with alpha (two tailed alpha) of 0.05 and power (1-beta) of 0.90. The children were randomly allotted into two groups (30 each), i.e, salbutamol nebulisation group (Group 1) and epinephrine nebulisation group (Group 2). Randomization was done by lottery method with the prospective cases being allotted serial numbers in cards. The cards were shuffled and picked to enter Group I or Group II alternatively.
Children received periodic (0, 20, 40 minutes) doses of either salbutamol (0.15mg/kg with 3ml saline subject to a minimum of 2.5mg) or laevo- epinephrine (1:1000, 0.5ml/kg subject to a maximum of 2.5ml with 3ml saline) via nebuliser along with oxygen (other than standard oxygen/IV fluids when indicated). Changes in heart rate (HR), respiratory rate (RR), respiratory distress assessment instrument (RDAI) (Table 1) and oxygen saturation (SpO2) were assessed along with measurement of airway resistance (Rint) (Expiratory phase in kPa/l/s) using interrupter method. The airway resistance was measured using the MicroRint module of the Micro-Loop spirometer (Micro Medical Ltd, Rochester, UK), a portable device including a shutter and pneumotachograph, connected to a palmtop computer with an online display showing mouth pressure, time of shutter closure, Rint values and the median value of all Rint data recorded during one session. All measurements were carried out with a filter (Micro Medical Ltd) in place for reasons of hygiene and to prevent dysfunction of the pneumotachograph due to any saliva.
Table 1.
Respiratory distress assessment instrument
| 0 | 1 | 2 | 3 | 4 | Max (17) points | |
|---|---|---|---|---|---|---|
| Wheeze | ||||||
| Expiration | None | End | 1/2 | 3/4 | Complete | 4 |
| Inspiration | None | Part | Complete | 2 | ||
| Location | None | Segmental : | Diffuse: | 2 | ||
| ≤2 of 4 lung fields | ≥ 3 of 4 lungfields | |||||
| Retractions | ||||||
| Supraclavicular | None | Mild | Moderate | Marked | 3 | |
| Intercostal | None | Mild | Moderate | Marked | 3 | |
| Subcostal | None | Mild | Moderate | Marked | 3 |
All children were assessed in the beginning and at 10 minutes post nebulisation for the initial three nebulisations with special emphasis on heart rate (HR), respiratory rate (RR), respiratory distress assessment instrument (RDAI) score [13], oxygen saturation by pulse oximetry (SpO2) and airway resistance (Rint) followed by closed monitoring based on the clinical condition. A comparison between observations before and after intervention in the given groups and between the two groups was done.
Data was recorded on a predetermined proforma and analysed using the Student's t-test.
Results
A total of 60 children in the age range of 2 to 60 months were included in the study with 30 in each group. The mean age of children was 16.13 ±14.26 months in Group 1 and 16.9 ±14.85 months in Group 2. A total of 59.4% children in Group 1 and 52.8% in Group 2 were males. The two groups were comparable with respect to their mean initial HR, RR, RDAI score, SpO2 and airway resistance (Rint) (Table 2).The trends of the various parameters through the initial three nebulisations (based on mean values at 0,10,30 and 50 minutes) in the two groups are shown in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5. At the end of three nebulisations, the mean (SD) changes in parameters in both groups are given in Table 3. In Group 1, the post nebulisation mean heart rate/min increased by 9.63 ± 2.58, the mean respiratory rate/min decreased by 8.13 ±1.63, the mean respiratory distress assessment score decreased by 4.40 ± 0.86, the mean SpO2% increased by 4.27 ± 0.94, the mean airway resistance (Rint) in kPa/l/s decreased by 0.31 ± 0.05. In Group 2 also there was a similar change after initial nebulisations with mean heart rate/min increasing by 9.50 ±2.46, mean respiratory rate/min falling by 14.3 ± 3.39, mean respiratory distress assessment instrument score falling by 5.57 ± 0.82, mean SpO2% increasing by 6.13 ± 1.91, the mean Rint in kPa/l/s decreased by 0.39 ± 0.03. All the parameters in both the groups (within the groups) had registered a statistically significant change (p<0.0001).
Table 2.
Initial Mean and Standard deviation (SD) of parameters in the two groups
| HR/min | RR/min | Mean ± SD RDAI score | SpO2% | Rint (kPa/l/s) | |
|---|---|---|---|---|---|
| Group 1 | 147.9 ±17.34 | 70 ± 16.52 | 11.83 ± 1.53 | 89.1 ± 2.14 | 1.63 ± 0.17 |
| Group 2 | 149.5 ± 16.71 | 71.2 ± 17.13 | 12.13 ± 1.46 | 88.8 ± 2.01 | 1.59 ± 0.11 |
| t | 0.36 | 0.28 | 0.77 | 0.56 | 0.9 |
| p | Not significant | Not significant | Not significant | Not significant | Not significant |
Fig. 1.
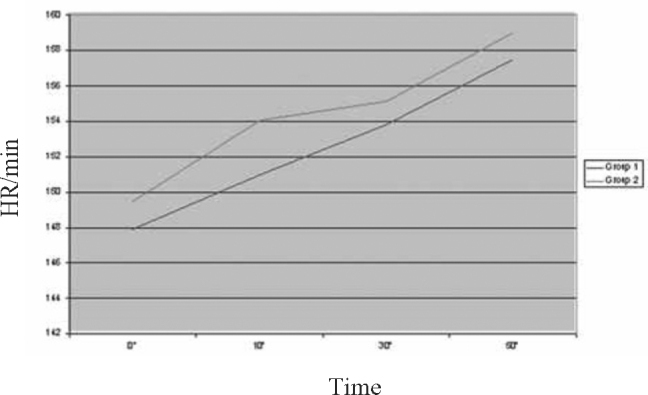
Trend of study parameter-Heart Rate (HR)
Fig. 2.
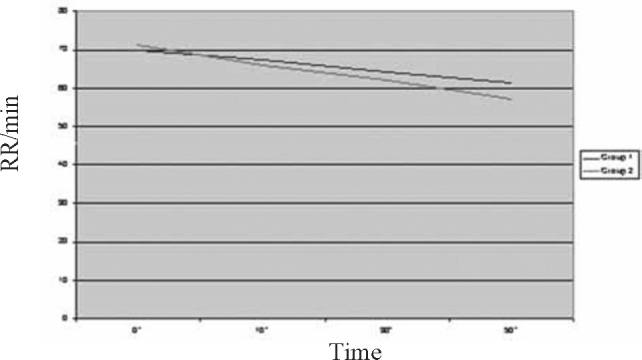
Trend of study parameter-Respiratory Rate (RR)
Fig. 3.
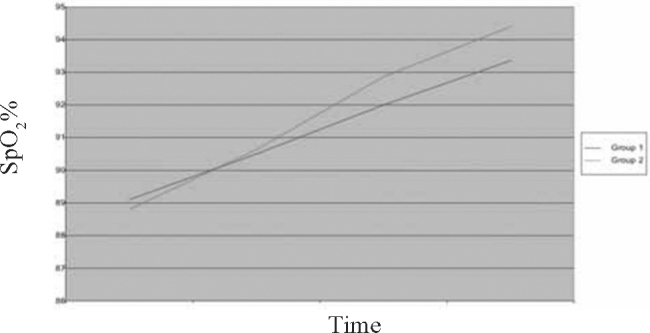
Trend of study parameter-Oxygen Saturation (SpO2)
Fig. 4.
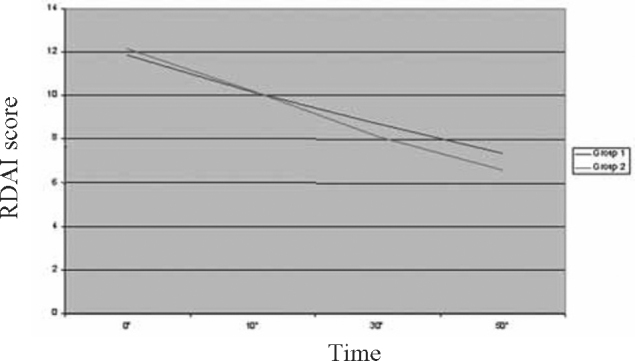
Trend of study parameter-Respiratory Distress Assessment Instrument (RDAI)
Fig. 5.
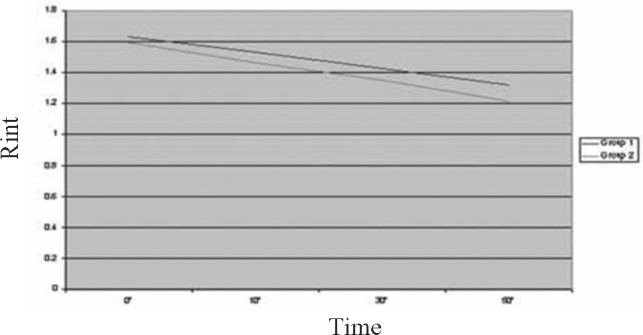
Trend of study parameter-Airway Resistance (Rint)
Table 3.
Change in parameters after three initial nebulisations in both groups
| Mean ± (SD) change in parameters |
|||||
|---|---|---|---|---|---|
| HR/min | RR/min | RDAI score | SpO2%. | Rint (kPa/l/s) | |
| Group1 | 9.63 ± 2.58 | 8.13 ± 1.63 | 4.40 ± 0.86 | 4.27 ± 0.94 | 0.31 ± 0.05 |
| t=20.45, p<0.0001 | t=22.50, p<0.0001 | t=29.8, p<0.0001 | t=24.74, p<0.0001 | t=34.50, p<0.0001 | |
| Group2 | 9.50 ± 2.46 | 14.3 ± 3.39 | 5.57 ± 0.82 | 6.13 ± 1.91 | 0.39 ± 0.03 |
| t=21.15, p<0.0001 | t=22.50, p<0.0001 | t=37.3, p<0.0001 | t=17.36, p<0.0001 | t=85.11, p<0.0001 | |
| t | 0.19 | 8.98 | 5.39 | 4.78 | 7.50 |
| p | Not significant | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
On comparing the two groups for difference in the change of parameters brought about, it was noticed that there was no significant difference in change of HR, there was a significant difference in change in RR, RDAI, SpO2 and Rint favouring epinephrine group (p<0.0001) (Table 3).
There were no significant side effects such as tachyarrythmia, irritability, tremors or facial blanching with either epinephrine /salbutamol initially or during subsequent nebulisations.
Discussion
Use of bronchodilators in bronchiolitis and first time WARI has been surrounded with controversy ever since it was tried as early as the late 1960's/early 1970's [1, 7]. Somehow, even after 30 years and many trials with bronchodilators, a consensus on their use in the management of bronchiolitis remains elusive till date though salbutamol is widely used for WARI. A variety of agents ranging from parenteral epinephrine to nebulised racemic epinephrine, albuterol, salbutamol and routinely available laevo-epinephrine have been tried [14, 15]. The interest in epinephrine has been significant owing to the following: (i) α–adrenergic vasoconstrictor action that can decongest the mucosa, limit its own absorption and regulate pulmonary blood flow, with little effect on ventilation–perfusion matching (ii) β2-adrenergic bronchial muscle relaxant effect (iii) β-adrenergic action to suppress release of chemical mediators (iv) physiological antihistamine effect that can reverse histamine effects, such as edema and (v) reduction of catarrhal secretions[16, 17].
Over the last 15 years the authors have come across 12 randomised controlled trials evaluating the effect of salbutamol or albuterol on bronchiolitis. Nine (75%) have shown that bronchodilators have no effect. There have been around five recent randomised trials evaluating the effect of nebulised epinephrine on bronchiolitis. All five (100%) have shown significant clinical improvement, with reductions in oxygen requirement, respiratory rate and wheeze after nebulised epinephrine [5]. The closest Indian study compared nebulised epinephrine and salbutamol in WARI and found short-term beneficial effects of both and more so with epinephrine [1]. This study however used 0.1ml/kg of 1:10000 epinephrine which is much lesser than the recommended useful dose.
There has been no study trying to analyse the usefulness of bronchodilators in wheezy children using airway resistance which is a good objective method of assessment. First time wheezers between the ages of 2-60 months were included to widen the spectrum of cases to include WARI other than bronchiolitis.
Analysis of the results showed a significant improvement in respiratory status (RR, RDAI, SpO2 and Rint) with both epinephrine and salbutamol with the benefit more marked in the epinephrine group. Unlike other studies [1, 18], this study did not see any significant difference in increase in heart rate in the two groups while there was a significant short term rise in HR in both groups after the intervention. There were no side effects of the bronchodilators used during the study which was similar to what was observed by other workers including those that did not find any benefit with the bronchodilators [1, 19, 20]. These findings are at variance to what has been mentioned in a recent multicentric trial which points to a lack of benefit of nebulized epinephrine in infants hospitalized with acute bronchiolitis, in either short-term or long-term clinically relevant outcomes [10]. The study mentions of a possibility of increased oxygen consumption with epinephrine based on the finding that among their infants with bronchiolitis who required supplemental oxygen and intravenous fluids, the time until the infant was ready for discharge was significantly longer in the epinephrine group than in the placebo group. This, however, doesn't explain the findings in infants in whom only oxygen was required (without IV fluids), wherein the epinephrine group consumed lesser oxygen than the placebo group. They also had more infants with moderately severe illness assigned to epinephrine than to placebo group which could have affected their results.
Cochrane analysis describes that there is insufficient evidence to support the use of epinephrine for the treatment of bronchiolitis among inpatients while there was some evidence to suggest that epinephrine may be favourable to salbutamol and placebo among outpatients [21]. The lack of consensus on usefulness of bronchodilators in bronchiolitis especially epinephrine is surprising when one considers the fact that approximately 68 to 96% of infants with bronchiolitis at tertiary paediatric centres in Canada are treated with bronchodilators [22, 23]. In a European survey of 88 paediatric centres, 54 centres reported using bronchodilators in all patients with bronchiolitis and 15 centres reported using bronchodilators only in high-risk patients [24]. In an Australian survey, 88% paediatricians used bronchodilators in infants with bronchiolitis [25].
This is the first study to conduct a randomized trial with inhaled bronchodilators using airway resistance apart from clinical parameters in first time wheezers. A study by Sanchez et al [6] using clinical scores and pulmonary mechanics in bronchiolitis comparing epinephrine and salbutamol had found epinephrine to be superior. A smaller study using only clinical parameters in cases of bronchiolitis was done by the author which showed a benefit of epinephrine over salbutamol [26]. The current study has demonstrated superiority of epinephrine over salbutamol in both bronchiolitis and WARI. The main drawbacks of this study is that at least few children in the study presenting with respiratory distress could be due to a first episode of bronchial asthma rather than bronchiolitis/WARI particularly as the age group taken was till 60 months and WARI is more likely to respond to bronchodilators than bronchiolitis. However attempts were made to exclude bronchial asthma by looking into the past and family history.
In conclusion, while it can be inferred that nebulised epinephrine and salbutamol are safe and useful in wheezy children with bronchiolitis/WARI, with epinephrine comparing better than salbutamol in relieving respiratory distress, there is a need for large multicentric randomized blinded studies to confirm our results.
Conflicts of Interest
This study has been financed by research grants from the O/o DGAFMS, New Delhi.
Intellectual Contribution of Authors
Study Concept : Wg Cdr BM John, Gp Capt D Singh
Drafting & Manuscript Revision : Wg Cdr BM John, Gp Capt D Singh
Statistical Analysis : Wg Cdr BM John
Study Supervision : Wg Cdr BM John, Gp Capt D Singh
References
- 1.Ray MS, Singh V. Comparison of nebulised adrenaline versus salbutamol in wheeze associated respiratory infection in infants. Indian Paediat. 2002;39:12–22. [PubMed] [Google Scholar]
- 2.Glezen WP, Denny FW. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 3.Wohl ME, Chernick V. State of the art: bronchiolitis. Am Rev Respir Dis. 1978;118:759–781. doi: 10.1164/arrd.1978.118.4.759. [DOI] [PubMed] [Google Scholar]
- 4.Soto ME, Sly PD, Uren E, Taussig LM, Landau LI. Bronchodilator response during acute viral bronchiolitis in infancy. Paediatr Pulmonol. 1985;1:85–90. doi: 10.1002/ppul.1950010206. [DOI] [PubMed] [Google Scholar]
- 5.Schindler Margrid. Do bronchodilators have an effect on bronchiolitis? Critical Care. 2002;6:111–112. doi: 10.1186/cc1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez I, De Coster J, Powell RE, Wolstein R, Chernick V. Effect of racemic epinephrine and salbutamol on clinical score and pulmonary mechanics in infants with bronchiolitis. J Paediatr. 1993;122:145–151. doi: 10.1016/s0022-3476(05)83508-5. [DOI] [PubMed] [Google Scholar]
- 7.Rusconi F, Sideri S. Efficacy of epinephrine and salbutamol in treatment of acute bronchiolitis. J Paediatr. 1996;128:441–443. doi: 10.1016/s0022-3476(96)70312-8. [DOI] [PubMed] [Google Scholar]
- 8.Numa AH, Williams GD, Dakin CJ. The effects of nebulized epinephrine on respiratory mechanics and gas exchange in bronchiolitis. Am J Respir Crit Care Med. 2001;164:86–91. doi: 10.1164/ajrccm.164.1.2008090. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand P, Aranibar H, Castro E, Sanchez I. Efficacy of nebulized epinephrine versus salbutamol in hospitalized infants with bronchiolitis. Paediatr Pulmonol. 2001;31:284–288. doi: 10.1002/ppul.1040. [DOI] [PubMed] [Google Scholar]
- 10.Wainwright C, Altamirano L, Marise Cheney M. A multicenter, randomized, double-blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349:27–35. doi: 10.1056/NEJMoa022226. [DOI] [PubMed] [Google Scholar]
- 11.Phagoo SB, Wilson NM, Silverman M. Evaluation of a new interrupter device for measuring bronchial responsiveness and the response to bronchodilator in 3-year-old children. Eur Respir J. 1996;9:1374–1380. doi: 10.1183/09031936.96.09071374. [DOI] [PubMed] [Google Scholar]
- 12.Beydon N, Trang-Pham H, Bernard A, Gaultier C. Measurements of resistance by the interrupter technique and of transcutaneous partial pressure of oxygen in young children during methacholine challenge. Paediatr Pulmonol. 2001;31:238–246. doi: 10.1002/ppul.1034. [DOI] [PubMed] [Google Scholar]
- 13.Lowell D, I, Lister G, Von Koss H, McCarthy P. Wheezing in infants: The response to epinephrine. Paediatrics. 1987;79:939–945. [PubMed] [Google Scholar]
- 14.Lenney W, Milner AD. Alpha and beta-adrenergic stimulants in bronchiolitis and wheezy bronchitis in children under 18 months of age. Arch Dis Child. 1978;53:707–709. doi: 10.1136/adc.53.9.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gadomski AM, Lichenstein R, Horton L, King J, Keane V, Permutt T. Efficiency of albuterol in the management of bronchiolitis. Pediatrics. 1994;93:907–912. [PubMed] [Google Scholar]
- 16.Katzung BG. Vol. 12. Appleton and Lange; Stamford: 1998. p. 1151. (Basic and clinical pharmacology). 7th ed. [Google Scholar]
- 17.Barr FE, Patel NR, Newth CJ. The pharmacologic mechanism by which inhaled epinephrine reduces airway obstruction in respiratory syncytial virus-associated bronchiolitis. J Paediatr. 2000;136:699–700. doi: 10.1067/mpd.2000.105358. [DOI] [PubMed] [Google Scholar]
- 18.Menon K, Sutcliffe T, Klassen T. A randomised trial comparing the efficacy of epinephrine with salbutamol in the treatment of acute bronchiolitis. J Paediatr. 1995;126:1004–1007. doi: 10.1016/s0022-3476(95)70234-2. [DOI] [PubMed] [Google Scholar]
- 19.Abul-Ainine A, Luyt D. Short term benefits of adrenaline in bronchiolitis: a randomised controlled trial. Arch Dis Child. 2002;86:276–279. doi: 10.1136/adc.86.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristjansson S, Carlsen K, Wennergren G. Nebulised racemic adrenaline in the treatment of acute bronchiolitis in infants and toddlers. Arch Dis Child. 1993;69:650–654. doi: 10.1136/adc.69.6.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartling L, Wiebe N, Russell K, Patel H, Klassen TP. The Cochrane Library. John Wiley and Sons; Chichester, UK: 1993. Epinephrine for bronchiolitis (Cochrane Review) Issue 4. [DOI] [PubMed] [Google Scholar]
- 22.Wang EE, Law BJ, Boucher FD. Paediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study of admission and management variation in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Paediatr. 1996;129:390–395. doi: 10.1016/s0022-3476(96)70071-9. [DOI] [PubMed] [Google Scholar]
- 23.Law BJ, De Carvalho V. Respiratory syncytial virus infections in hospitalized Canadian children: regional differences in patient populations and management practices. Paediatr Infect Dis J. 1993;12:659–663. [PubMed] [Google Scholar]
- 24.Kimpen JL, Schaad UB. Treatment of respiratory syncytial virus bronchiolitis: 1995 poll of members of the European Society for Paediatric Infectious Diseases. Paediatr Infect Dis J. 1997;16:479–481. doi: 10.1097/00006454-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Barben JU, Robertson CF, Robinson PJ. Implementation of evidence-based management of acute bronchiolitis. J Paediatr Child Health. 2000;36:491–497. doi: 10.1046/j.1440-1754.2000.00558.x. [DOI] [PubMed] [Google Scholar]
- 26.John BM, Patnaik SK, Prasad PL. Efficacy of nebulised epinephrine versus salbutamol in hospitalized children with bronchiolitis. MJAFI. 2006;62:354–357. doi: 10.1016/S0377-1237(06)80107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


