Abstract
Background
Therapeutic decisions in systemic lupus erythematosus (SLE) are based on the disease activity and nature of organ involvement. There are various clinical and laboratory methods to assess the lupus flares.
Methods
Fifty one SLE patients with active disease (lupus flare) were studied. Systemic lupus erythematosus disease activity index (SLEDAI), C3, C4 and anti-double stranded DNA levels were estimated and repeated monthly till remission. After remission these tests were done three monthly. Values of serological parameters were then correlated with SLEDAI score.
Result
Thirteen (25.4%) patients had predominantly renal involvement while 38 (74.6%) patients had non-renal affliction. Musculoskeletal and mucocutaneous symptoms were the commonest features of lupus flare (90%). It was observed that 12 out of 13 (92.3%) patients with active renal involvement had low C3 levels and 11 (84.6%) had low C4 levels. The anti-dsDNA levels were elevated in all patients with predominant renal flare. In non-renal flare anti-dsDNA titre was raised only in 35% cases. Low C3 and C4 levels were noticed in 43% and 53% of non-renal flares respectively. Significant positive correlation was noticed between SLEDAI score and anti-dsDNA levels (0.01 level two-tailed prediction) and a significant negative correlation was observed with SLEDAI and C3, C4 levels (0.01 and 0.05 levels, two-tailed prediction) in our patients. On subgroup analysis it was noticed that this correlation is stronger for renal lupus. Negative correlation of SLEDAI and complement levels was not observed in non-renal flares.
Conclusion
Calculation of SLEDAI is a vital clinical tool for assessment of SLE patients. Serial estimation of anti-dsDNA titre, C3 and C4 levels help us diagnose lupus flare and make appropriate therapeutic decisions in patients with high SLEDAI score.
Key Words: Systemic lupus erythematosus, Lupus flare, Complement, Anti-dsDNA
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease with a wide spectrum of clinical manifestations characterized by remissions and exacerbations. Tissue damage in SLE is caused by autoantibodies and complement fixing immune complex deposition. Therapeutic decisions are based on the estimation of the degree of damage that may result from untreated disease activity. There are various methods to quantify disease activity, identify flares and to predict flares.
SLE Disease Activity Index (SLEDAI), developed at the University of Toronto in 1992, is a global score reflecting all aspects of disease activity [1]. It is a weighted scale for 24 parameters and the score can range from zero to 105. Various manifestations are scored based on their presence or absence in the previous ten days of evaluation. Higher scores indicate more severe disease activity. SLEDAI has certain limitations in that it does not score some life threatening manifestations such as pulmonary haemorrhage and haemolytic anaemia. It is heavily weighted for central nervous system and does not take into account the severity of manifestations. Gladman et al [2] defined that an increase in SLEDAI score of more than three was a flare, SLEDAI score that was within three points of the previous score was persistent disease and a score of zero was remission. A change of SLEDAI score of more than 12 is a severe flare according to another study [3]. Global scores like SLEDAI can be problematic at times in that the score may be the same whether the patients are improving, stable or worsening. For instance a rash can improve and still be present, or deteriorate and yet the score may be same [4].
Serological tests are commonly used to assess the disease activity and predict lupus flare. During active disease, usually there is a fall in complement levels and a rise in anti-double stranded deoxyribonucleic acid (anti dsDNA) levels. Literature suggests strong correlation between disease activity and a rise in dsDNA and fall in complement (C3 and C4) levels [5]. However it may not be true in all patients. Studying correlation between SLEDAI, anti- dsDNA, C3 and C4 in different clinical subsets of SLE during disease flare and in remission will be useful. There are no prospective studies available in Indian patients on this subject.
This study was undertaken to correlate SLEDAI scores with C3, C4 and anti-dsDNA antibody levels in patients with active SLE (during lupus flare) and during remission. These serological changes are analysed in various clinical presentations of SLE. Patients with predominantly renal involvement are compared with those having non-renal flares.
Material and Methods
This study was a prospective study conducted in the Department of Rheumatology, Army Hospital (Research & Referral), New Delhi from 31 Jul 05 to 31 Jul 08. Patients satisfying the 1982 American College of Rheumatology (ACR) criteria (updated in 1997) for SLE were included in the study. Patients below 16 years and pregnant women were excluded from the study. Approval of the hospital ethics committee was taken. Informed consent was taken from every patient.
All patients underwent baseline investigations for haematological and biochemical parameters, chest radiograph and electrocardiogram (ECG). Immunological investigations included antinuclear antibodies (ANA) by immunofluroscence method, anti-dsDNA by enzyme linked immunosorbent assay (ELISA) and complement levels (C3, C4) by Nephelometry. Other special investigations were done as per clinical indication.
SLEDAI score was calculated on initial visit and then during subsequent monthly visits till clinical remission. Once the patient was identified to have active SLE, C3, C4 and anti-dsDNA levels were estimated and repeated monthly till remission. After remission these tests were repeated at three monthly intervals. These values were correlated with SLEDAI score.
It was planned to study at least 50 lupus flares in this project. Ideally one should correlate SLEDAI with C3, C4 and anti-dsDNA antibodies levels in different organ system flares (renal, musculoskeletal, neuropsychiatric, mucocutaneous, haematological, pulmonary etc). As more than one organ system can get involved at one time, the correlation with each organ system is difficult. Hence the flares were grouped into predominantly renal and non-renal flares. Statistical analysis was done using statistical package for social sciences (SPSS) software.
Results
In all, 51 flares were studied. Out of these, 13 (25.4%) patients had renal involvement, while 38 (74.6%) patients had non-renal affliction. Females accorded for 45 (88.24%) flares and six (11.76%) were males. Age of the patients ranged from 17 to 51 years (mean age 27 years). No patients were lost to follow up during the study period.
Among patients with renal flares, only two patients had isolated renal involvement while the other 11 patients had more than one organ involvement. Musculoskeletal and mucocutaneous flare occurred together in 28 patients. Mean values of SLEDAI, C3, C4 and anti-dsDNA of all cases during activity and in remission and group wise (renal and non-renal) mean values are shown in Table 1, Table 2, Table 3.
Table 1.
Mean SLEDAI, C3, C4 and anti-dsDNA levels of all patients (n=51)
| S. No | SLEDAI | C3 (mg/dl) (Normal:89-187mg/dl) | C4 (mg/dl) (Normal:16.5-38mg/dl) | Anti-dsDNA (Normal:0-40 IU/L) | |||
|---|---|---|---|---|---|---|---|
| Active disease | Remission | Active disease | Remission | Active disease | Remission | ||
| Mean | 10.88 | 81.08 | 106.06 | 15.05 | 18.89 | 82.24 | 30.97 |
| SD | 5.66 | 41.24 | 29.83 | 6.72 | 4.95 | 92.70 | 15.10 |
| Median | 10 | 86 | 98 | 14 | 18 | 40 | 30 |
| Q1 | 7 | 53 | 90 | 10.5 | 16.95 | 28 | 20 |
| Q3 | 13 | 102 | 112 | 18.65 | 22 | 94 | 36 |
Q1-Quartile 1, Q3-Quartile 3, IQR= Q3-Q1 (eg IQR for SLEDAI=13-7).
Table 2.
Mean SLEDAI, C3, C4 and anti-dsDNA levels of renal lupus patients (n=13)
| S. No | SLEDAI | C3 (mg/dl) (Normal:89-187mg/dl) | C4 (mg/dl) (Normal:16.5-38mg/dl) | Anti-dsDNA (Normal:0-40 IU/L) | |||
|---|---|---|---|---|---|---|---|
| Active disease | Remission | Active disease | Remission | Active disease | Remission | ||
| Mean | 18.85 | 46.80 | 99.54 | 12.13 | 19.88 | 196.46 | 39.77 |
| SD | 4.65 | 20.00 | 30.68 | 7.54 | 5.27 | 114.69 | 13.76 |
| Median | 10 | 86 | 98 | 14 | 18 | 40 | 30 |
| Q1 | 7 | 53 | 90 | 10.5 | 16.95 | 28 | 20 |
| Q3 | 13 | 102 | 112 | 18.65 | 22 | 94 | 36 |
Table 3.
Mean SLEDAI, C3, C4 and anti-dsDNA levels of non-renal patients (n=38)
| S. No | SLEDAI | C3 (mg/dl) (Normal:89-187mg/dl) | C4 (mg/dl) (Normal:16.5-38mg/dl) | Anti-dsDNA (Normal:0-40 IU/L) | |||
|---|---|---|---|---|---|---|---|
| Active disease | Remission | Active disease | Remission | Active disease | Remission | ||
| Mean | 8.16 | 92.81 | 108.29 | 16.05 | 18.54 | 43.17 | 27.96 |
| SD | 2.51 | 40.18 | 29.62 | 6.22 | 4.86 | 34.55 | 14.50 |
| Median | 10 | 86 | 98 | 14 | 18 | 40 | 30 |
| Q1 | 7 | 53 | 90 | 10.5 | 16.95 | 28 | 20 |
| Q3 | 13 | 102 | 112 | 18.65 | 22 | 94 | 36 |
Mean SLEDAI score of all patients was 10.88 (SD=5.66) whereas in lupus nephritis it was 18.85 (SD= 4.65) and in non-renal lupus it was 8.16 (SD= 2.51). Mean anti-dsDNA level in renal lupus flare was 196.46 IU /L (SD=114.69) and non-renal flare was 43.17 IU/L (SD=34.55). During remission this level decreased to 39.77 IU/L (SD=13.76) in renal and 27.96 IU/L (SD=14.50) in non-renal lupus. Mean C3 and C4 levels of inactive renal lupus patients were 46.8mg/dl (SD=20) and 12.13mg/dl (SD=7.54) respectively.
It was observed that 12 out of 13 (92.3%) patients with active renal involvement had low C3 levels and 11 (84.6%) had low C4 levels. However C3 levels remained low in four (30.7%) patients and C4 in one (7.7%) patient during remission. The anti-dsDNA levels were elevated in all patients with predominant renal flare. In five (38.5%) patients, the levels remained above normal even after clinical remission.
In non-renal flares anti-dsDNA titre was raised only in 13 (35%) cases whereas C3 and C4 levels were low in 15 (43%) and 21 (53%) cases respectively. During remission anti-dsDNA remained elevated in three (8%) cases of non-renal lupus flares and C3 and C4 levels remained low in five (13%) and 10 (26.5%) cases respectively.
The Pearson correlation tests showed significant negative correlation between SLEDAI scores and C3 (p < 0.01) and C4 (p < 0.05) for two-tailed prediction among all patients. There was significant positive correlation between SLEDAI and anti-dsDNA values at 0.01 levels (two tailed). On sub group analysis positive correlation between SLEDAI and anti-dsDNA titre was stronger for renal lupus compared to non-renal flares. Significant negative correlation between SLEDAI and complement levels (C3 and C4) was observed in renal flares (Pearson correlation –0.576 and –0.677) whereas in non-renal flares there was no negative correlation (Pearson correlation 0.058 and 0.028). Details of Pearson correlation tests are shown in Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12 and Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5.
Table 4.
Correlation of SLEDAI and anti-dsDNA of all patients (n=51)
| Correlations | SLEDAI | Anti-dsDNA | |
|---|---|---|---|
| SLEDAI | Pearson correlation | 1 | 0.775 |
| Significance (2-tailed) | - | 0.000 | |
| n | 51 | 51 | |
| Anti-dsDNA | Pearson correlation | 0.775 | 1 |
| Significance (2-tailed) | 0.000 | - | |
| n | 51 | 51 |
95% Confidence interval on the population correlation is from 0.64 to 0.87.
Table 5.
Correlation SLEDAI with C3 of all patients
| Correlations | SLEDAI | C3 | |
|---|---|---|---|
| SLEDAI | Pearson correlation | 1 | 0.445 |
| Significance (2-tailed) | - | 0.001 | |
| n | 51 | 51 | |
| C3 | Pearson correlation | 0.445 | 1 |
| Significance (2-tailed) | 0.001 | - | |
| n | 51 | 51 |
95% confidence interval on the population correlation is from-0.28 to 0.28.
Table 6.
Correlation of SLEDAI with C4 of all patients
| Correlations | SLEDAI | C4 | |
|---|---|---|---|
| SLEDAI | Pearson correlation | 1 | 0.355 |
| Significance (2-tailed) | - | 0.011 | |
| n | 51 | 51 | |
| C4 | Pearson correlation | 0.355 | 1 |
| Significance (2-tailed) | 0.011 | - | |
| n | 51 | 51 |
95% confidence interval on the population correlation is from-0.28 to 0.28.
Table 7.
Subgroup analysis: Renal lupus - correlation of SLEDAI with C3
| Correlation | SLEDAI | C3 | |
|---|---|---|---|
| SLEDAI | Pearson correlation | 1 | 0.576 |
| Significance (2-tailed) | - | 0.039 | |
| n | 13 | 13 | |
| C3 | Pearson correlation | 0.576 | 1 |
| Significance. (2-tailed) | 0.039 | - | |
| n | 13 | 13 |
95% confidence interval on the population correlation is from-0.53 to 0.58.
Table 8.
Subgroup analysis: renal lupus - correlation of SLEDAI with C4
| Correlations | SLEDAI | C4 | |
|---|---|---|---|
| SLEDAI | Pearson correlation | 1 | 0.677 |
| Significance (2-tailed) | - | 0.011 | |
| n | 13 | 13 | |
| C4 | Pearson correlation | 0.677 | 1 |
| Significance. (2-tailed) | 0.011 | - | |
| n | 13 | 13 |
95% confidence interval on the population correlation is from-0.55 to 0.56.
Table 9.
Subgroup analysis: Renal lupus - Correlation of SLEDAI with anti-dsDNA
| Correlations | SLEDAI | Anti-Ds DNA | |
|---|---|---|---|
| SLEDAI | Pearson correlation | 1 | 0.515 |
| Significance (2-tailed) | - | 0.072 | |
| n | 13 | 13 | |
| Anti-ds DNA | Pearson correlation | 0.515 | 1 |
| Significance (2-tailed) | 0.072 | - | |
| n | 13 | 13 |
95% confidence interval on the population correlation is from −0.50 to 0.60.
Table 10.
Subgroup analysis: non renal lupus - correlation of SLEDAI with C4
| SLEDAI | C4 | ||
|---|---|---|---|
| SLEDAI | Pearson correlation | 1 | 0.028 |
| Significance (2-tailed) | - | 0.869 | |
| n | 38 | 38 | |
| C4 | Pearson correlation | 0.028 | 1 |
| Significance (2-tailed) | 0.869 | - | |
| n | 38 | 38 |
95% confidence interval on the population correlation is from −0.24 to 0.40.
Table 11.
Subgroup analysis: non renal lupus - correlation of SLEDAI with C3
| SLEDAI | C3 | ||
|---|---|---|---|
| SLEDAI | Pearson correlation | 1 | 0.058 |
| Significance (2-tailed) | - | 0.729 | |
| n | 38 | 38 | |
| C3 | Pearson correlation | 0.058 | 1 |
| Significance (2-tailed) | 0.729 | - | |
| n | 38 | 38 |
95% confidence interval on the population correlation is from 0.26 to 0.38.
Table 12.
Subgroup analysis: non renal lupus - correlation of SLEDAI with Anti-dsDNA
| SLEDAI | Anti-dsDNA | ||
|---|---|---|---|
| SLEDAI | Pearson correlation | 1 | 0.356 |
| Significance (2-tailed) | - | 0.028 | |
| n | 38 | 38 | |
| Anti-dsDNA | Pearson correlation | 0.356 | 1 |
| Significance (2-tailed) | 0.028 | - | |
| n | 38 | 38 |
95% confidence interval on the population correlation is from 0.29 to 0.35.
Fig. 1.
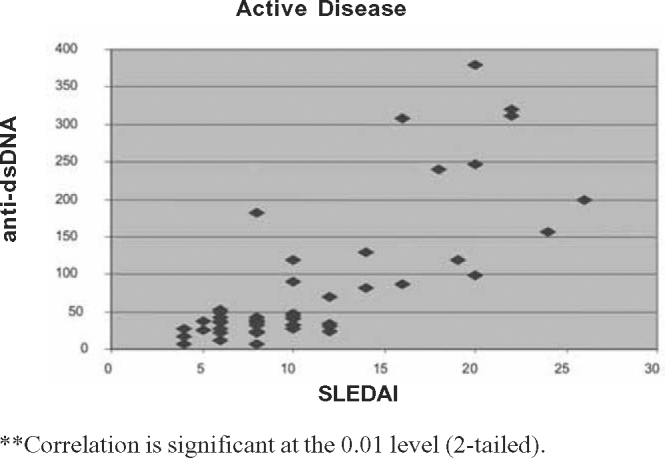
Correlation between SLEDAI and anti ds-DNA in active disease.
Fig. 2.
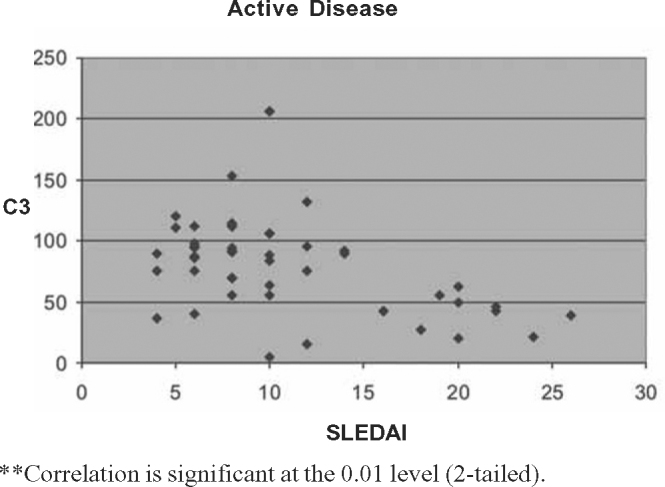
Correlation between SLEDAI and C3 levels in active disease.
Fig. 3.
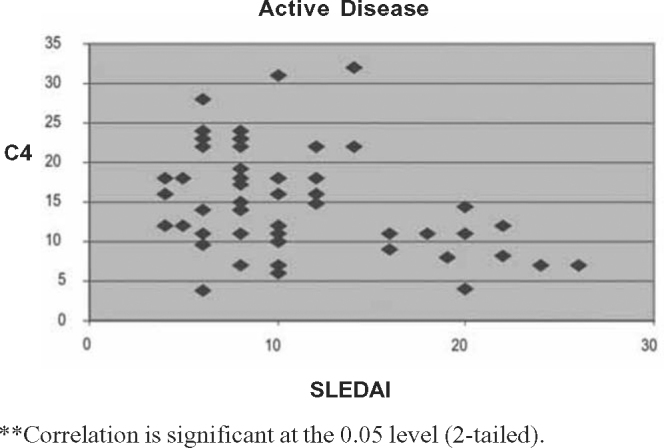
Correlation between SLEDAI and C4 levels in active disease.
Fig. 4.
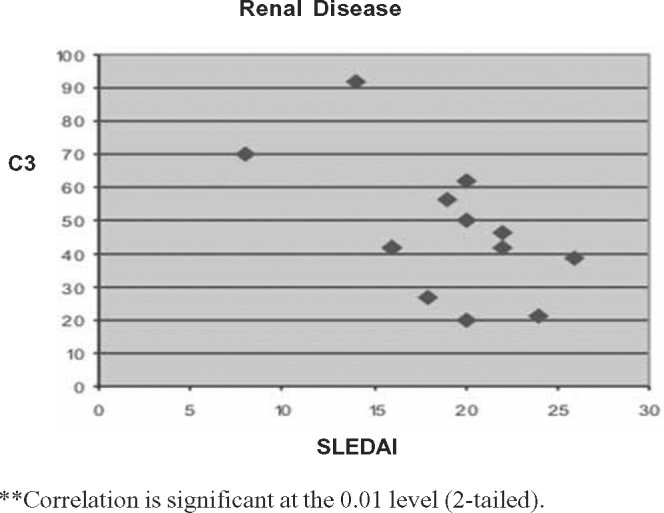
Correlation between SLEDAI and C3 levels in renal disease.
Fig. 5.
Correlation between SLEDAI and C4 levels in renal disease.
Discussion
Determination of serum anti-dsDNA titre and complement levels (C3, C4 and CH 50) are the most common and useful tests available for assessing disease activity and predicting flares in SLE. However both these tests have limitations in that elevated anti-dsDNA antibodies and hypocomplementemia do not occur in all patients and their correlation with disease activity is not absolute. Patients can have persistently elevated anti-dsDNA antibody titers without evidence of clinical disease for several months [6]. Predictive value of various serologic tests in SLE depends on many factors such as criteria used to define and measure disease activity, effect of drug therapy, immunologic methods used to measure serologic parameters and the type of study, whether cross sectional or long term prospective study. Hence comparison of the results of various studies is difficult.
Petri et al [7] in their prospective cohort study of 185 patients with SLE had only 10% flares associated with new appearance of anti-dsDNA antibodies and 17% with an increase in antibody titre. Decrease in C3 and C4 titres were observed in 44% and 41% of the disease flares respectively. Minter et al studied 70 patients longitudinally over three years and only half of the active disease episodes were associated with a high anti-dsDNA level and a low CH 50 level. Most episodes of CNS lupus flares occurred without significant changes in anti-dsDNA antibody titre or CH 50 level whereas active lupus nephritis was associated with these changes. Ter Borg et al [8] showed 89% of all disease flares that occurred in 72 SLE patients were preceded by a rise in anti-dsDNA titer by 8–10 weeks. The anti-dsDNA antibody titer was more sensitive than serum C3 or C4 levels in predicting exacerbations. Swaak et al [9] in their prospective study of 143 SLE patients noticed a progressive rise and a sharp drop in anti-dsDNA titer in all 33 major disease flares. A drop in serum C4 level followed by C3 level occurred 20 to 25 weeks before the onset of lupus nephritis.
There are some investigators who have concluded that fluctuations in the values of anti-dsDNA, C3 and C4 are poor predictors of disease. In these studies the laboratory data were obtained every three months whereas in the prospective studies that found anti-dsDNA to be predictive of disease flares, laboratory values were obtained every 4–6 weeks. Several studies have proposed that qualitative properties of the anti-dsDNA antibodies, such as the complement fixing property, avidity, dissociation constant and immunoglobulin class are more important determinants than the total antibody content in regard to pathogenicity and correlation with disease activity [10, 11]. Linnik et al [12] in a multicenter study of 487 lupus nephritis cases found that changes in the titer of anti-dsDNA measured by a Farr assay correlated with a risk of renal flares and was inversely correlated with serum C3 levels. However, in a prospective study of 53 SLE patients, Ho et al [13] reported that at the time of lupus flares including renal flares, the serum titer of anti-dsDNA often decreased after a previous rise. As per the authors, the decrease may represent deposition of immune complexes during the disease flare.
In our study we have observed that musculoskeletal and mucocutaneous symptoms are the commonest presentation of a lupus flare. It was seen in 90% cases, followed by renal involvement in about 26%. Anti-dsDNA titre was raised in all cases of renal flare. C3 and C4 levels were low in 92% and 85% cases of renal flare respectively. In non-renal flare anti-dsDNA titre was raised only in 35% cases. Low C3 and C4 levels were noticed in 43% and 53% of non-renal flares respectively. There was significant positive correlation noticed with anti-ds DNA levels and SLEDAI in lupus flare at 0.01 levels on two-tailed prediction. The Pearson correlation was stronger in renal lupus compared to non-renal flares. There was significant negative prediction for SLEDAI and C3, C4 levels at 0.01 (two-tailed prediction) for all patient and renal lupus. However analysis of no renal lupus did not show negative correlation of SLEDAI with C3 and C4 levels.
During remission anti-dsDNA remained elevated in 38.5% of renal lupus and 8% of non-renal lupus flares. C3 and C4 levels remained low in 13% and 26.5% of patients respectively without renal involvement during remission. In patients with lupus nephritis C3 and C4 levels remained low in 30% and 7.5% cases respectively.
Monitoring SLEDAI is an important clinical tool for assessment and follow up of disease activity in lupus patients. The most useful laboratory parameters for assessing disease activity are anti-dsDNA and serum complement levels. These parameters should be measured frequently every 4–6 weeks. Serial measurements of these serological markers are useful in predicting lupus flare and during follow up. There is a strong positive correlation between SLEDAI scores and anti-dsDNA levels and a negative correlation with C3 and C4 levels in renal flares. However in non-renal flares negative correlation of C3 and C4 levels with SLEDAI could not be established. This could be explained by the heterogeneous nature of the pathogenesis of the disease.
Conflicts of Interest
This study has been funded by research grants from the O/o DGAFMS.
Intellectual Contribution of Authors
Study Concept: Col K Narayanan Col V Marwaha
Drafting & Manuscript Revision: Col K Narayanan, Col V Marwaha
Statistical Analysis: Gp Capt S Shankar, Col K Shanmuganandan
Study Supervision: Col K Narayanan Col V Marwaha
References
- 1.Bombardier C, Gladman DD, Hurwitz MB, Caron D, Chang CH. The committee on prognosis studies in SLE. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD, Goldsmith CH, Urowitz MB. Sensitivity to change of 3 systemic lupus erythematosus disease activity Indices: Internal validation. J Rheumatol. 1994;21:1468–1471. [PubMed] [Google Scholar]
- 3.Petri M, Buyon J, Skovron ML. Reliability of SELENA SLEDAI and flares as a clinical trial outcome measure. Arthritis Rheum. 1998;41:S218. [Google Scholar]
- 4.The American College of Rheumatology response criteria for SLE clinical trials: measures of overall disease activity. Arthritis Rheum. 2004;50:3418–3426. doi: 10.1002/art.20628. [DOI] [PubMed] [Google Scholar]
- 5.Davis P, Cumming RH, Verri Jones J. Relationship between anti-DNA antibodies, complement consumption and circulating immune complexes in systemic lupus erythematosus. Clini Exp Immunol. 1977;28:226–232. [PMC free article] [PubMed] [Google Scholar]
- 6.Gladman DD, Urowitz MB, Keystone CC. Serologically active clinically quiescent systemic lupus erythematosus. Am J Med. 1979;66:210–215. doi: 10.1016/0002-9343(79)90529-1. [DOI] [PubMed] [Google Scholar]
- 7.Petri M, Genovese M, Engle E, Hochberg M. Definition, incidence and clinical description of flare in systemic lupus erythematosus: A prospective cohort study. Arthritis Rheum. 1991;34:937–944. doi: 10.1002/art.1780340802. [DOI] [PubMed] [Google Scholar]
- 8.Ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long term prospective study. Arthritis Rheum. 1990;33:634–643. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]
- 9.Swaak AJ, Aarden LA, Statius van Eps LW, Feltkamp TE. Anti-ds DNA and complement profiles as prognostic guides in systemic lupus erythematosus. Arthritis Rheum. 1979;22:226–235. doi: 10.1002/art.1780220304. [DOI] [PubMed] [Google Scholar]
- 10.Esdaile JM, Joseph L, Abrahamowicz M. Routine immunologic tests in systemic lupus erythematosus: Is there a need for more studies? J Rheumatol. 1996;23:1891–1896. [PubMed] [Google Scholar]
- 11.Kavanaugh AF, Solomon DH. American College of Rheumatology Ad Hoc Committee on Immunologic testing guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-DNA antibody tests. Arthritis Rheum. 2002;47:546–555. doi: 10.1002/art.10558. [DOI] [PubMed] [Google Scholar]
- 12.Linnik MD, Hu JZ, Heilbrunn KR. Relationship between antidsDNA antibodies and exacerbation of renal disease in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:1129–1137. doi: 10.1002/art.20980. [DOI] [PubMed] [Google Scholar]
- 13.Ho A, Magder LS, Barr SG, Petri M. Decreases in anti-dsDNA levels are associated with concurrent flares in patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:2342–2349. doi: 10.1002/1529-0131(200110)44:10<2342::aid-art397>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]


