Abstract
Background
Gluten sensitive enteropathy or celiac disease (CD) is a disorder of small bowel that occurs upon exposure to gluten. A total of 67 children of either sex in the age group of 1–12 years with unexplained failure to thrive were studied for the prevalence of CD.
Methods
This was a cross-sectional study. It included detailed history, clinical assessment, estimation of anti gliadin (AGA), tissue transglutaminase antibodies (tTGA) and duodenal biopsy. Treatment with gluten free diet and follow-up of diagnosed cases was done for one year.
Result
Sixteen cases (23.88%) had villous atrophy and positive serology, essential criteria for the diagnosis of CD. Forty six (69%) children were between 4–12 years of age. Male to female ratio was 2.3:1. Main symptoms were irritability (63%), diarrhea (56%) and weight loss (56%). Thirty seven (56%) children had weight less than 3rd percentile. tTGA was 100% sensitive and 90.2% specific. Duodenal biopsy showed decreased villious-crypt ratio in 81.25% and intra epithelial lymphocytosis in 81% children (p<0.000001). All the confirmed cases were advised strict gluten free diet for one year. On follow-up at six months, all children showed improvement in their symptoms and weight gain.
Conclusion
CD is an important cause of unexplained failure to thrive in children.
Key Words: Failure to thrive, Celiac disease
Introduction
Gluten sensitive enteropathy, also known as celiac disease (CD) is a disorder of small bowel, characterized by mucosal inflammation and villous atrophy. It occurs upon exposure to gluten and clinical and histological improvement is seen with withdrawal of gluten from the diet [1, 2]. Both T and B-cells play an important role [3]. Genetic susceptibility is suggested by a high concordance among monozygotic twins. Recent evidence suggests that an auto-antibody to transglutaminase (tTG), a connective tissue element surrounding smooth muscle called endomysium, is highly specific for CD [3, 4]. True prevalence of CD is difficult to estimate because of the inconsistent presentation of the disease [5]. Main clinical features are diarrhea (84%), failure to thrive (91%) and anaemia (84%). Diverse serologic markers with high sensitivity and specificity are now available for the diagnosis of CD with varying sensitivity and specificity (70 to 99%). Essential diagnostic criteria for the diagnosis of CD are villous atrophy, positive serology and improvement after gluten free diet [5].
Present study is an attempt to detect CD in a population of children presenting as unexplained failure to thrive. Failure to thrive is diagnosed in an infant or child whose physical growth is significantly less than that of his or her peers (usually refers to growth below the 3rd or 5th percentiles or a fall in growth from above the 75th percentile to below the 25th percentile in a short time) [6]. Objectives of the study are to study the prevalence of gluten sensitive enteropathy in children with unexplained failure to thrive by screening tests and histopathology and to determine clinical improvement after dietary modification.
Material and Methods
This was a prospective study done over two years time. The study population constituted children of either sex with unexplained failure to thrive either admitted in the paediatric ward or attending the paediatric out patient department (OPD) at a tertiary care centre. Inclusion criteria was children aged 1–12 years of either sex diagnosed as unexplained failure to thrive [6]. Exclusion criteria were at risk population (family members with celiac disease) and children with chronic infections and chronic systemic diseases.
Detailed history including dietary, family development and immunization history was taken. History was also taken to rule out chronic infections, chronic systemic disorders and stress factors at home or in the school, abnormal behaviour and disturbed sleep, chronic illness in the family, separation or death and child abuse for possible non organic cause of failure to thrive.
Nutritional history included duration of exclusive breast feeding, timing of introduction of wheat products in diet and relationship between introduction of wheat products in diet and appearance of symptoms. Detailed anthropometric examination including weight, height, head circumference and mid-arm circumference were recorded to assess grade of malnutrition. Detailed systemic examination was done to exclude chronic systemic diseases. Laboratory Investigations included complete blood counts, liver function tests, renal function tests, serum electrolytes, arterial blood gas, iron studies, routine examination of urine, three stool- samples for ova and cysts, stool for pH, fat and reducing substances, Mantoux (Mx) test, enzyme linked immuno sorbent assay (ELISA) for human immunodeficiency virus (HIV) in the mother and child. Imaging studies included radiograph chest for pulmonary tuberculosis and ultrasonography (USG) abdomen for organomegaly, free fluid and lymphadenoapthy. Serological investigations included anti gliadin (AGA) and anti tissue transglutaminase IgA (tTGA) antibodies. Endoscopic duodenal biopsy with the help of gastroenterology physician was done in all cases under sedation and after obtaining written consent from the parents and sent for histopathological examination. Biopsy confirmed CD cases were advised strict gluten free diet. Children with anaemia were given iron and folic acid for three months. Parents were given food charts indicating foods without gluten and calories content. Follow-up was done once in three months for one year. It included improvement/deterioration in symptoms, possible complications, anthropometry and relevant laboratory examination. No biopsy was done on follow-up due to lack of parental consent in most cases. Detailed statistical evaluation was done by using statistical package for social sciences (SPSS) software (version16) and Microsoft office Excel 2003.
Results
In our study, 38.8% of cases were in the age group of 4 - 7 years, 31.4% were between 1–3 years, 29.8% between 8–12 years with male predominance. Male: female ratio was 2.3: 1. Youngest child was 1.5 years. Sixteen cases (23.88%) had villous atrophy, out of whom 56.25% had weight less than 3rd percentile (Table 1). Of these, 65% children were short in stature in CD group and 67% in no CD group children.
Table 1.
Distribution of cases according to the degree of failure to thrive
| Weight less than 3rd percentile n (%) | Weight between 3rd and 5th percentile n (%) | |
|---|---|---|
| n = 67 | 43 (64.17) | 24 (35.82) |
| Villous atrophy n= 16 | 9 (56.25) | 7 (43.75) |
| No villous atrophy n=51 | 27 (52.94) | 24 (47.05) |
Common symptoms observed were weight loss (77.61%), irritability (55.22%) and anorexia (53.73%) in study group. In children with CD, main symptoms were irritability (63 %), weight loss (56%) and diarrhea (56%) (Table 2).
Table 2.
Clinical Profile of children in study group
| Variables | Weight loss | Diarrhea | Abdominal pain | Vomiting | Anorexia | Feverish feeling | Irritability |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Total cases n= 67 | 52 (77.61) | 30 (44.77) | 27 (40.29) | 12 (17.19) | 36 (53.73) | 21 (31.34) | 37 (55.22) |
| Villous atrophy group n= 16 | 9 (56.25) | 9 (56.25) | 4 (25.00) | 4 (25.00) | 6 (37.50) | 5 (31.25) | 10 (62.50) |
| No villous atrophy group n= 51 | 43 (84.31) | 21 (41.17) | 23 (45.09) | 8 (15.68) | 30 (58.82) | 16 (31.33) | 27 (52.98) |
| p | 0.12 | 0.29 | 0.25 | 0.46 | 0.11 | 0.76 | 0.71 |
Anaemia was observed in 51% of study population (Table 3). In the villous atrophy group 63% had anaemia. Mean haemoglobin in children with villous atrophy was 10.67 g/100ml and it was 10.68 g/100ml in children with no villous atrophy (p 0.107). Difference in mean total leucocyte count (TLC) and serum albumin in both groups was also not significant.
Fig. 1.
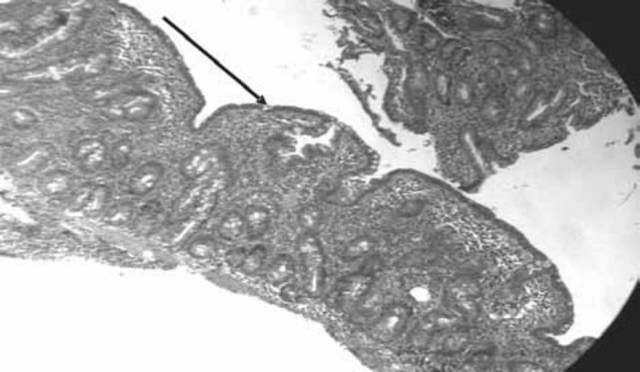
Duodenal biopsy under light microscope showing blunting of villi
Table 3.
Laboratory parameters of children in study group
| Variables | Hemoglobin (g/dl) | Total leucocyte count (per cmm) | Serum Albumin (g/dl) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | SD | Range | Mean | SD | Range | Mean | SD | |
| Villous atrophy (Group 1) n=16 | 5.6-12.9 | 10.67 | +1.54 | 6600-25900 | 9890.9 | +3018.69 | 1.2-4.3 | 3.88 | +0.44 |
| No villous atrophy (Group 2) n=51 | 7.3-13.9 | 10.68 | +1.54 | 5800-19300 | 9865.15 | +3022.4 | 3.4-4.6 | 3.87 | +0.43 |
| p value | 0.107 (not significant) | 0.142 (not significant) | 0.188 (not significant) | ||||||
In serology, anti tTGA was positive in 16 (100%) cases in villous atrophy group and four (7.84%) in no villous atrophy group. AGA was positive in 12 (75%) cases in villous atrophy group and six (11.76%) cases with no villous atrophy. Sensitivity of tTGA was 100% and specificity 90.2%. Sensitivity of AGA was 75% and specificity 88.2% in detecting CD.
Villous-crypt ratio was decreased in children with villous atrophy group in 81.25% while it was 1.96% in no villous atrophy group (p < 0.0000001). Intra epithelial lymphocytosis was seen in 81% children with villous atrophy group and 3.92% in no villous atrophy group (p <0.000001). Lymphomononuclear infiltration was observed in 87.5% children with villous atrophy group and 9.8% in no villous atrophy group (p <0.000001).
Out of 16 confirmed cases of CD, five (31.25%) did not come for follow-up. This was because of the posting out of the parents. Four (25%) cases did not improve. The reason for not improving was poor dietary compliance. Remaining seven cases were followed up for one year. In all these cases irritability, pain abdomen and feverish feeling disappeared after six months of dietary restriction. Three cases had diarrhea that stopped after two months. All seven cases had anorexia which improved in two months. Weight gain was observed in all and it ranged between 2 to 4 kg in one year. Children with anaemia were given oral iron with folic acid in the dose of 6mg/kg for three months. At the end of one year all had haemoglobin between 11.5g to 12.5 g/100ml.
Discussion
Early and correct diagnosis of CD is critical. Early diagnosis is essential to avoid complications like local malignancies, other autoimmune disorders, strictures and ulceration. Correct diagnosis is important because of strict life long adherence to gluten free diet. Risk factors for CD are rota and adeno virus infection and early introduction of grains containing prolamins. Breast feeding has protective role. Children presenting with atypical form of CD are usually missed. Strong suspicion and screening is essential. Incidence of celiac disease has varied from 1.1 to 50%, depending upon selection criteria [7]. In our study 24% children who presented with unexplained failure to thrive were diagnosed as CD. In India, presentation of CD is comparatively late; mean age of presentation being 6.7 years [5]. In our study 69% presented after four years of age. In the western studies there is no sex preponderance [8]. Present study showed significant male predominance. This could be due to better care and attention given to males in our country who are brought earlier to the hospital. Late detection is due to poor suspicion, atypical presentations and non availability of diagnostic tools.
Common symptoms observed were irritability (63%), weight loss and diarrhea (56%). Poddar et al [5] and Pooni et al [8] found diarrhea (84%), failure to thrive (91%), anaemia (84%) and stunting (60%) in north Indian children. Atypical cases generally present as iron deficiency anaemia, short stature and osteopenia [2]. In our study children with CD had anaemia in 63% and short stature in 65%. Anaemia was due to iron deficiency in 91% and dimorphic in remaining. The causes of anaemia in these children are iron deficiency and in some cases folic acid and B12 deficiency. CD disease can also be associated with growth hormone deficiency and that may be one of the causes of short stature [9, 10]. In our study growth hormone estimation was not done.
Prolamins in the food (wheat, barley and rye) are responsible for immune reaction in CD in genetically susceptible children. Gliadin in wheat is the best-understood member of this family. On exposure to these prolamins, the enzyme tissue transglutaminase modifies the protein and the immune system cross reacts with the small bowel tissue, causing an inflammatory reaction and villous atrophy. Detection of antibodies against these proteins is useful for screening. Serological markers available have high sensitivity and specificity [11, 12, 13]. Infections and cow's milk allergy can give false positive result with AGA. Variability and low accuracy associated with AGA tests make them unsuitable for diagnostic or screening purpose. Discovery of ELISA based assays that detect anti tTGA is a major breakthrough. The sensitivity and specificity of anti tTGA is more than 99% and 90% respectively. While the performance of the anti-epithelial membrane agent (EMA) is superior to anti tTGA, interpretation of immunofluorescence assay for anti-EMA is operator dependant and liable to error. The disadvantage of anti tTGA and anti-EMA is that these are unreliable before two years of age and will be false negative in patients with selective IgA deficiency. In our study, sensitivity of anti tTG was 100% and specificity 90.2% for detection of CD while sensitivity of AGA was 75% and specificity 88.2%.
Intestinal biopsy is essential for the diagnosis of CD. Endoscopic findings associated with a high specificity for CD include scalloping of the small bowel folds, paucity in the folds, mosaic pattern, prominence of the sub mucosal blood vessels and a nodular pattern of the mucosa [7, 11]. Sensitivity of detection of CD with standard endoscope is 100% but the specificity is only 61% [12]. Histological examination further demonstrates a cellular infiltrate in lamina propria and blunting of villi. In the present study, 24% children had villous atrophy with decreased villous-crypt ratio in 81.25% and intra epithelial lymphocytic infiltration in 81% of them. These changes were statistically highly significant when compared with biopsies of children with no CD (p<0.000001). Troncone observed villous atrophy in 16% children suspected to be having CD clinically [19]. Intra epithelial lymphocytosis was observed in 38–100% by others [14, 15, 16, 17, 18]. Villous atrophy is not specific for CD. It can also be seen in other conditions like infections, infestations, tropical sprue etc. There is strong genetic association of CD (90–95%) with HLA-DQ2 (sensitivity 94%, specificity 73%) and DQ8 (sensitivity 12% and specificity 81%). While HLA typing is not recommended for routine diagnosis as it is cumbersome and costly, it can be used for ruling out CD where the diagnosis is equivocal. Screening is advised in high risk cases (Diabetes type-1, first degree relative of CD case, Down's syndrome), unexplained anaemia, unexplained failure to thrive, autoimmune thyroid diseases and unexplained neurological disorders. Gluten challenge is indicated when there is doubt about the diagnosis.
Treatment of CD is life long gluten free diet. This diet is cumbersome. Some children while on modified diet develop mouth ulcers, osteoporosis and fractures [19, 20]. In the present study, 25% did not improve, probably because of poor compliance. Parents in these cases did not agree for repeat biopsy or serology. Remaining children showed 100% improvement in their symptoms of pain abdomen, anxiety, diarrhea and anaemia. None developed any complication. Steroids or other immunosuppressants can be considered in children who do not improve on gluten-free diet [15]. Poor dietary compliance is the main cause for poor response. Genetically engineered wheat species free of gluten may be available in future where compliance will not be a problem [21]. Larger studies with better follow-up are required in our country so that recommendations on the management of these children can be made.
Conflicts of Interest
None identified
Intellectual Contribution of Authors
Study Concept: Col KS Rana, Col P Puri, Lt Col S Badwal
Drafting & Manuscript Revision: Col KS Rana, Col P Puri
Statistical Analysis: Col KS Rana
Study Supervision: Col KS Rana
References
- 1.Marsh MN. Gluten, major histocompatibility complex and the small intestine: A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 2.Walker JA, Guandalini S, Schmitz J, Shmerling DH, Visakorpi JK. Revised criteria for the diagnosis of celiac disease. Arch Dis Child. 1990;65:909–911. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van WY, Kooy Y, Van VP, Vader W, Koning F, Pena S. Celiac disease: it takes three to tango. Gut. 2000;46:734–737. doi: 10.1136/gut.46.5.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sollid LM, McAdam SN, Molberg O, Quarsten H, Arentz-Hansen H, Louka AS. Genes and environment in celiac disease. Acta Odontologica Scandinavica. 2001;59:183–186. doi: 10.1080/000163501750266792. [DOI] [PubMed] [Google Scholar]
- 5.Poddar U, Thapa BR, Singh K. Clinical features of CD in Indian children: Are they different from the West? J Paediatr Gastroenterol Nutr. 2006;43:313–317. doi: 10.1097/01.mpg.0000231589.32114.9d. [DOI] [PubMed] [Google Scholar]
- 6.Howard B. Failure to thrive. In: Behrman RC, Kliegman Robert M, Jenson HB, editors. Nelson text book of Pediatrics. 17th edition. WB Saunders; Philadelphia: 2004. pp. 133–134. [Google Scholar]
- 7.Hoffenberg EJ, MacKenzie T, Barriga KJ, Eisenbarth GS, Bao F, Haas JE. A prospective study of the incidence of childhood celiac disease. J Paediatr. 2003;143:308–314. doi: 10.1067/s0022-3476(03)00282-8. [DOI] [PubMed] [Google Scholar]
- 8.Pooni PA, Chinna RS, Jaina BK, Singh D, Gautam A. Clinical and anthropometric profile of celiac disease in Punjab. Journal of Tropical Paediatrics. 2006;52:30–33. doi: 10.1093/tropej/fmi054. [DOI] [PubMed] [Google Scholar]
- 9.Halfdonarson TR, Litzow MR, Murry JA. Hematological manifestations of celiac disease. Blood. 2007;109:412–421. doi: 10.1182/blood-2006-07-031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giovenale D, Cristina M, Cardinale G, Spastito M, Mastreglilo C, Messini B. The prevalence of growth hormone deficiency and celiac disease in short children. Clinical Medicine and Research. 2006;4:180–183. doi: 10.3121/cmr.4.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catassi C, Fabiani E, Corrao G, Barbato M, De Renzo A, Carella AM. Risk of non-Hodgkin lymphoma in celiac disease. JAMA. 2002;287:1413–1419. doi: 10.1001/jama.287.11.1413. [DOI] [PubMed] [Google Scholar]
- 12.Murrey JA, Tapia AR, Vanyke CT, Brogan DL, Brianlahr K, Rumalla A. Mucosal atrophy in CD: Extent of involvement, correlation with clinical presentation and response to treatment. Clin Gastroentrol Hepatol. 2008;6:186–225. doi: 10.1016/j.cgh.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatnagar S, Tandon N. Diagnosis of Celiac disease. IJP. 2006;73:703–710. doi: 10.1007/BF02898449. [DOI] [PubMed] [Google Scholar]
- 14.Van HD, West J. Recent advances in celiac disease. Gut. 2006;55:1037–1046. doi: 10.1136/gut.2005.075119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Layrisse Z, Guedez Y, Domínguez E, Paz N, Matos M, Herrera F. Extended HLA haplotypes in a Carib Amerindian population: The Yucpa of the Perija Range. Hum Immunol. 2001;62:992–1000. doi: 10.1016/s0198-8859(01)00297-x. [DOI] [PubMed] [Google Scholar]
- 16.Kotze LMS, Utiyama SRR, Nisihara RM, De CV, Ioshii SO. IgA class anti-endomysial and anti-tissue transglutaminase antibodies in relation to duodenal mucosa changes in celiac disease. Pathology. 2003;35:56–60. [PubMed] [Google Scholar]
- 17.Wahab PJ, Meijer Jos WR, Dumitra D, Goerres MS, Mulder Chris JJ. Celiac disease: More than villous atrophy. Romanian Journal of Gastroenterology. 2002;11:121–127. [PubMed] [Google Scholar]
- 18.Mahadeva S, Wyatt JI, Howdle PD. Is a raised intraepithelial lymphocyte count with normal duodenal villous architecture clinically relevant? Journal of Clinical Pathology. 2002;55:424–428. doi: 10.1136/jcp.55.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troncone R, Catassi C, Lambertini A, Zaniboni MG, Lazzari R, Bottaro G. Latent celiac disease in Italy. Acta Paediatr Int J Paediatr. 1995;84:1252–1257. doi: 10.1111/j.1651-2227.1995.tb13543.x. [DOI] [PubMed] [Google Scholar]
- 20.Häuser W, Gold J, Stein J, Caspary W, Stallmach A. Healthrelated quality of life in adult celiac disease in Germany: Results of a national survey. Eur J Gastroenterol Hepatol. 2006;7:747–754. doi: 10.1097/01.meg.0000221855.19201.e8. [DOI] [PubMed] [Google Scholar]
- 21.Selby WS, Painter D, Collins A, Faulkner-Hogg KB, Loblay RH. Persistent mucosal abnormalities in celiac disease are not related to the ingestion of trace amounts of gluten. Scand J Gastroenterol. 1999;34:909–914. doi: 10.1080/003655299750025390. [DOI] [PubMed] [Google Scholar]


