Abstract
Meconium aspiration syndrome (MAS) is respiratory distress in a newborn baby caused by the presence of meconium in the tracheobronchial airways. The aspiration of meconium stained amniotic fluid by the fetus can happen during antepartum or intrapartum periods and can result in airway obstruction, interference with alveolar gas exchange, chemical pneumonitis as well as surfactant dysfunction. These pulmonary effects cause gross ventilation-perfusion mismatching. To complicate matters further, many infants with MAS have primary or secondary persistent pulmonary hypertension of the newborn as a result of chronic in utero stress and thickening of the pulmonary vessels. Although meconium is sterile, its presence in the air passages can predispose the infant to pulmonary infection. MAS is essentially a clinical diagnosis and should always be suspected in a child with respiratory distress and meconium-stained amniotic fluid at delivery. Though a known entity for a long time, its management still remains contentious. Intubation and direct tracheal suction is performed when meconium is observed in the amniotic fluid and the infant is not vigorous. Subsequent management involves ventilation, surfactant instillation and lavage, inhaled nitric oxide and high frequency ventilation. The role of steroids continues to be controversial.
Key Words: Meconium aspiration, Amnioinfusion, Surfactant, Ventilation, Tracheal suction
Introduction
Since when first described by the ancient Greeks to the present day, meconium and its effect on the newborn infant, has remained an enigma. The term was coined by Aristotle from the Greek word “meconium arion” meaning “opium like” as he believed that the substance induced foetal sleep [1]. In spite of rapid advances in investigative and management modalities, meconium and its effects on the foetus and neonate have over decades remained a cause of worry. To date debates continue to rage regarding the optimum obstetrical approach, resuscitation measures at birth and subsequent management of the critically ill neonate with meconium aspiration syndrome (MAS).
Definition
The most popularly used definition of MAS is respiratory distress occurring soon after birth in an infant born from a meconium stained milieu with compatible radiological findings which cannot be otherwise explained.
Incidence
Despite changing strategies, meconium staining of the amniotic fluid (MSAF) happens in approximately 10-15% of childbirths with incidences ranging from 5-25% [2]. MAS develops in approximately 4-10% of the infants born from a MSAF milieu. Of late however, there are encouraging trends of a progressive decline in the incidence of MAS from Australia and New Zealand [3]. Of these neonates who develop MAS, one third require ventilatory support, 10% develop air leaks and in spite of appropriate management strategies, 5-10% of them have a fatal outcome. Of the babies who suffer persistent pulmonary hypertension of the newborn (PPHN), 5-6 % are related to MAS [2].
Pathophysiology
Meconium which comprises of gastrointestinal, hepatic and pancreatic secretions, cellular debris, swallowed amniotic fluid, lanugo, vernix caseosa and blood begins to appear in the foetal intestines by the 10th week of life gradually increasing in amount to reach 200 gms at birth. However due to lack of strong peristalsis, good anal sphincter tone, low levels of motilin and a cap of viscous meconium in the rectum, in utero passage is uncommon till term. In utero hypoxia and acidosis lead to a vagal response with resultant increased peristalsis and a relaxed anal sphincter leading to meconium passage. Integrity of the parasympathetic system therefore appears to be pre requisite for meconium passage making it a maturational event and rare before term.
It has been seen in animal experiments that when hypoxia occurs, deep intrauterine gasping ensues placing the foetus in a MSAF milieu at risk for aspiration [4].
Autopsies have revealed meconium in the terminal airways of stillborn foetuses. However the relationship between intrauterine passage of meconium, development of foetal distress and the pathophysiology of foetal asphyxia has not been determined precisely. Although the passage of meconium may be a physiologic event related to increasing gastrointestinal maturity and increased motilin levels, it also occurs as a result of acidosis and hypoxaemia both acute as well as chronic, its aspiration being more likely in the depressed foetus and in the one which is post-term.
The clinical features of MAS appear to be related to the viscosity of meconium; thick meconium being more associated with complications. This thick particulate meconium can either block the airways completely leading to atelectasis and ventilation perfusion mismatch or partially resulting in ball valve air trapping (Table 1). These obstructive properties lead to the classical radiological picture of areas of atelectasis and consolidation interspersed with hyperexpanded zones along with air leaks (Fig. 1, Fig. 2). Meconium in the airways initiates an inflammatory reaction which was first described by Clark et al [5] who showed the effect of meconium on neutrophil function by inhibiting oxidative burst and phagocytosis.
Table 1.
Pathophysiology of meconium aspiration syndrome
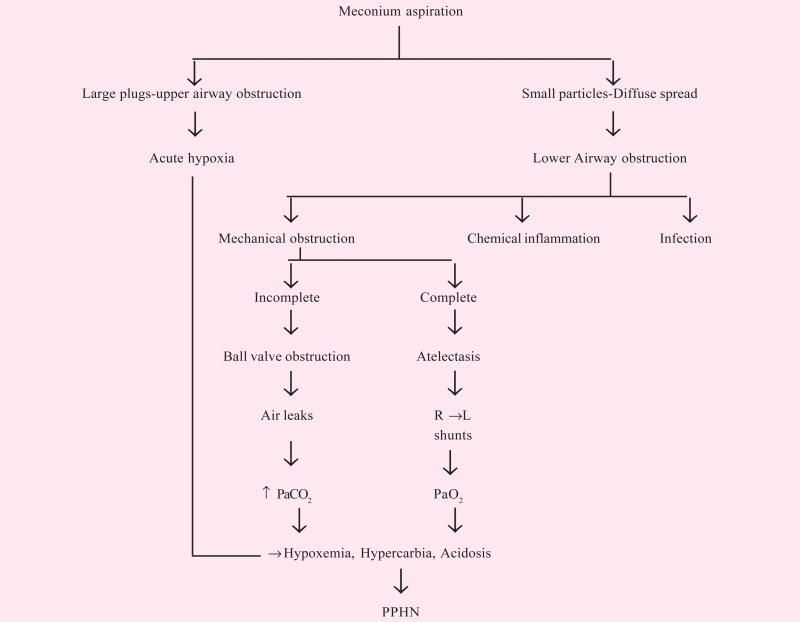 |
Fig. 1.

Chest X-Ray showing zones of hyper-inflation and atelectasis.
Fig. 2.

Chest X-ray showing hyperinflation and Pneumothorax (Left).
Others have found that meconium induces lung injury by activating alveolar macrophages and generates increased production of superoxide anions in these cells. Increased levels of inflammatory cytokines have been found in broncheoalveolar lavage (BAL) in meconium aspirated infants suggesting that meconium induces production of inflammatory cytokines and such an induction can occur in utero [6]. The contained bile salts have been implicated in displacing surfactant as well as damaging type 2 pneumocytes with resultant surfactant deficiency.
All of these mechanisms lead to hypoxemia, acidosis and hypercapnea which result in pulmonary vasoconstriction. Pulmonary hypertension ensues which in turn aggravates the hypoxemia and acidosis creating a vicious cycle.
The meconium from the airways gets absorbed and is excreted in urine which may give urine a turbid green colour in extreme cases (Fig. 3). Meconium aspiration can occur both by in utero gasping as well as postpartum aspiration with the initial breaths of the baby, it being difficult to distinguish which mechanism was responsible in a given case. However, the babies who follow a more severe clinical course are likely to have aspirated in utero, are depressed at birth and develop respiratory distress early.
Fig. 3.

Meconium stained urine in meconium aspiration syndrome.
Obstetrical management
Foetal Monitoring: The assessment of foetal condition is a major priority for all birth attendants. However this assessment is complicated by the high false positive rate of the indicators. Several workers have studied the relationship between cardiotocogram (CTG) abnormality, MSAF, umbilical cord pH and apgar scores. It has been observed that CTG while picking up foetal jeopardy does provide a high false positivity [7]. The presence of thick meconium, late decelerations, tachycardia on CTG, cord pH <7.16, apgar scores of <6 at 1 and 5 minutes and meconium in the trachea correctly predicted 50% of the infants of MAS. Absence of these symptoms correctly predicted 97% of the infants who were healthy. It has been observed that normal CTG records combined with adequate amniotic fluid volume have a high predictive accuracy of foetal well being, in spite of heavy meconium contamination [8]. It has also been observed that compared with healthy neonates with MSAF, those with MAS had higher rate of non reassuring foetal heart record tracing, thick meconium, apgar scores of < 5 at five minutes and lower birth weights. Thus it appears that CTG abnormality and MSAF are insufficiently correlated with apgar scores or cord pH and have more value as predictors of foetal well being rather than jeopardy. However additional measures such as foetal ECG, head compression force analysis, CPK-BB and maternal-foetal temperature difference may improve our predictive ability [9].
Amnioinfusion: Initial reports indicated that amnioinfusion by thinning the MSAF would reduce the incidence and severity of subsequent MAS [10]. However later studies reported that this procedure was not accompanied by any statistically significant reduction in adverse foetal outcomes. Moreover this procedure had fallen into disrepute for its increased association with foetal heart rate abnormalities, operative/instrument deliveries and infection. The current consensus is that in clinical settings with standard perinatal surveillance, evidence does not support the use of amnioinfusion for MSAF. However, when there is limited perinatal surveillance, where complications of MSAF are common, it appears to reduce the incidence of MAS [11, 12].
Neonatal Management
Although meconium staining and the MAS are common neonatal problems, the appropriate management in the delivery room and subsequently remains controversial.
Airway Clearing: There was in the past a universal agreement that suctioning of the mouth, nose and posterior pharynx before the delivery of the thorax should be performed in all cases with MSAF regardless of the meconium consistency. However the current evidence suggests that intrapartum suctioning of the oro/nasopharynx may not reduce the risk of aspiration. Therefore the present reccomendations no longer advise routine intrapartum upper airway suctioning in infants born from a milieu of meconium. Subsequent tracheal toileting which was earlier advocated has been challenged on the precincts that it is only the depressed neonate who runs the risk of MAS. Therefore the vigorous infant at low risk should not be subjected to this potentially risky procedure. It is likely that MAS will develop in a small minority of apparently healthy meconium stained infants, but there is no way of identifying these neonates at risk during childbirth. The 2005 Joint Committee of the American Academy of Paediatrics and American Heart Association delineated neonatal resuscitation guidelines recommend that tracheal toileting be performed on meconium stained newborns soon after delivery if the neonate is depressed [13]. The features of foetal depression delineated are absent/depressed respiration, heart rate < 100/minute and hypotonia. It is further recommended that the initial suctioning should not exceed five seconds. If no meconium is retrieved, repetitive suctioning is not required. However if meconium is retrieved and no bradycardia is present, it is recommended to reintubate and perform suction under oxygen cover. In case of bradycardia, positive pressure ventilation is to be administered and airway toileting considered later. Because moderate amounts of meconium may remain in the stomach and be aspirated later, it is advisable to perform a gastric lavage after the baby has stabilized. Saline lavage and chest physiotherapy performed with due caution in the stabilized baby may assist the removal of tenacious secretions [14].
Ventilatory Support: One third of the infants with MAS require ventilatory support. Because air leaks are a major problem in this condition, high concentrations of oxygen are necessary initially. Continuous Positive Airway Pressure (CPAP) / Bubble CPAP could be beneficial if air trapping is not a major problem. If CPAP does not suffice, mechanical ventilation using low inspiratory pressures, short inspiratory and long expiratory times and rapid rates have been advocated to maintain blood gases within normal limits. Elevation of positive end expiratory pressure (PEEP) while improving oxygenation may worsen air trapping and the risk of pneumothorax. Hence low PEEP appears to be a more beneficial option. This strategy while preventing air leaks also limits pulmonary hypertension, a major problem with MAS. Sedation and neuromuscular blockade aid mechanical ventilation.
High Frequency Ventilation (HFV) by providing effective gas exchange at low tidal volumes has been found advantageous in treating MAS. Its benefits include less barotraumas, increased mobilization of airway secretions, quicker attainment of respiratory alkalosis and fewer histopathological changes. In a large retrospective study, it was observed that HFV was being increasingly utilized in treating babies with MAS (12.2% in 1996 to 25.2% in 2003). It was also observed that infants on HFV spent longer on respiratory support and there was an increased mortality rate albeit a declining one, as compared to infants who were provided other ventilation modalities. This increased morbidity and mortality was perhaps because HFV was being applied to infants at the greatest risk of serious adverse outcomes, more as a rescue therapy [15].
Liquid Ventilation with perfluorocarbons has been found to be beneficial in lamb models of MAS with improved survival, gas exchange and haemodynamic stability. Quality scientific proof of human trials is eagerly awaited.
Surfactant Therapy: Surfactant deficiency in MAS is a consequence of altered function rather than a deficiency state. Meconium displaces surfactant from the alveolar surface and inhibits its surface tension lowering function. In high concentrations, it has a direct cytotoxic effect on the type 2 pneumocytes. Human trials have shown varying results from marked improvement to a marginal one in oxygenation when used in this condition. It has also been observed that surfactant therapy in MAS restored the distended terminal airspaces of the lungs and kept the spaces from irregular overdistension [16]. Surfactant replacement by bolus or slow infusion in infants with severe meconium aspiration syndrome improved oxygenation and reduced the severity of respiratory failure, air leaks and need for extracorporeal membrane oxygenation (RR: 0.64; 95% CI: 0.46–0.91; NNT:6). Doses from 100-200mg/kg of phospholipid have been used in various studies with repeat dosages being provided 6-8 hourly till oxygenation improves. However no clear consensus on the optimal dosage or the number of doses to be provided exists as yet [17, 18]. Although there was no increase in acute morbidity in these infants, transient oxygen desaturation and endotracheal tube obstruction occurred during bolus administration in nearly one third of the surfactant treated infants. A review of randomised control trials (RCT's) evaluating its effects in infants with MAS suggested that surfactant administration may reduce the severity of respiratory illness and decrease the number of infants with progressive respiratory failure requiring support with Extra corporeal membrane oxygenation (ECMO). The relative efficacy of surfactant therapy including KL-4 surfactant compared to, or in conjunction with, other approaches to treatment including nitric oxide, liquid ventilation, surfactant lavage and high frequency ventilation remains to be tested [19, 20]. There are reports which suggest that surfactant when combined with an adjuvant-PEG/dextran is more efficacious.
Broncho Alveolar Lavage (BAL): The efficacy of lung lavage by bronchoscopy in removing large quantities of meconium and improving lung functions is increasingly being documented. Surfactant lavage for meconium aspiration was evaluated in a small, randomized trial. Trends toward lower duration of ventilation and severity of illness were reported [17]. The recent reports suggest that surfactant is more effective than saline as a lavage fluid [21, 22]. The use of surfactant/dextran mixture has been reported to aid the meconium clearance ability of surfactant [23]. There are reports of perfluorocarbon lavage followed by partial liquid ventilation being a more efficacious method as compared to surfactant lavage alone [24].
Inhaled Nitric Oxide (INO) is currently considered the most effective therapy in the management of PPHN which often accompanies MAS. The recommended dosage of INO is 20 parts per million (PPM). Effective use of INO requires adequate lung expansion to optimize its delivery within the lungs. Hence effective ventilation is required to achieve the full benefits of INO when there is significant parenchymal disease of the lungs as occurs in MAS [25].
Steroid Therapy: Meconium in the airway evokes an inflammatory response characterized by the presence of elevated cell counts and pro inflammatory cytokines viz. interleukin (IL-1B), IL-6, tumour necrosis factor (TNF-α). [26]. Reduction in the levels of these cytokines has been found to correlate with improved lung function [27]. Steroids provided by both the intravenous as well as inhaled routes have been observed to suppress this inflammatory response and thus improve pulmonary functions in babies with MAS [28]. Given its easy availability and inexpensive nature, this form of therapy holds promise in its application in the neonatal intensive care unit (NICUs) of the developing nations.
Extra corporeal membrane oxygenation (ECMO): In the 1990's, substantial work has been done assessing the usefulness of ECMO in neonates with MAS wherein it has been proved effective in reducing both death and severe disability in neonates. Further studies have indicated that MAS patients had a significantly lower number of complications vs no MAS patients on ECMO. These data support the consideration of relaxed ECMO entry criteria for MAS [29].
Antibiotics: Meconium is almost always sterile. Yet several workers routinely administer antibiotics to the babies with MAS, the rationale being:-
-
(a)
Meconium produces a chemical pneumonitis with segmental atelectasis mimicking bacterial pneumonitis.
-
(b)
There is the possibility that infection may be the stimulation for in utero meconium passage.
-
(c)
In vitro enhancement of bacterial growth by meconium suggests the increased risk of superimposed bacterial infection in MAS.
However the consensus opinion does not favour the routine use of antibiotics in babies with MAS [30].
Supportive Care: It is necessary to maintain an optimal thermal environment and minimal handling because these infants are agitated easily and become hypoxemic and acidotic quickly. Careful attention should be paid to systemic blood pressure and blood volume. Volume expansion, transfusion therapy and systemic vasopressors are critical in maintaining systemic blood pressure greater than pulmonary blood pressure, thereby decreasing the right to left shunt through the patent ductus arteriosus.
Newer Therapies: INO as a pulmonary vascular relaxing agent has been used to treat PPHN, a common accompaniment of MAS. Studies suggest that though mortality statistics did not alter significantly, sustained improvement in oxygenation with nitric oxide and better oxygenation at initiation with ECMO may have important clinical benefits. It has been speculated that adopting specific lung expansion strategies with nitric oxide may lead to reduced use of the more invasive ECMO. Novel pharmacologic interventions like pentoxiphylline by antiinflamatory property of preventing meconium induced polymorph degranulation, CC10 and tezosentan are awaiting trials with sufficient power before they come to be used regularly in this scenario [31, 32].
Medicolegal Implications
With increasing consumer awareness, there is an enhanced risk of lawsuits when a baby born from a meconium stained milieu is found to develop long term sequelae. It is as yet not clear if MSAF per se indicates foetal jeopardy. There is no quality proof to suggest that MAS leads to sequelae. An accurate calendar for timing the passage of meconium to delivery time does not exist. It is known however that many of the infants who have experienced MAS have suffered pre and postnatal periods of hypoxia and acidosis which are risk factors for CNS damage. Though the delivering obstetrician is the primary focus of such lawsuits, the neonatologist also gets involved..
Conclusions
Though well recognised and common problems, MSAF and MAS continue to occur with the same frequency over the years. The management of the afflicted neonate with MAS is a daunting task requiring critical care support. Several modalities of monitoring and treatment are available, but these are yet to be substantiated with quality scientific investigation. Our understanding of this rather complex though common entity is as yet incomplete, making it a fertile ground for research.
MSAF though a sign of foetal maturity, could in the presence of abnormal CTG and acidemia suggest foetal jeopardy. Oropharyngeal suctioning at birth, followed by tracheal toileting in depressed and hypotonic babies limits the occurrence and severity of MAS.
Management modalities in MAS include mechanical ventilation, surfactant therapy and supportive care. Steroid therapy is as yet controversial. Early and appropriate respiratory support limits PPHN, treatment of which can be challenging.
Conflicts of Interest
None identified
References
- 1.Antonowiez I, Schwachman H. Meconium in health and disease. Adv Paediatr. 1979;26:275–292. [PubMed] [Google Scholar]
- 2.Carbine DN. Meconium aspiration. Paediatr Rev. 2008;29:212–213. doi: 10.1542/pir.29-6-212. [DOI] [PubMed] [Google Scholar]
- 3.Dargaville PA, Copnell B. The epidemiology of MAS: the incidence, risk factors, therapies and outcome. Paediatrics. 2006;117:1712–1721. doi: 10.1542/peds.2005-2215. [DOI] [PubMed] [Google Scholar]
- 4.Rossi EM, Philipson EH, Williams TG, Kathen SC. MASIntrapartum and neonatal attributes. Am J Obstet Gynaecol. 1989;5:161–166. doi: 10.1016/0002-9378(89)90643-1. [DOI] [PubMed] [Google Scholar]
- 5.Clark P, Doff P. Inhibition of neutrophil oxidative burst and phagocytosis by meconium. Am J Obstet Gynecol. 1995;173:1301–1305. doi: 10.1016/0002-9378(95)91375-0. [DOI] [PubMed] [Google Scholar]
- 6.Jones CA. Early production of pro inflamatory cytokines in the pathogenesis of neonatal ARDS associated with meconium aspiration. Pediatr Res. 1994;35:339. [Google Scholar]
- 7.Steer PJ, Eigbe F, Lissauer TJ, Beard RW. Inter relationships among abnormal CTG's in labor, meconium staining of the amniotic fluid, arterial cord blood pH and Apgar scores. Obstet Gynaecol. 1989;74:715–719. [PubMed] [Google Scholar]
- 8.Miller FC, Sacks DA, Yeh SY. Significance of meconium during labor. Am J Obstet Gynaecol. 1975;122:573–577. doi: 10.1016/0002-9378(75)90052-6. [DOI] [PubMed] [Google Scholar]
- 9.Khazardoost S, Hantoushzadeh S, Khooshideh M, Borna S. Risk factors for MAS in MSAF. J Obstet Gynecol. 2007;27:577–579. doi: 10.1080/01443610701469636. [DOI] [PubMed] [Google Scholar]
- 10.Wiswell TE, Bent RC. Meconium staining and the MAS. PCNA. 1993;40:955–961. doi: 10.1016/s0031-3955(16)38618-7. [DOI] [PubMed] [Google Scholar]
- 11.Jena N, Barik S, Arora N. Intrapartum amnioinfusion for meconium-stained amniotic fluid: a systematic review of randomised controlled trials. BJOG. 2007;114:1582–1583. doi: 10.1111/j.1471-0528.2007.01536.x. [DOI] [PubMed] [Google Scholar]
- 12.Das AK, Jena N, Dasgupta S, Samanta B. Intrapartum transcervical amnioinfusion for MSAF. Int J Gynaecol Obstet. 2007;97:182–186. doi: 10.1016/j.ijgo.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 13.2005 AHA guidelines of CPR and emergency cardiovascular care of the paediatric and neonatal patient: Neonatal Resuscitation Guidelines. Paediatrics. 2006;117:1029–1038. [Google Scholar]
- 14.Dargaville PA, Copnell B, Tingay DG, Gordon JI, Morley CJ. Refining methods of therapeutic lung levage in meconium aspiration. Neonatology. 2008;94:160–163. doi: 10.1159/000143394. [DOI] [PubMed] [Google Scholar]
- 15.Tingay DG, Mills JF, Morley CJ, Dargaville PA. Trends in use and outcome of newborn infants treated with high frequency ventilation in Australia and New Zealand 1996-2003. J Pediatr Child Health. 2007;43:160–166. doi: 10.1111/j.1440-1754.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T, Wu H, Cui X, Li W, Matsuhisa D, Tanaki N. Effects of surfactant replacement on irregular over distension of meconium injured lungs in rats. Neonatology. 2007;93:117–124. doi: 10.1159/000107401. [DOI] [PubMed] [Google Scholar]
- 17.Chinese Collaborative Study Group for Neonatal Respiratory Distress Treatment of severe meconium aspiration syndrome with porcine surfactant: a multicenter, randomized, controlled trial. Acta Paediatr. 2005;94:896–902. doi: 10.1111/j.1651-2227.2005.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 18.Engle WA. The Committee on Fetus and Newborn. Surfactantreplacement therapy for respiratory distress in the preterm and term neonate. Paediatrics. 2008;121:419–432. doi: 10.1542/peds.2007-3283. [DOI] [PubMed] [Google Scholar]
- 19.Shahed AI, Dargaville P, Ohisson A, Soll RF. Surfactant for meconium aspiration syndrome in full term/near term infants. Cochrane Database Syst Rev. 2007;18:CD 002054. [Google Scholar]
- 20.Wiswell TE, Knight GR, Finer NN. A multicentric, randomised controlled trial comparing Surfaxin (Lucinactant) levage with standard care for treatment of meconium aspiration syndrome. Paediatrics. 2002;109:1081–1087. doi: 10.1542/peds.109.6.1081. [DOI] [PubMed] [Google Scholar]
- 21.Colvero MO, Fiori HH, Fiori RM. Bronchoalveolar lavage plus surfactant in a piglet model of meconium aspiration syndrome. Neonatology. 2007;93:188–192. doi: 10.1159/000110866. [DOI] [PubMed] [Google Scholar]
- 22.Dargaville PA, Mills JF, Copnell B, Loughnan PM, McDougall PN, Morley CJ. Therapeutic lung lavage in meconium aspiration syndrome: a preliminary report. J Paediatry Child Health. 2007;43:539–545. doi: 10.1111/j.1440-1754.2007.01130.x. [DOI] [PubMed] [Google Scholar]
- 23.Calkovska A, Mokra D, Drgova A, Zila I, Javorka K. Bronchoalveolar lavage with surfactant/dextran mixture improves meconium clearance and lung functions in experimental meconium aspiration syndrome. Eur J Paediatry. 2007 doi: 10.1007/s00431-007-0596-7. E pub. [DOI] [PubMed] [Google Scholar]
- 24.Jeng MJ, Soong WJ, Lee YS. Effects of therapeutic bronchalveolar lavage and partial liquid ventilation on meconium aspirated piglets. Crit Care Med. 2006;34:1099–1105. doi: 10.1097/01.CCM.0000205662.60832.35. [DOI] [PubMed] [Google Scholar]
- 25.Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev. 2001:CD 000399. doi: 10.1002/14651858.CD000399. [DOI] [PubMed] [Google Scholar]
- 26.Okazaki K, Kondo M, Kato M, Kakinuma R, Nishida A, Noda M. Serum cytokine and chemokine profiles in neonates with meconium aspiration syndrome. Paediatrics. 2008;121:748–753. doi: 10.1542/peds.2007-1697. [DOI] [PubMed] [Google Scholar]
- 27.Cayabyab RG, Kwong K, Jones C, Minoo P, Durand M. Lung inflammation and pulmonary function in infants with meconium aspiration syndrome. Paediatry Pulmonol. 2007;42:898–905. doi: 10.1002/ppul.20675. [DOI] [PubMed] [Google Scholar]
- 28.Tripathi S, Saili A, Dutta R. Inflammatory markers in meconium induced lung injury in neonates and effect of steroids on their levels: A randomized controlled trial. Indian J Med Microbiol. 2007;25:103–107. doi: 10.4103/0255-0857.32714. [DOI] [PubMed] [Google Scholar]
- 29.Radhakrishnan RS, Lally PA, Lally KP, Cox CS., Jr ECMO for meconium aspiration syndrome: support for relaxed entry criteria. ASAIO J. 2007;53:489–491. doi: 10.1097/MAT.0b013e318063c602. [DOI] [PubMed] [Google Scholar]
- 30.Basu S, Kumar A, Bhatia BD. Role of antibiotics in meconium aspiration syndrome. Ann Trop Paediatr. 2007;27:107–113. doi: 10.1179/146532807X192471. [DOI] [PubMed] [Google Scholar]
- 31.Angert RM, Pilon AL, Chester D, Davis JM. CC10 reduces inflammation in meconium aspiration syndrome in newborn piglets. Paediatr Res. 2007;62:684–688. doi: 10.1203/PDR.0b013e31815a5632. [DOI] [PubMed] [Google Scholar]
- 32.Geiger R, Kleinsasser A, Meier S. Intravenous tezosentan improves gas exchange and hemodynamics in acute lung injury secondary to meconium aspiration. Intensive Care Med. 2008;34:368–376. doi: 10.1007/s00134-007-0857-y. [DOI] [PubMed] [Google Scholar]


