Abstract
Background
For the increasing development of type 2 diabetes dietary habits play an important role. In this regard, dietary supplements are of growing interest to influence the progression of this disease.
Objective
The aim of this study was to investigate the effect of a cascade-fermented dietary supplement based on fruits, nuts, and vegetables fortified with chromium and zinc on metabolic control in patients with type 2 diabetes mellitus.
Methods
This was a randomized, placebo-controlled, double-blind, intervention study under free-living conditions using a cross-over design. Thirty-six patients with type 2 diabetes mellitus were enrolled and randomized either to receive a cascade-fermented dietary supplement enriched with chromium (100 µg/d) and zinc (15 mg/d) or a placebo similar in taste but without supplements, over a period of 12 weeks. After a wash-out period of 12 weeks, the patients received the other test product. The main outcome variable was the levels of glycated hemoglobin (HbA1c). Other outcome variables were fasting blood glucose, fructosamine, and lipid parameters.
Results
Thirty-one patients completed the study. HbA1c showed no relevant changes during both treatment periods, nor was there a relevant difference between the two treatments (HbA1c: p=0.48). The same results were found for fructosamine and fasting glucose (fructosamine: p=0.9; fasting glucose: p=0.31). In addition, there was no effect on lipid metabolism.
Conclusion
This intervention study does not provide evidence that a cascade-fermented plant-based dietary supplement enriched with a combination of chromium and zinc improves glucose metabolism in patients with type 2 diabetes mellitus under free-living conditions.
Keywords: intervention study, human, free-living condition, type 2 diabetes, HbA1c
Type 2 diabetes mellitus is a major health concern with increasing incidence and prevalence almost worldwide. Up to 30% of the elderly population in Germany is currently treated for diabetes mellitus (1), resulting in an enormous economic burden for the health care system (2). This unfavorable development is a result of the modern lifestyle partly characterized by overnutrition leading to overweight and/or obesity (3). Therefore, dietary habits play an important role in the development and progression of this disease. In this context, dietary supplements have received growing interest as a potential supportive measure to tackle this challenge (4–6).
Chromium is thought to be essential for normal glucose homeostasis (7). Mechanistic studies have shown that chromium improves insulin receptor and post-receptor signaling, resulting in an increased glucose uptake by enhancing the activity of the glucose transporter type 4 (GLUT 4) (8). Severe chromium deficiency can lead to insulin resistance and diabetes, which is reversible by chromium supplementation (9). Some clinical trials with chromium supplementation could show beneficial effects on fasting plasma glucose and/or glycated hemoglobin (HbA1c) (10–15). A recent systematic review by Balk et al. (16) concluded that chromium supplementation in diabetic patients is associated with improvements in HbA1c levels by −0.6% and fasting glucose by −1.0 mmol/l. However, in most of these studies, rather high doses of chromium were supplemented (16).
Zinc is a micronutrient that plays multiple roles in a number of cellular and systemic functions (17). Concerning type 2 diabetes mellitus, zinc is an important component for insulin synthesis that functions by stabilizing insulin hexamers and their pancreatic storage (18, 19). Zinc also appears to have an insulinomimetic effect owing to its inhibition of the glycogen-regulating enzyme glycogen synthase kinase 3 (GSK3) (20). In addition, zinc may act as an antioxidant and anti-inflammatory compound (21, 22). Due to hyperglycemia, increased urinary excretion is found in patients with type 2 diabetes resulting in a zinc deficiency (23, 24). Clinical studies with zinc supplementation could show a lowering of HbA1c (25, 26). A meta-analysis by Jayawardena et al. (27) reported mean reductions of HbA1c by 0.54% and fasting blood glucose by 18.13 mg/dl after zinc supplementation.
Additionally, organic acids present in vinegar were shown to exhibit antiglycemic effects (28). However, the mechanism by which the effect is carried out is yet unclear. For example, the acetic acid in vinegar might interfere with disaccharidase activity (29) and/or gastric emptying might be delayed (30). A reduction of postprandial glycemia could be shown, for example, by Östmann et al. (31), who tried out three levels of vinegar (18, 23, 28 mmol), and Johnston et al. (32), who described a ~20% reduction after the ingestion of 10 g of vinegar.
In the present study, we investigated the effect of a dietary product representing a combination of chromium, zinc, and organic acids from the cascade fermentation of vegetable raw materials on glucose metabolism. We used a randomized, placebo-controlled, double-blinded cross-over design to assess the effect of this product on glycemia in well-controlled patients with type 2 diabetes, for 12 weeks under free-living conditions.
Methods
Patients
Patients with type 2 diabetes mellitus were screened through advertisements and by personal invitation in general practitioner's practices. Thirty-six non-insulin-dependent diabetic individuals, who met the eligibility criteria, were enrolled. Inclusion criteria were diabetes mellitus type 2 for at least 1 year, treated by diet alone or with oral antidiabetic agents; age between 25 and 73 years; HbA1c >6.5%; and <9.0%, body mass index (BMI) between 25 and 40 kg/m2. Exclusion criteria were insulin treatment, uncontrolled arterial hypertension, instable coronary heart disease, or any other severe general diseases, including psychiatric diseases.
Study design
The study was carried out in the Human Study Center of the Unit of Nutritional Medicine at the Technical University of Munich in Freising-Weihenstephan, Germany. It was registered in the German Clinical Trials Register (DRKS 00003111) and the study protocol was approved by the ethical committee of the Technical University of Munich (Project number: 2866/10). Written informed consent was obtained from all subjects prior to the beginning of the study.
This was a randomized, double-blinded, placebo-controlled trial using a cross-over design with two treatment periods of 12 weeks, each under free-living conditions and separated by a wash-out phase of 12 weeks. The randomization and allocation procedure (blockwise) was prepared by a person not involved in the study. The patients were handed out boxes with their designated number. Sealed envelopes were stored in order to be able to unblind.
Intervention
The participants received a daily dose of 20 ml of either the test product (Regulat Spezial Diabetic) or a placebo handed out in 20 ml bottles. Ten milliliters had to be taken before breakfast and 10 ml before dinner. Regulat Spezial Diabetic and placebo were produced and provided by Dr. Niedermaier Pharma GmbH (Hohenbrunn, Germany). The test product was prepared by the cascade fermentation of defined vegetables, fruits, and nuts and subsequently enriched with chromium and zinc. The raw ingredients were artichokes, curcuma, dates, peas, millet seeds, figs, coconuts, saffron, celery, soybeans, walnuts, bean sprouts, lemons, and onions. The product provided 100 µg chromium(III)chloride hexahydrate and 15 mg zinc chloride per day. Other ingredients were vitamin C (40 mg/20 ml), niacin (4.8 mg/20 ml), pantothenic acid (1.8 mg/20 ml), pyridoxine hydrochloride (0.51 mg/20 ml), riboflavin (0.42 mg/20 ml), thiamine (0.33 mg/20 ml), folic acid (0.06 mg/20 ml), and cyanocobalamin (0.75 µg/20 ml). The placebo product was designed to have a similar taste and appearance based on orange juice concentrate without any supplements. Compliance was monitored by weighing and counting the number of empty bottles returned by the participants. Adverse effects, changes in body weight, medication, changes in diet or physical activity, and acute illnesses were documented at each visit in week 0, 4, and 12. An additional telephone visit was conducted at week 8. The patients were asked to maintain their normal lifestyle and keep their body weight stable.
Blood samples
Venous blood samples were drawn in the fasting state (12 h overnight) at baseline and after 4 and 12 weeks in each treatment period. Blood glucose was measured with the HemoCue® system (Großostheim, Germany). Blood samples were centrifuged immediately and stored at −80°C until further analysis. Insulin was determined with a commercially available insulin ELISA kit (DAKO, Denmark) on the ELISA-star platform of Hamilton (Bonaduz, Swiss). All other serum variables were analyzed by a certified laboratory for clinical studies (synlab Labor München Zentrum, Munich, Germany).
Statistical analysis
Sample size was chosen to detect a difference between treatment and placebo for the primary outcome variable HbA1c with a power of 90% on a two-sided level of significance of 5%. Under the assumption of a true effect size (mean difference divided by standard deviation of the difference) of 0.6 and a drop-out rate of 10%, the sample size resulted in 36 patients.
The method described by Hills and Armitage (33) was used to analyze the data adjusting for a possible period effect. Period effect and treatment by period interaction as well as for differences between the two groups were tested using two sample t-test or Mann Whitney U test depending on the distribution of the parameters.
Statistical analyses were conducted with the use of R software, version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria). All statistical tests were two-sided, with a significance level of 0.05. Data are presented as mean and standard deviation or median and range, where appropriate.
Results
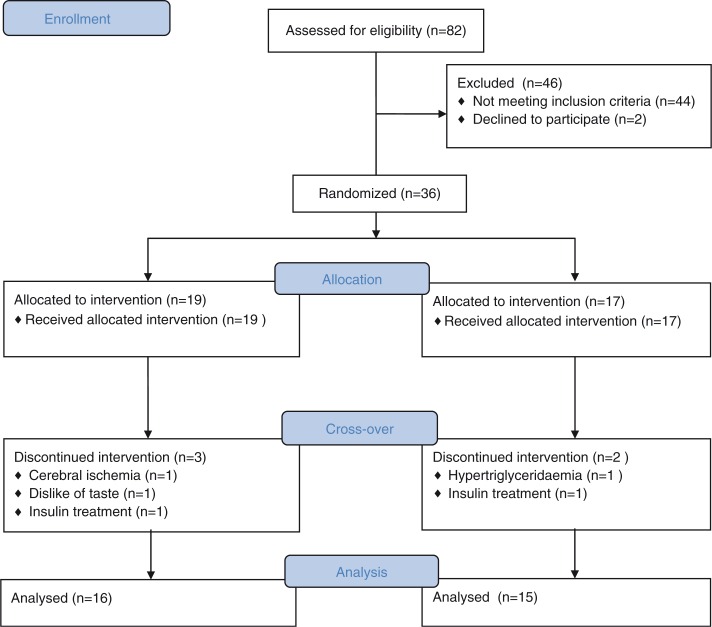
The baseline characteristics of the participants at the initiation of the study are presented in Table 1. At the beginning of the study, 50.1% of the patients were treated with metformin, 24.9% with sulfonyl urea, 4.5% with acarbose, 4.5% with dipeptidyl peptidase-4 inhibitor (DPP-4 inhibitor), 11.5% with a combination of DPP-4 inhibitor and metformin, and 4.5% by diet alone. Thirty-one subjects completed the study as presented in the flow diagram (Fig. 1). Reasons for dropping out were elevated triglyceride level (n=1), initiation of insulin treatment (n=2), cerebral ischemia (n=1), and dislike of the taste (n=1).
Table 1.
Baseline characteristics (mean±SD) of the diabetic patients at initiation of the intervention
| Characteristic | Test product (n=19) |
Placebo (n=17) |
|---|---|---|
| Age (years) | 65.0±6.0 | 61.9±7.6 |
| BMI (kg/m2) | 30.9±4.0 | 30.0±5.5 |
| Gender (female/male) | 12/7 | 6/11 |
| Duration of diabetes (years) | 12.4±8.0 | 9.1±6.5 |
| Fasting blood glucose (mmol/l) | 141.5±37.3 | 125.2±19.8 |
| HbA1c (%) | 7.3±0.9 | 7.2±0.6 |
| Fructosamine (µmol/l) | 284.3±33.1 | 267.2±33.1 |
| Cholesterol (mg/dl) | 198.0±45.2 | 206.7±36.7 |
| LDL (mg/dl) | 111.8±39.9 | 117.4±33.8 |
| HDL (mg/dl) | 53.8±13.8 | 48.6±14.0 |
| Triglycerides (mg/dl) | 192.9±124.1 | 202.3±103.4 |
| Uric acid (mg/dl) | 5.8±1.6 | 6.3±1.6 |
| GOT (U/l) | 29.6±7.5 | 27.2±7.7 |
| GPT (U/l) | 31.0±11.0 | 27.2±11.1 |
| Gamma-GT (U/l) | 52.0±49.6 | 41.6±32.0 |
Fig. 1.
CONSORT 2010 flow diagram of a randomized, placebo-controlled, double-blinded cross-over study of a fermented dietary supplement containing chromium and zinc in type 2 diabetic patients.
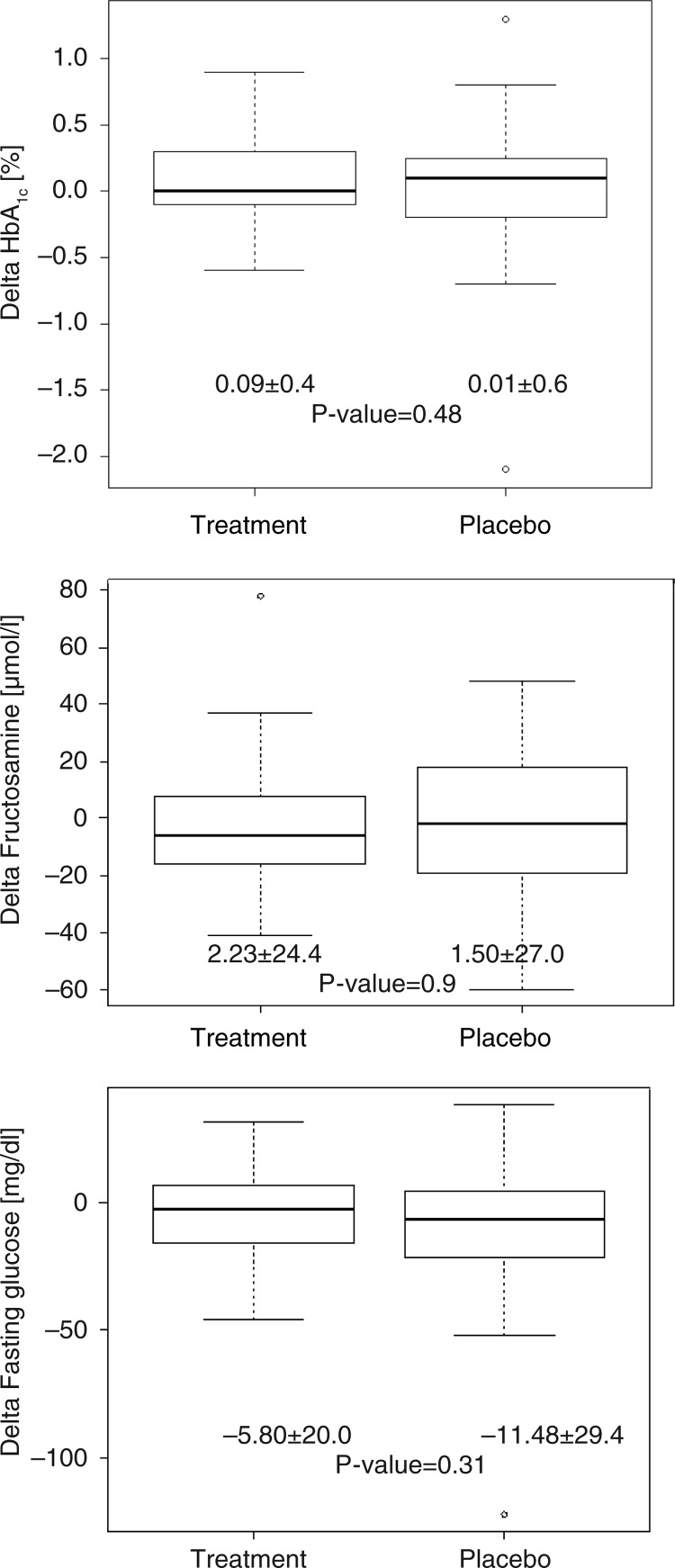
HbA1c was the primary outcome variable and was used as a parameter of glycemic control. There was no relevant change in the average values during both treatment periods for the test product (0.09%±0.4) and placebo (0.01%±0.6), respectively. No relevant difference was found between verum and placebo treatment (p=0.48) (Fig. 2). We additionally measured fructosamine as another surrogate parameter of glycemia with a shorter elimination rate compared to HbA1c. Again, there was no relevant change in the average values for the test product (−2.23 µmol/l±24.4) and placebo (−1.50 µmol/l±27.0), and no difference between the two interventions (p=0.9) (Fig. 2). Fasting glucose concentrations also were relevantly affected by neither the test product (−5.80 mg/dl±20.0) and placebo (−11.48 mg/dl±29.4) nor the interventions (p=0.31) (Fig. 2).
Fig. 2.
Treatment effect (delta) on HbA1c, fructosamine, and fasting glucose for placebo and test product. Data are presented as mean±SD. N=31.
As other groups could show beneficial changes in blood lipid profiles after chromium or zinc supplementation (12, 34), we also measured the lipid profile as a secondary outcome. However, no relevant differences were observed for total cholesterol (p=0.885), HDL (p=0.649), LDL (p=0.642), and triglycerides (p=0.312) at any time point (Table 2). Similar non-relevant results were measured after 4 weeks of treatment (data not shown).
Table 2.
Treatment effect (Δ) on lipid metabolism, insulin, and zinc levels for the test product and the placebo
| Test product | Placebo | |
|---|---|---|
| Cholesterol (mg/dl) | −6.5 (−43 to 64) | 5.5 (−106 to 108) |
| HDL cholesterol (mg/dl) | 1.0 (−9.0 to 31) | 0.5 (−9.0 to 8.0) |
| LDL cholesterol (mg/dl) | −5.0 (−47 to 71) | 3.0 (−92 to 95) |
| Triglycerides (mg/dl) | −8.5 (−131 to 119) | −11.0 (−128 to 360) |
| Insulin (U/ml) | −0.45 (−23 to 9.6) | −1.0 (−33 to 15) |
| Zinc (µg/l) | −4.0 (−128 to 78) | −1.0 (−38 to 29) |
Data are presented as median (range). N=31.
Both Regulat Spezial Diabetic and a placebo were taken regularly before breakfast and dinner. The test product was well tolerated. During the active intervention period, three patients reported constipation and maldigestion, and two patients mentioned heartache or retrosternal burning after swallowing. During placebo treatment, four patients reported gastrointestinal symptoms, e.g. diarrhea, constipation; and two patients mentioned retrosternal pain or burning after taking the solution. One patient had pustules both under the placebo and the test product. Body weight, dietary habits, and physical activity remained unchanged during both intervention periods (data not shown).
Discussion
Dietary factors play a pivotal role in the treatment of type 2 diabetes and have acute and long-term effects on blood glucose excursions and finally on HbA1c. Certain nutritional supplements like chromium, zinc, and vinegar have been shown to support the control of hyperglycemia in patients with type 2 diabetes suggested by data from different sources, including animal experiments and human studies (8, 10, 12, 25, 26, 34).
This fermented extract of fruits, nuts, and vegetables has been promoted as a natural product with a glucose-lowering activity, although not yet formally proven. Therefore, the initial concept was to combine different glucose-lowering dietary components, namely chromium, zinc, and organic acids. This was achieved by adding chromium and zinc to the fermented extract. As to the used doses of the micronutrients, the recommendations of the German Nutrition Society (DGE) (35) were followed. These allowed doses were moderate compared to those of other studies, but as this study aimed to have a combined glucose-lowering effect, moderate doses were chosen.
The strategy for chromium supplementation is controversially discussed with regard to the daily dosage. The dosages in studies reporting positive effects on glycemic parameters ranged from 100 up to 1,000 µg chromium daily. However, there was no clear dose-effect relationship (16). Our test product provided 100 µg/d and was administered as chromium(III)chloride hexahydrate. This supplementation seems justified to compensate for the higher loss of chromium (III) via the urine reported for patients with type 2 diabetes (24, 36), but there was no effect on any metabolic parameter measured in our study.
Regarding zinc supplementation, clinical studies showed positive effects on glycemic control like the one by Al-Maroof and Al-Sharbatti (25) and Gunasekara et al. (26), who administered 30 mg and 22 mg zinc sulfate, respectively. There are also studies with a daily dose of 30 mg zinc gluconate administered for 6 months, reporting no effect on HbA1c (37, 38). We used an amount of 15 mg zinc chloride per day but could not detect any significant effect.
The test product also contained organic acids derived from cascade fermentation of various fruits, nuts, and vegetables, which resulted in a vinegar-like composition. Johnston et al. (32) were able to show a reduction of postprandial glycemia of ~20% by ingesting 2 tablespoons of vinegar per day. We did not observe any effect on blood glucose levels in our study. This discrepancy may be explained by differences in the composition of organic acids. The main organic acid in the test product was lactic acid in contrast to acetic acid in vinegar. It was hypothesized by Ogawa et al. (29) that acetic acid, but not organic acids such as lactic and citric acid, inhibit disaccharidase activity in the small intestine.
The combination of different glucose-lowering dietary components in moderate doses did not show any significant positive effects on glucose metabolism. The doses might have been too low. Thus, we cannot fully exclude the possibility that higher doses of chromium, zinc, and organic acids may be necessary to elicit significant effects on glucose and lipid metabolism in subjects with type 2 diabetes.
The strength of this intervention is the study design itself. Cross-over studies are advantageous for studies of chronic diseases like type 2 diabetes mellitus and the influence of confounding covariates is reduced because each cross-over patient serves as his or her own control.
A limitation of this trial is that we were not able to show any positive effects on glucose and lipid metabolism, even though a power calculation had been performed and the study was carefully conducted according to the protocol.
Conclusion
Taken together, our study does not provide evidence that a product based on cascade-fermented fruits, nuts, and vegetables combined with chromium and zinc supplementation at moderate doses has a beneficial effect on glucose and lipid metabolism in subjects with type 2 diabetes.
Acknowledgements
We would like to thank Dr. Dörfler-Schmidt (Freising), Drs Haslbeck (Kranzberg), Dr. Koch (Freising), Dr. Miedl (Freising), Drs. Peller (Straubing), and Dr. Weyerer (Freising) for their valuable contributions in recruiting participants. The study was funded by Dr. Niedermaier Pharma GmbH, Hohenbrunn, Germany. Furthermore, we like to thank Manuela Hubersberger for her assistance in laboratory analyses.
Conflict of interest and funding
Yu-Mi Lee's work was funded by Dr. Niedermaier Pharma GmbH, Hohenbrunn, Germany. The funding body had no influence on design of the study; the collection and analysis of the data, and the decision to publish. Dr Wolf, Dr Hauner, and Dr Skurk declare no potential conflict of interest.
References
- 1.Hauner H, Bramlage P, Lösch C, Steinhagen-Thiessen E, Schunkert H, Wasem J, et al. Prevalence of obesity in primary care using different anthropometric measures – results of the German Metabolic and Cardiovascular Risk Project (GEMCAS) BMC Public Health. 2008;8(1):282. doi: 10.1186/1471-2458-8-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Köster I, Huppertz E, Hauner H, Schubert I. Direct costs of diabetes mellitus in Germany CoDiM 2000–2007. Exp Clin Endocrinol Diabetes. 2011;119(6):377–85. doi: 10.1055/s-0030-1269847. [DOI] [PubMed] [Google Scholar]
- 3.Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159:1150–9. doi: 10.1093/aje/kwh167. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett HE, Eperjesi F. Nutritional supplementation for type 2 diabetes: a systematic review. Ophthalmic Physiol Opt. 2008;28:503–23. doi: 10.1111/j.1475-1313.2008.00595.x. [DOI] [PubMed] [Google Scholar]
- 5.Wiernsperger N, Rapin JR. Trace elements in glucometabolic disorders. Diabetol Metab Syndr. 2010;2:70–8. doi: 10.1186/1758-5996-2-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davi G, Santilli F, Patrono C. Nutraceuticals in diabetes and metabolic syndrome. Cardiovasc Ther. 2010;28:216–26. doi: 10.1111/j.1755-5922.2010.00179.x. [DOI] [PubMed] [Google Scholar]
- 7.Cefalu WT, Hu FB. Role of chromium in human health and in diabetes. Diabetes Care. 2004;27:2741–51. doi: 10.2337/diacare.27.11.2741. [DOI] [PubMed] [Google Scholar]
- 8.Cefalu WT, Wang ZQ, Zhang XH, Baldor LC, Russell JC. Oral chromium picolinate improves carbohydrate and lipid metabolism and enhances skeletal muscle Glut-4 translocation in obese, hyperinsulinemic (JCR-LA corpulent) rats. J Nutr. 2002;132(6):1107–14. doi: 10.1093/jn/132.6.1107. [DOI] [PubMed] [Google Scholar]
- 9.Jeejeebhoy KN, Chu RC, Marliss EB, Greenberg GR, Bruce-Robertson A. Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation, in a patient receiving long-term total parenteral nutrition. Am J Clin Nutr. 1977;30:531–8. doi: 10.1093/ajcn/30.4.531. [DOI] [PubMed] [Google Scholar]
- 10.Martin J, Wang ZQ, Zhang XH, Wachtel D, Volaufova J, Matthews DE, et al. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Diabetes Care. 2001;29:1826–32. doi: 10.2337/dc06-0254. [DOI] [PubMed] [Google Scholar]
- 11.Racek J, Trefil L, Rajdl D, Mudrova V, Hunter D, Senfrl V. Influence of chromium-enriched yeast on blood glucose and insulin variables, blood lipids, and markers of oxidative stress in subjects with type 2 diabetes mellitus. Biol Trace Elem Res. 2006;109:215–30. doi: 10.1385/BTER:109:3:215. [DOI] [PubMed] [Google Scholar]
- 12.Rabinovitz H, Friedensohn A, Leibovitz A, Gabay G, Rocas C, Habot B. Effect of chromium supplementation on blood glucose and lipid levels in type 2 diabetes mellitus elderly patients. Int J Vitam Nutr Res. 2004;74:178–82. doi: 10.1024/0300-9831.74.3.178. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh D, Bhattacharya B, Mukherjee B, Manna B. Role of chromium supplementation in Indians with type 2 diabetes mellitus. J Nutr Biochem. 2002;13:690–7. doi: 10.1016/s0955-2863(02)00220-6. [DOI] [PubMed] [Google Scholar]
- 14.Bahijiri SM, Mira SA, Mufti AM, Ajabnoor MA. The effects of inorganic chromium and brewers yeast supplementation on glucose tolerance, serum lipids and drug dosage in individuals with type 2 diabetes. Saudi Med J. 2000;21:831–7. [PubMed] [Google Scholar]
- 15.Anderson RA, Cheng NZ, Bryden NA, Polansky MM, Cheng NP, Chi JM, et al. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–91. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 16.Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154–63. doi: 10.2337/dc06-0996. [DOI] [PubMed] [Google Scholar]
- 17.Haase H, Overbeck S, Rink L. Zinc supplementation for the treatment or prevention of disease: current status and future perspectives. Exp Gerontol. 2008;43(5):394–408. doi: 10.1016/j.exger.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Arnaud J, Faure H, Bourlard P, Denis B, Favier AE. Longitudinal changes in serum zinc concentration and distribution after acute myocardial infarction. Clin Chim Acta. 1994;230(2):147–56. doi: 10.1016/0009-8981(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 19.Wijesekara N, Chimienti F, Wheeler MB. Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes Meta. 2009;11:202–14. doi: 10.1111/j.1463-1326.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- 20.Ilouz R, Kaidanovich O, Gurwitz D, Eldar-Finkelman H. Inhibition of glycogen synthase kinase-3ß by bivalent zinc ions: insight into the insulin-mimetic action of zinc. Biochem Biophys Res Commun. 2002;295(1):102–6. doi: 10.1016/s0006-291x(02)00636-8. [DOI] [PubMed] [Google Scholar]
- 21.Saper RB, Rash R. Zinc: an essential micronutrient. Am Fam Physician. 2009;79(9):768–72. [PMC free article] [PubMed] [Google Scholar]
- 22.Prasad A. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008;43(5):370–7. doi: 10.1016/j.exger.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Afridi HI, Kazi TG, Kazi N, Baig JA, Jamali MK, Arain MB, et al. Status of essential trace metals in biological samples of diabetic mother and their neonates. Arch Gynecol Obstet. 2009;280(3):415–23. doi: 10.1007/s00404-009-0955-x. [DOI] [PubMed] [Google Scholar]
- 24.Kazi TG, Afridi HI, Kazi N, Jamali MK, Arain MB, Jalbani N, et al. Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol Trace Elem Res. 2008;122(1):1–18. doi: 10.1007/s12011-007-8062-y. [DOI] [PubMed] [Google Scholar]
- 25.Al-Maroof RA, Al-Sharbatti SS. Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med J. 2006;27(3):344–50. [PubMed] [Google Scholar]
- 26.Gunasekara P, Hettiarachchi M, Liyanage C, Lekamwasam S. Effects of zinc and multimineral vitamin supplementation on glycemic and lipid control in adult diabetes. Diabetes Metab Syndr Obes. 2011;4:53–60. doi: 10.2147/DMSO.S16691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayawardena R, Ranasinghe P, Galappathy P, Malkanthi RLDK, Constantine GR, Katulanda P. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 2012;4:13–24. doi: 10.1186/1758-5996-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latis S, Grammatikou S, Poulia KA, Perrea D, Makrilakis K, Diakoumopoulou E, et al. Vinegar reduces postprandial hyperglycaemia in patients with type II diabetes when added to a high, but not a low glycaemic index meal. Eur J Clin Nutr. 2010;64:727–32. doi: 10.1038/ejcn.2010.89. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa N, Satsu H, Watanabe H, Fukaya M, Tsukamoto Y, Miyamoto Y, et al. Acetic acid suppresses the increase in disaccharidase activity that occurs during culture of caco-2 cells. J Nutr. 2000;130:507–13. doi: 10.1093/jn/130.3.507. [DOI] [PubMed] [Google Scholar]
- 30.Liljeberg H, Bjorck I. Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur J Clin Nutr. 1998;52:368–71. doi: 10.1038/sj.ejcn.1600572. [DOI] [PubMed] [Google Scholar]
- 31.Östmann E, Granfeldt Y, Persson L, Björck I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr. 2005;59:983–8. doi: 10.1038/sj.ejcn.1602197. [DOI] [PubMed] [Google Scholar]
- 32.Johnston CS, Steplewska I, Long CA, Harris LN. Examination of the antiglycemic properties of vinegar in healthy adults. Ann Nutr Metab. 2010;56:74–9. doi: 10.1159/000272133. [DOI] [PubMed] [Google Scholar]
- 33.Hills M, Armitage P. The two-period cross-over clinical trial. Br J Clin Pharmacol. 1979;8(1):7–20. doi: 10.1111/j.1365-2125.1979.tb05903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clodfelder BJ, Gullick BM, Lukaski HC, Neggers Y, Vincent JB. Oral administration of the biomimetic [Cr3O(O2CCH2CH3)6(H2O)3]+ increases insulin sensitivity and improves blood plasma variables in healthy and type 2 diabetic rats. J Biol Inorg Chem. 2005;10(2):119–30. doi: 10.1007/s00775-004-0618-0. [DOI] [PubMed] [Google Scholar]
- 35.Deutsche Gesellschaft für Ernährung (DGE) D-A-CH: Referenzwerte für die Nährstoffzufuhr. 5th ed. Neustadt: Umschau Buchverlag; 2013. [Google Scholar]
- 36.Morris BW, MacNeil S, Hardisty CA, Heller S, Burgin C, Gray TA. Chromium homeostasis in patients with type II (NIDDM) diabetes. J Trace Elem Med Biol. 1999;13(1–2):57–61. doi: 10.1016/S0946-672X(99)80024-8. [DOI] [PubMed] [Google Scholar]
- 37.Anderson RA, Roussel A, Zouari N, Mahjoub S, Matheau J, Kerkeni A. Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J Am Coll Nutr. 2001;20:212–18. doi: 10.1080/07315724.2001.10719034. [DOI] [PubMed] [Google Scholar]
- 38.Roussel A, Kerkeni A, Zouari N, Mahjoub S, Matheau J, Anderson RA. Antioxidant effects of zinc supplementation in Tunisians with type 2 diabetes mellitus. J Am Coll Nutr. 2003;22:316–21. doi: 10.1080/07315724.2003.10719310. [DOI] [PubMed] [Google Scholar]