Abstract
Radiation therapy (RT) is a clinical modality dealing with the use of ionizing radiations to treat malignant neoplasias (and occasionally benign diseases). Since its inception, the goal of RT has been to cure cancer locally without excessive side effects. The most important factors affecting the results of RT are the tumor type, its location and regional extent, the anatomic area of involvement and the geometric accuracy with which a calculated radiation dose is delivered. Although higher doses of radiation can produce better tumor control, the dosage which can be given is limited by the possibility of normal tissue damage. Approximately 60-65% of all cancer patients require RT as the sole treatment modality and / or in combination with surgery or chemotherapeutic drugs. There is a huge gap between demand and supply of radiotherapy facilities and infrastructure. Most of the oncocentres are located in urban areas in private sector and are beyond the reach of the common man.
Key Words: Radiotherapy, Radiation biology, Radiation physics
Inroduction
Cancer prevalence in India is estimated to be around 2.5 million, with over 8,00,000 new cases and 5,50,000 deaths occurring each year due to this disease in the country [1, 2]. Carcinoma breast and cervix are the most common malignancies noted in Indian females; while in males the most common malignancies are those of aerodigestive tract i.e. lung, stomach, esophagus and head and neck [1, 3]. About 2/3 of cancer patients need radiation therapy (RT) i.e. 500,000 patients per year. Like surgery, RT is a locoregional treatment modality. The main aim of RT is to maximize tumour control whilst minimizing damage to normal tissues. Over the last 20 years major technological advances have helped greatly to improve the accuracy of treatment with resulting improvements in the outcome.
Basics of Radiation Physics
The term radiation applies to the emission and propagation of energy through space or a material medium. Broadly, it may be classified into electromagnetic radiation and particulate radiation. Electromagnetic radiation is characterized by oscillating electrical and magnetic fields and has a dual nature. X-rays and Gamma (γ) rays are the two major forms of electromagnetic radiation used in radiotherapy. X-rays are produced when high speed electrons collide with a material of high atomic number like Tungsten-Molybdenum in the anode of a x-ray tube, while γ rays are produced by intra-nuclear disintegration. Particulate radiation refers to the energy propagated by travelling corpuscles, which have definite rest mass, definite momentum and a defined position at any instant, examples include electron, proton, neutron etc. Despite several decades of research, photon-beam still constitutes the main therapeutic modality in RT, because of several unresolved technical problems with the use of particulate radiation [4, 5].
When an x-ray or γ-ray beam passes through a medium, interactions occur between the photon and the matter and energy is transferred to the medium. The photon-beam may undergo attenuation, absorption, scattering or transmission. The three major forms of interaction of radiation with matter, which are of clinical importance in RT, are Compton effect, photoelectric effect and pair production. Compton effect is the most important in modern-day megavoltage RT. The photoelectric effect is of primary importance in diagnostic radiology and has only historical importance in present day RT. In Compton effect (Fig. 1), photons interact with free electrons and hand over part of their energy to it. The angle through which the photon is scattered, the energy handed on to the electron and energy lost by the photon is interconnected. The wavelength change depends neither on the material being irradiated nor on the radiation energy, but only upon the angle through which the radiation is scattered. It has several important implications in designing radiation protection. The reduced scattering suffered by high-energy radiation as well as the almost homogeneous tissue dosage is primarily due to the Compton effect. In photoelectric effect (Fig. 2), the photon disappears altogether after interacting with the bound electron, some of the energy being used to remove the electrons from the shell, while the rest is imparted as kinetic energy to the photoelectron. The energy of the characteristic radiation (fluorescent radiation) varies from atom to atom and for low atomic number elements, which make up most of the biological materials, it is of such low energy that it is probably absorbed by the same cell in which the initial event occurs. Pair production results from an interaction with the electromagnetic field of the nucleus and as such the probability of this process increases rapidly with the atomic number (Z2). When the photon with energy in excess of 1.02 MeV (million electron volt) passes close to the nucleus of an atom, the photon disappears and a positron and an electron appear [5].
Fig. 1.
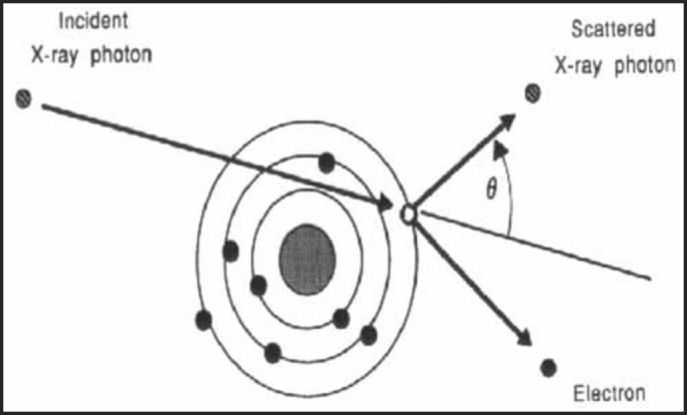
Compton effect: Interaction of photons with free electrons and scattering by an angle θ.
Fig. 2.
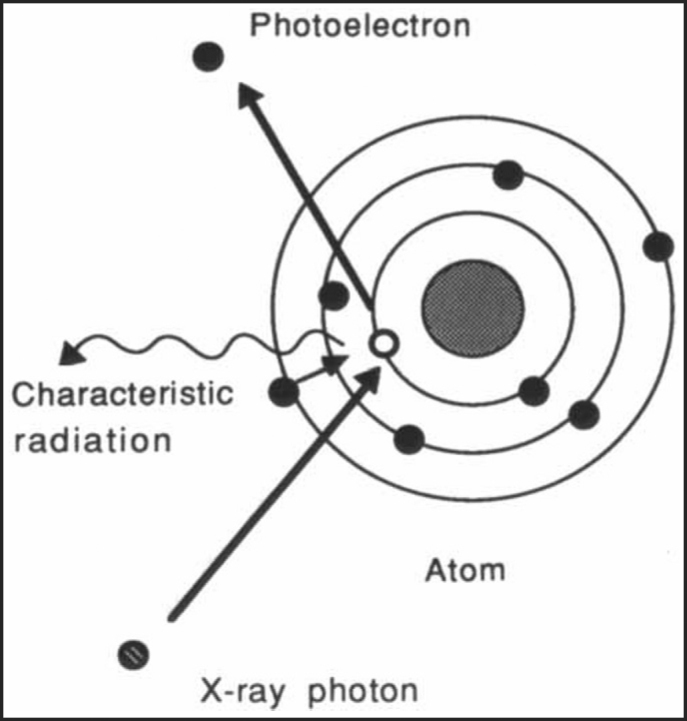
Photoelectric effect: The photon disappears altogether after interacting with the bound electron and emit characteristic radiation.
Biologic Basis of Radiation Therapy
The exact mechanism of cell death due to radiation is still an area of active investigation. A large body of evidence supports double-stranded breaks of nuclear deoxyribose nucleic acid (DNA) as the most important cellular effect of radiation. This breakage leads to irreversible loss of the reproductive integrity of the cell and eventual cell death. Radiation damage can be directly ionizing. However, in clinical therapy, damage is most commonly indirect ionizing via free-radical intermediaries formed from the radiolysis of cellular water. Radiation can also affect the processes of the cell cycle necessary for cell growth, cell senescence and apoptosis [6].
In RT the success of eradicating tumor depends on radio sensitivity of tumour as well as surrounding normal tissue tolerance (NTT) [7]. Tumour lethal dose (TLD) is defined as the dose of radiation that produces complete and permanent regression of tumor in vivo in zone irradiated. Therapeutic index (TI) is the ratio of NTT/TLD and it determines whether a particular disease can be treated or not. Radiosensitivity expresses the response of the tumour to irradiation and is greater for highly mitotic, undifferentiated cells like malignant cells. For highly radiosensitive tumours, NTT is much greater than TLD and TI is high (e.g. lymphoma, seminoma, dysgerminoma, leukemia etc). For moderately radiosensitive tumours, NTT exceeds TLD by a few fractions and TI is low (e.g. most squamous cell carcinomas and adenocarcinomas). For radioresistant tumours, TLD is much higher than NTT and TI is very low e.g. soft tissue sarcomas, bone tumours, melanoma etc.
Delivery of tumorocidal dose in small dose fractions in conventional multifraction regimen is based on 4R's of radiobiology namely, repair of sub lethal damage, repopulation, redistribution and reoxygenation. Repair is considered as the most important rationale for fractionation. Dose fractionation enables normal tissue to recover between two fractions reducing damage to normal tissues. Redistribution of proliferating cell populations from radioresistant to radiosensitive phase throughout the cell cycle increases cell kill in fractionated treatment relative to a single session treatment. If interval is more than six hours then cells will repopulate and results in increase of surviving fraction, referred to as repopulation. Cells at the centre of tumor are hypoxic and are resistant to radiation. Hypoxic cells get reoxygenated which occurs during a fractionated course of treatment, making them more radiosensitive to subsequent doses of radiation [8].
Radiation Delivery Techniques
External beam radiotherapy or teletherapy is the most frequently used form of RT. The patient lies on a couch and an external source of radiation is pointed at a particular part of the body. Cobalt units have been the traditional teletherapy equipments and are still in widespread use worldwide, since the machinery is relatively reliable and simple to maintain compared to the modern linear accelerator. They produce stable, dichromatic beams of 1.17 and 1.33 MeV, resulting in average beam energy of 1.25 MeV. The role of the cobalt unit has partly been replaced by the linear accelerator (linse, which can generate higher energy X-rays as well as electrons; with energy range of 4, 6, 15 and 18 MeV. The shape and intensity of the beam produced by a linac may be modified or collimated by a variety of means. Thus, conventional, conformal, intensity-modulated, tomographic and stereotactic RT are all produced by specially modified linear accelerators. Electron beams are useful for treating superficial lesions because the maximum of dose deposition occurs near the surface. The dose then decreases rapidly with depth, sparing underlying tissue. Although the X-ray target is removed in electron mode, the beam must be fanned out by sets of thin scattering foils in order to achieve flat and symmetric dose profiles in the treated tissue [9].
Brachytherapy involves placing a radioactive material directly inside or next to the tumor. It allows a physician to use a higher total dose of radiation to treat a smaller area and in a shorter time than is possible with external radiation treatment [10]. The delivery device may be inserted into a body cavity such as the vagina or uterus (intracavitary brachytherapy) or into a lumen like esophagus (intraluminal brachytherapy) or applicators may be inserted into body tissues as in prostate or breast (interstitial brachytherapy). “Conventional” brachytherapy may be more suitable in routine usage than “temporary” brachytherapy. In temporary brachytherapy, the radioactive material is placed inside or near a tumour for a specific amount of time and then withdrawn. It uses a delivery device, such as a catheter, needle, or applicator; placed into the tumour using fluoroscopy, ultrasound, magnetic resonance imaging (MRI) or computed tomography (CT) to help position the radiation sources. The radiation sources are then inserted by the radiation oncologist either manually causing high exposure risk or the source of radiation may be inserted using a computer-controlled remote afterloading machine.
For treatment planning, a computer is used to help calculate the source position and the amount of time needed to deliver the correct dose of radiation to the tumor. Treatment may be delivered at a high dose-rate (HDR) or a low dose-rate (LDR). The dose rate in LDR ranges from 0.4 to 2.0 Gy/hr, while that in HDR is > 12 Gy/hr. HDR brachytherapy is usually an outpatient procedure lasting only a few minutes. With LDR brachytherapy, the in-patient is treated with radiation delivered at a continuous rate over several hours or days. Permanent brachytherapy, also called seed implantation, involves placing radioactive seeds or pellets (about the size of a grain of rice) in or near the tumour and leaving them there permanently. After several weeks or months, the radioactivity level of the implants eventually diminishes to nothing [11].
Three-dimensional Conformal Radiotherapy (3-DCRT)
Here the radiation field conforms to the shape of the volume to be treated. 3-DCRT is most useful for tumours that are close to important organs and structures, examples include carcinomas of prostate, spine, esophagus, lung, bladder, pancreas, head and neck etc. Most 3-DCRT cases begin with a “virtual simulation” session that lasts between 30 and 90 minutes. CT scans are taken of the patient in the treatment position and the images are transferred into the treatment-planning computer. The clinician can then mark on each CT slice the required volume to be treated. The computer generates a 3-D image of the volume to be treated and critical structures at risk can be highlighted [12]. This helps define the best beam arrangement and the computer then calculates the optimum dose distribution. A beam's-eye view can be generated digitally to give an image of how the simulation film should look and this is also used in treatment verification. Critical structures can be shielded by beam shaping, which can be achieved with customized lead blocks or the use of multileaf collimators which are computer controlled motorized movable lead leaves within the treatment machine which can block part of the radiation field. A typical treatment session lasts about 15-30 minutes.
Intensity Modulated Radiotherapy (IMRT) and Image Guided Radiotherapy (IGRT)
Intensity-modulated radiation therapy (IMRT) is an advanced form of 3-DCRT. It uses sophisticated software and hardware to vary the shape and intensity of radiation delivered to different parts of the treatment area. Regular 3-DCRT and IMRT differ in how the pattern and volume of radiation delivered to the tumor is determined. In conventional 3-DCRT, clinicians input delivery patterns into the computer. In IMRT, the physician designates specific doses of radiation (constraints) that the tumor and normal surrounding tissues should receive [13]. The physics team then uses a sophisticated computer program to develop an individualized plan to meet the constraints. This process is termed “inverse treatment planning”. Typically, combinations of several intensity-modulated fields coming from different beam directions produce a custom tailored radiation dose that maximizes tumour dose while also protecting adjacent normal tissues. The area's most commonly treated with IMRT are prostate, spine, lung, breast, kidney, pancreas, liver, tongue and larynx. Patients who have previously received the maximum amount of radiation delivered by conventional radiation therapy can also be treated with IMRT. The disadvantages include stringent patient set-up and immobilization, cost escalation and increased treatment time for the patients. Some Linacs have an on-board Imager, an automated system that uses high-resolution X-rays to produce contrasting images of cancerous tumours and surrounding soft tissue, allowing physicians to target the cancerous tumor more precisely during treatment and decreasing radiation exposure of healthy tissues. Before the on-board imager, physicians would have to treat a larger area of the body near the cancerous tumour to compensate for any tumor movement, exposing healthy tissue to the radiation. This technique is called image guided radiotherapy (IGRT) [14]. The imaging equipment can also be kept inside the treatment room separately (CT on rail) to acquire the scans in the treatment position. Thus IMRT improves the radiation delivery precision and IGRT improves the radiation delivery accuracy; thereby decreasing the volume of normal tissue being irradiated.
Stereotactic Radiosurgery (Gamma Knife)
The gamma knife works by a process called stereotactic radiosurgery, which uses multiple beams of radiation converging in three dimensions to focus precisely on a small volume, such as a tumor, permitting intense doses of radiation to be delivered to that volume safely while largely sparing the surrounding tissues. It can be used for a wide variety of problems including selected malignant tumours that arise in or spread to the brain (primary brain tumours or metastatic tumours), benign brain tumours (meningiomas, pituitary adenomas, acoustic neuromas), blood vessel defects (arterio-venous malformations) and functional problems (trigeminal neuralgia) [15]. A special headframe that has three-dimensional coordinates built into it is attached to the patient's skull with four screws, CT/MRI is done and the images are sent to the gamma knife's planning computer system to determine the exact relationship between the target lesions and the frame. The frame is then precisely attached to the gamma knife unit so that when the unit is activated, the target is placed exactly in the centre of 201 precision-aimed, converging Co-60 beams. Treatment takes anywhere from several minutes to a few hours to complete depending on the shape of the target and the dose required.
Gamma knife is operated by a multidisciplinary team consisting of neurosurgeons, radiation oncologists, medical physicists, neuroradiologists and anaesthetist. The gamma knife is limited in use by its high cost, limited access, and inability to treat extracranial lesions or multiple lesions and unsuitability to treat targets larger than 2.5 centimetres in size. In India, the cost of gamma knife treatment package is around 2.5 lacs as the cost of equipment, set-up costs and operating costs are very high. It will be cost competitive only if demand for SRS services is high enough to fully use equipment working time.
X-knife is another form of stereotactic radiosurgery where linear accelerator is used to deliver treatment. In addition to certain brain lesions, X-knife can be used to treat selected extra-cranial lesions like spine, lung and liver; though precision is less as compared to Gamma knife [16].
Cyber Knife or Robotic Radiosurgery is a frameless robotic radiosurgery system which uses real time image guidance technology and computer-controlled robotics to deliver a very high dosage of precisely targeted radiation to kill cancer cells in 1-5 fractions [17]. The biggest advantage is that this painless treatment can be completed in a week's time but the treatment cost ranges from 3-5 lacs.
Acute and Late Normal Tissue Reactions
The acute effects occur within 90 days and late normal tissue complications of RT can occur even months and years after RT. The tissues that divide rapidly (e.g, mucous membranes) respond acutely to radiation and are responsible for much of the acute morbidity of the treatment. Late side effects are attributed to the damage of the microvasculature or to stem cell depletion. The acute and chronic effects are related to site, dose, volume and time of treatment. Other therapies, such as surgery and chemotherapy, can increase the probability and severity of radiation-related morbidity. The tolerance dose of various critical organs is given in Table 1 [18].
Table 1.
Tolerance doses of various critical organs: Minimal tolerance TD5/5 is the dose resulting in no more than 5% severe complication rate within 5 years treatment, while maximum tolerance TD50/5 is the dose that results in a 50% severe complication rate 5 years after treatment [18].
| Target cells | Complication end point | TD5/5 to td50/5 (Gy) |
|---|---|---|
| 2 − 10Gy | ||
| Lymphocytes and lymphoid tissue | Lymphopenia | 2-10 |
| Testes, spermatagonia | Sterility | 1-2 |
| Ovarian, oocytes | Sterility | 6-10 |
| Diseased bone marrow | Severe leukopenia, thrombo-cytopenia | 3-5 |
| 10 − 20Gy | ||
| Lens | Cataract | 6-12 |
| Bone marrow stem cells | Acute aplasia | 15-20 |
| 20 − 30Gy | ||
| Kidney: glomeruli | Arterionephrosclerosis | 23-38 |
| Lung: type 2 cells, vascular connective tissue stroma | Pneumonitis or fibrosis | 20-30 |
| 30 − 40Gy | ||
| Liver: central veins | Hepatopathy | 35-40 |
| Bone marrow | Hypoplasia | 25-35 |
| 40 − 50Gy | ||
| Heart (whole organ) | Pericarditis or pancarditis | 43-50 |
| Bone marrow micro-environments | Permanent aplasia | 45-50 |
| 60 − 70Gy | ||
| Brain | Encephalopathy | 60-70 |
| Mucosa | Ulcer | 65-75 |
| Rectum, bladder | Ulcer | 65-75 |
| Mature bones | Fracture | 65-70 |
| Pancreas | Pancreatitis | >70 |
Indian Scenario
In our country, there is a big divide in the facilities available in rural and urban areas. Presently only 337 teletherapy units are functional in the entire country. Based on a rate of nearly one machine per every 800 - 1000 new cases of cancer, the number of treatment units available at present is only about one fourth of the requirement. Approximately 500,000 patients need RT every year but only 1/3 of these estimated patients actually receive RT due to major shortfall in number of therapy units and urban-centric distribution of centres [1, 2]. The past decade has witnessed a dramatic improvement in the field of radiotherapy in some select urban oncocenters which are poised for a major leap towards technology oriented treatment delivery. However, the need of the hour in our country is to provide affordable and accessible diagnostic, therapeutic and palliative care services in most districts. Stress should be given on cancer prevention, community participation, involvement of non governmental organisations (NGOs), reducing the existent socio-demographic disparity in RT infrastructure, promoting sharing of resources across adjacent centers, development of indigenous telecobalt units/Linacs and use of teleconferencing between tertiary care centers and peripheral hospitals.
Conflicts of Interest
None identified
References
- 1.Dinshaw KA, Shastri SS, Patil SS. Cancer control programme in India: Challenges in new millennium. Health Administrator. 2004;17:10–13. [Google Scholar]
- 2.Biswas LN, Deb AR, Pal S. Radiation therapy: experience in Indian patients. J Indian Med Assoc. 2005;103:486–488. [PubMed] [Google Scholar]
- 3.Consolidated Report of the population Based Cancer Registries. ICMR; New Delhi: 2001. National Cancer Registry Programme. 1990-1996. [Google Scholar]
- 4.Amols HI. New technologies in radiation therapy: ensuring patient safety, radiation safety and regulatory issues in radiation oncology. Health Phys. 2008;95:658–665. doi: 10.1097/01.HP.0000326334.64242.46. [DOI] [PubMed] [Google Scholar]
- 5.Seibert JA. X-ray imaging physics for nuclear medicine technologists. Part 1: Basic principles of x-ray production. J Nucl Med Technol. 2004;32:139–147. [PubMed] [Google Scholar]
- 6.Joubert A, Foray N. Intrinsic radiosensitivity and DNA doublestrand breaks in human cells. Cancer Radiother. 2007;11:129–142. doi: 10.1016/j.canrad.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Fernet M, Hall J. Genetic biomarkers of therapeutic radiation sensitivity. DNA Repair. 2004;3:1237–1243. doi: 10.1016/j.dnarep.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Wilson GD. Cell kinetics. Clin Oncol. 2007;19:370–384. doi: 10.1016/j.clon.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Klein EE, Mamalui-Hunter M, Low DA. Delivery of modulated electron beams with conventional photon multi-leaf collimators. Phys Med Biol. 2009;54:327–339. doi: 10.1088/0031-9155/54/2/010. [DOI] [PubMed] [Google Scholar]
- 10.Van DS, Byram D, Bernshaw D. Brachytherapy for cancer of the cervix: an Australian and New Zealand survey of current treatment techniques. J Med Imaging Radiat Oncol. 2008;52:588–597. doi: 10.1111/j.1440-1673.2008.02025.x. [DOI] [PubMed] [Google Scholar]
- 11.Pearce A, Craighead P, Kay I, Traptow L, Doll C. Brachytherapy for carcinoma of the cervix: a Canadian survey of practice patterns in a changing era. Radiother Oncol. 2009;91:194–196. doi: 10.1016/j.radonc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Prabhakar R, Ganesh T, Rath GK, Julka PK, Sridhar PS, Joshi SC. Impact of different CT slice thickness on clinical target volume for 3D conformal radiation therapy. Med Dosim. 2009;34:36–41. doi: 10.1016/j.meddos.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Ding M, Newman F, Chen C, Stuhr K, Gaspar LE. Dosimetric comparison between 3DCRT and IMRT using different multileaf collimators in the treatment of brain tumours. Med Dosim. 2009;34:1–8. doi: 10.1016/j.meddos.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Webster GJ, Rowbottom CG, Mackay RI. Accuracy and precision of an IGRT solution. Med Dosim. 2009;34:99–106. doi: 10.1016/j.meddos.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Combs SE, Widmer V, Thilmann C. Stereotactic radiosurgery (SRS): treatment option for recurrent option for recurrent glioblastoma multi-forme. Cancer. 2005;104:2168–2173. doi: 10.1002/cncr.21429. [DOI] [PubMed] [Google Scholar]
- 16.Ahn YC, Lee KC, Kim DY. Fractionated stereotactic radiation therapy for extracranial head and neck tumours. Int J Radiat Oncol Biol Phys. 2000;48:501–505. doi: 10.1016/s0360-3016(00)00612-x. [DOI] [PubMed] [Google Scholar]
- 17.Dieterich S, Pawlicki T. Cyberknife image-guided delivery and quality assurance. Int J Radiat Oncol Biol Phys. 2008;71:S126–S130. doi: 10.1016/j.ijrobp.2007.08.081. [DOI] [PubMed] [Google Scholar]
- 18.Williams J, Chen Y, Rubin P, Finkelstein J, Okunieff P. The biological basis of a comprehensive grading system for the adverse effects of cancer treatment. Acute and late effects. Semin Radiat Oncol. 2003;13:182–188. doi: 10.1016/S1053-4296(03)00045-6. [DOI] [PubMed] [Google Scholar]


