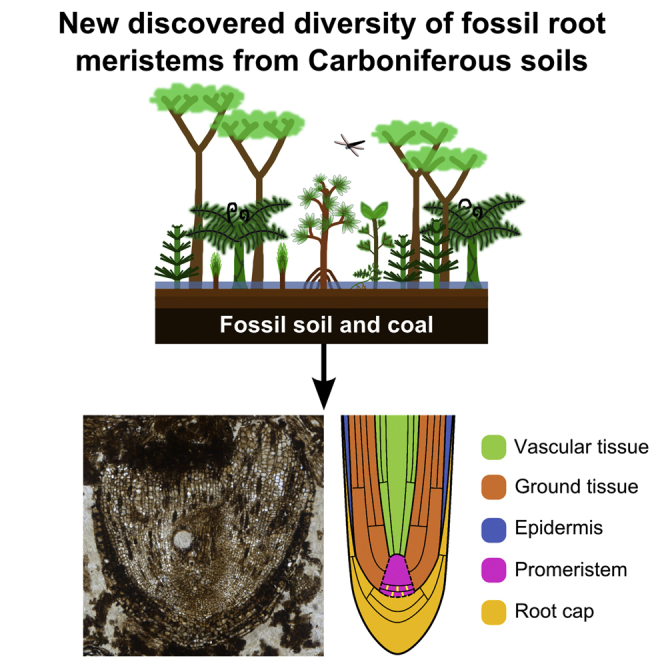
Summary
Roots and shoots of plant bodies develop from meristems—cell populations that self-renew and produce cells that undergo differentiation—located at the apices of axes [1].The oldest preserved root apices in which cellular anatomy can be imaged are found in nodules of permineralized fossil soils called coal balls [2], which formed in the Carboniferous coal swamp forests over 300 million years ago [3, 4, 5, 6, 7, 8, 9]. However, no fossil root apices described to date were actively growing at the time of preservation [3, 4, 5, 6, 7, 8, 9, 10]. Because the cellular organization of meristems changes when root growth stops, it has been impossible to compare cellular dynamics as stem cells transition to differentiated cells in extinct and extant taxa [11]. We predicted that meristems of actively growing roots would be preserved in coal balls. Here we report the discovery of the first fossilized remains of an actively growing root meristem from permineralized Carboniferous soil with detail of the stem cells and differentiating cells preserved. The cellular organization of the meristem is unique. The position of the Körper-Kappe boundary, discrete root cap, and presence of many anticlinal cell divisions within a broad promeristem distinguish it from all other known root meristems. This discovery is important because it demonstrates that the same general cellular dynamics are conserved between the oldest extinct and extant root meristems. However, its unique cellular organization demonstrates that extant root meristem organization and development represents only a subset of the diversity that has existed since roots first evolved.
Graphical Abstract
Highlights
-
•
The oldest fossilized root meristem is described from >300-million-year-old soil
-
•
The discovery allows the first description of a fossilized root stem cell niche
-
•
The cellular organization and therefore development of the meristem is unique
-
•
The discovery reveals previously unknown diversity in plant meristem types
Hetherington et al. report the discovery of the oldest fossilized remains of an actively growing root meristem from Carboniferous (>300-million-year-old) soil. The cellular organization of stem cells and differentiating cells is unique. This discovery reveals new but now extinct meristem diversity in Carboniferous plants.
Results and Discussion
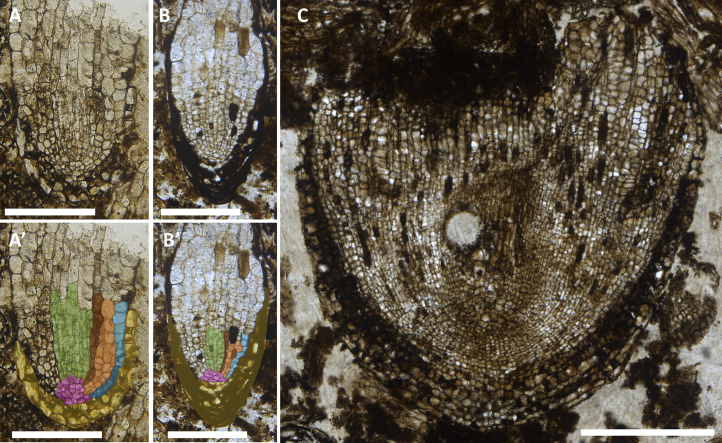
To characterize cellular development in the oldest root apices [3, 4, 5, 6, 7], we inspected 139 thin sections made from Carboniferous coal balls from Britain (see Supplemental Information). We identified two new apices (Figures 1A and 1C). The presence of root caps covering each demonstrated that they were root apices. The first apex was the tip of a differentiated, non-growing root (Figure 1A). It was designated Apex 76.1 and tentatively assigned to Lyginopteris oldhamia on the basis of cellular organization (Figures 1A and 1B [3]; see Supplemental Information). Finding Apex 76.1 validated our search for root meristems in this coal ball material. The second apex (Figure 1C) was larger, blunt, and represents an entirely new root apex type; it was named Radix carbonica (see Supplemental Information for systematic paleobotany and comparisons with other fossil apices).
Figure 1.
Two New Fossil Root Apices from the Carboniferous Period
(A and A′) Thin section 76, shown by permission of the Oxford University Herbaria. (A) Apex 76.1 tentatively assigned to Lyginopteris oldhamia. (A′) Apex 76.1 is shown with the main tissue types color coded (yellow, root cap; blue, epidermis; pink, differentiated cells at the position of the promeristem; orange, cortex; brown, endodermis; and green, procambium).
(B and B′) Thin section R646, shown courtesy of the Manchester Museum, The University of Manchester. (B) Lyginopteris oldhamia root apex discovered by Stopes and Watson [3]. (B′) L. oldhamia is shown overlaid with colors to represent the major tissues types as shown in (A′).
(C) Thin section 81, shown by permission of the Oxford University Herbaria. Holotype of the root apex of Radix carbonica (produced by the assembly of a series of continuous images of the root apex).
Scale bars, 200 μm (A and B) and 500 μm (C).
The cellular organization of Apex 76.1 and R. carbonica can be compared with root meristems of extant species, because both thin sections are near median longitudinal in orientation. However, meristem organization of extant plants can be investigated only in actively growing roots, because meristem structure changes when root growth stops [11]. It is therefore essential to establish if the root apices were fossilized during active growth. In roots that have stopped growing, differentiated tissues, including thickened xylem cells, are found very close to the promeristem as in the fossil meristems of Apex 76.1, Lyginopteris, Amyelon, and Psaronius, (Figures 1A and 1B) [3, 4, 6, 7, 9], a feature not found in actively growing roots [11]. By contrast, there were no distinguishable tissue types within the ground tissues or differentiated vascular cells found near the tip of R. carbonica (Figure 1C).
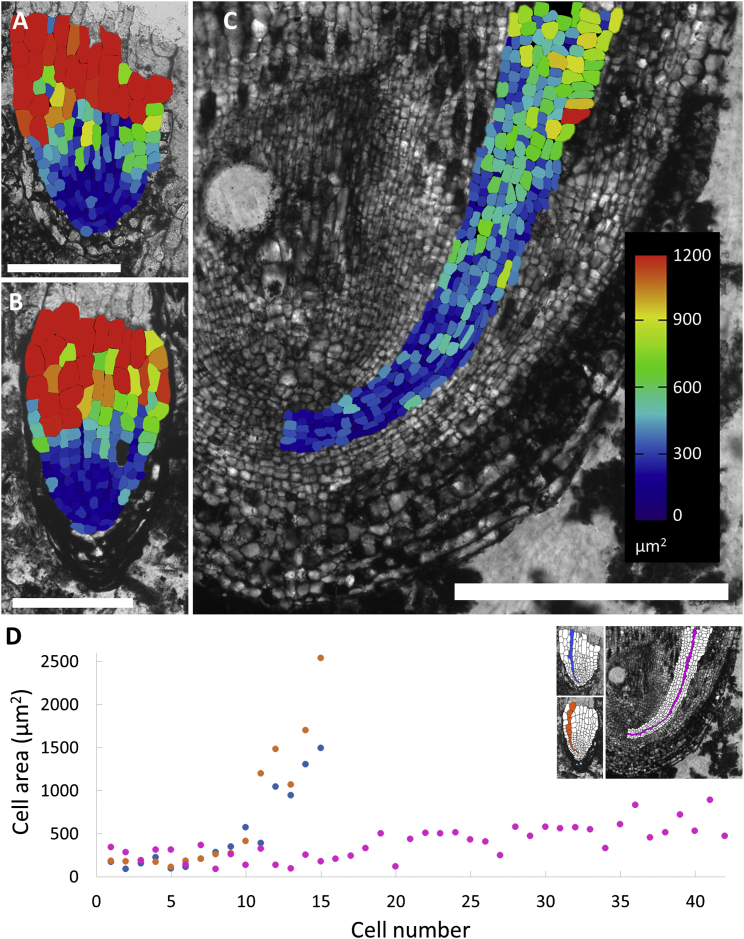
There is clear zonation of cell sizes in active root meristems of growing roots; meristematic cells are relatively small and vary in size by ∼2-fold as dividing cells go through the cell cycle. Then cells expand as they differentiate. Consequently, there is a gradient from the smaller cells of the promeristem to the larger cells in the differentiating tissues. In contrast, there is no cell size gradient in inactive meristems where cell size abruptly increases from the small inactive initials, which abut much larger differentiated cells close to the apex (Figures 2A, 2B, and 2D).The distribution of cell areas in the differentiating ground tissues of R. carbonica indicated that there was a gradual increase and a roughly 2-fold difference in cell area (see heatmap in Figures 2C and 2D; there is a 2-fold difference in cell size between blue [<300 μm2] and turquoise cells [300–600 μm2] throughout the majority of the body of the root), typical of actively growing root apices. These data indicate that R. carbonica is the first and only example of a root fossilized during active growth, which has preserved the cellular organization of the meristem.
Figure 2.
R. carbonica Is the First Fossil of an Active Meristem in a Growing Root
(A–C) Cell surface area heat plots show (A) Apex 76.1 (Figure 1A), (B) L. oldhamia [3] (Figure 1B), and (C) R. carbonica (Figure 1C). Scale bars, 200 μm (A and B) and 500 μm (C).
(D) Cell area increase along a single-cell file in Apex 76.1 (blue), L. oldhamia [3] (orange), and R. carbonica (pink). Note the gradual increase in cell size within the ground tissue of R. carbonica compared to Apex 76.1 and L. oldhamia.
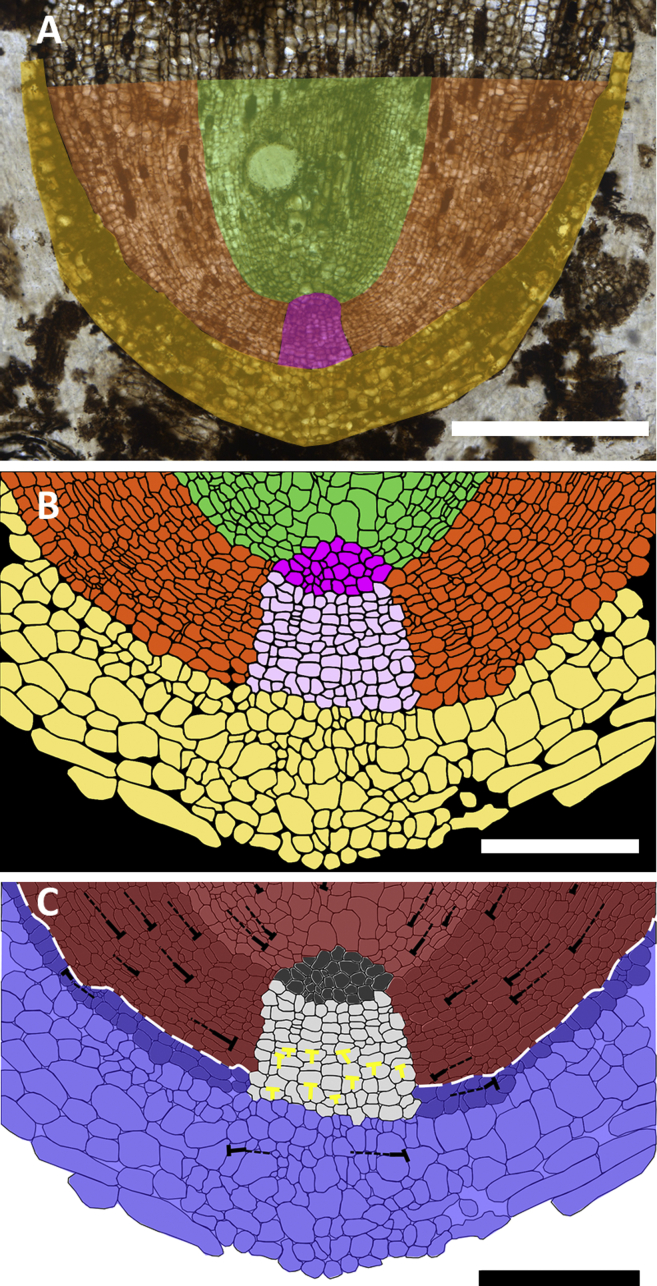
Comparison of the cellular organization of the different regions of the root apex indicates that the cellular dynamics in R. carbonica conform to that observed in extant root meristems. The root apices of all extant roots are covered by a protective cap. Root caps are typically tapered (they are thinner in proximal positions than in distal positions) because older cell layers are sloughed off as the root grows through the soil. R. carbonica is covered by a protective root cap that tapers rapidly, indicating that cells were sloughing, typical of roots of extant species [1] (Figure 3A, yellow). The promeristem is the group of cells in a growing root that gives rise to all tissues [1], and it is identified in R. carbonica as the region where the files of cells of fundamental tissues converge (Figure 3A, pink). The R. carbonica promeristem is large, consisting of 138 cells arranged in 10–15 tiers when imaged in the longitudinal plane of section (Figures 3A, pink, and 3B, pink and lilac). It comprises two morphologically distinct pools of initials (Figure 3B, pink and lilac) (see Supplemental Information), and initials give rise to many mature cell files, meaning that discrete initials for each cell layer do not exist. The R. carbonica promeristem is different from all extant vascular plant root meristems (see Supplemental Information), because of its large size and the spatial organization of cells that are arranged in more than ten tiers of initials. The distal promeristem (Figure 3B, lilac) of R. carbonica takes the form of a regular block of cells; a similar organization of the promeristem is found in almost all extant gymnosperms [12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23]. However, the structure of R. carbonica differs from that of extant gymnosperm root meristems in two ways.
Figure 3.
R. carbonica Has a Unique Cellular Organization
(A) R. carbonica (Figure 1C) is overlaid with colors representing the four major tissues found within the apex (yellow, root cap; pink, promeristem; orange, ground tissues and epidermis; and green, procambium).
(B) Line drawing of the apical portion of the R. carbonica holotype (Figure 1C) is color coded to represent the major tissue types as in (A), except the promeristem is further divided (pink, proximal promeristem; and lilac, distal columella-like promeristem).
(C) Same line drawing as in (B) (blue, Kappe complex; red, Körper complex; and gray, promeristem). Black Ts indicate T-divisions in both complexes with dashed lines showing the cell files that make the vertical stroke of the T. White dashed line marks the boundary between the Körper-Kappe complexes. Yellow Ts mark positions of anticlinal cell divisions within the central columella-like region of the promeristem.
Scale bars, 500 μm (A) and 200 μm (B and C).
The first feature that distinguishes R. carbonica from extant gymnosperm root meristems is the discrete root cap that is not continuous with the distal promeristem in R. carbonica (Figure 3B, lilac). In extant gymnosperms, it is not possible to distinguish a boundary between the promeristem and the root cap [12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23]. However, in R. carbonica, the promeristem is broad and not continuous with the root cap, which is discrete from other tissues. Furthermore, within this broad promeristem there are large numbers of anticlinal cell divisions (marked in yellow on Figure 3C), which lead to the loss of the columnar organization of cell files between the promeristem and the cap. While some gymnosperm promeristems are columellar [14, 15, 16, 17, 18, 19, 20, 21], and anticlinal division occurs in the promeristem of others [19, 20, 22, 23], numerous anticlinal divisions within a columellar promeristem have not been described in any species. No similar organization with broad promeristem and discrete root cap has been described in any root meristem to date (see Supplemental Information).
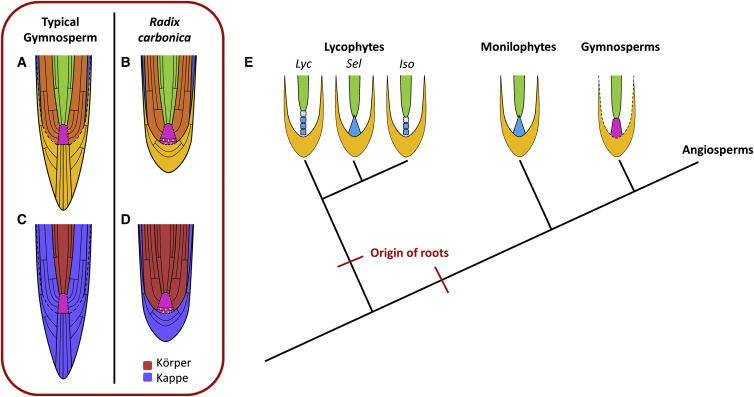
The second feature that marks R. carbonica as distinct from the meristems of extant gymnosperms is the position of the Körper-Kappe boundary [1, 13, 24, 25] (see Supplemental Information for an extended description of the Körper-Kappe theory). The boundary between the Körper and Kappe complex is a highly conserved feature of all extant gymnosperms. In gymnosperms the Körper complex contains the vascular tissue and, in some cases, a small number of layers of the ground tissues [13, 14, 15, 16] (Figures 4A and 4C, red). The Kappe complex, on the other hand, makes up the majority of the tissues (remainder of the ground tissues, epidermis, and root cap) of the root meristem [13, 14, 15, 16] (Figures 4A and 4C, blue). The Körper-Kappe boundary is, therefore, located very close to the junction between the provascular tissues and the ground tissue (Figures 4A and 4C). However, the Körper-Kappe boundary is markedly different in R. carbonica. The R. carbonica Körper complex constitutes the stele and almost all the ground tissue (Figure 3C, red; Figure 4D, red). The Kappe complex (Figure 3C, blue shading; Figure 4D, blue) comprises the root cap and the cell file abutting the root cap interpreted as the epidermis. Therefore, the position of the Körper-Kappe boundary of R. carbonica is structurally different from all extant gymnosperm root meristems (Figure 4).
Figure 4.
R. carbonica Is Distinct from All Extant Root Meristems
Schematic diagrams show the cellular organization of a typical gymnosperm (A and C) and Radix carbonica meristem (B and D).
(A and B) Schematics are color coded for the major tissue types within the meristem (yellow, root cap; pink, promeristem [yellow lines in the R. carbonica promeristem indicate the positions of anticlinal cell divisions within the promeristem]; orange, ground tissue; blue, epidermis; and green, procambium).
(C and D) Same schematics as in (A) and (B) but color coded to mark the position of the Körper- (red) Kappe (blue) complexes. Note the difference in the Körper-Kappe boundary between R. carbonica (D) and the gymnosperm meristem (C).
(E) A simplified vascular plant cladogram [26], showing the two hypothesized origins of roots [27], and schematics of lycophyte, monilophyte, and gymnosperm root meristems. Lyc stands for the Lycopodiales that typically have multicellular promeristems consisting of either three or four tiers of initials. Sel stands for the Selaginellales that typically have a single initial cell (apical cell). Iso stands for the Isoetales that typically have multicellular promeristems consisting of either two or three tiers of initials. Monilophyte root meristems typically have a single initial cell (apical cell). Gymnosperm root meristems have multicellular promeristems consisting of a zone of common initials for all tissues, or common initials for all non-vascular tissues and a separate set for all vascular tissues.
For a detailed review of meristem types, see Supplemental Information.
R. carbonica is the only root meristem that has been preserved in which the patterns of cell division in the active apex can be elucidated. This allowed us for the first time to compare the organization of cells in the promeristem of an extinct Carboniferous root with the organization of cells in root meristems of extant plants. The organization of stem cells and differentiating cells suggests that the same general cellular dynamics in the self-renewing populations and their derivatives occurred in R. carbonica as in extant root meristems. However, the discrete root cap, zone of anticlinal cell divisions in the group of columella-like promeristem, and the position of the Körper-Kappe boundary mark R. carbonica as structurally distinct from all other previously described meristems (Figure 4).
Using the organization of cells in the promeristem and the meristem as criteria, Schüepp [13] identified nine classes of vascular plant root meristems. There were five classes of meristems in non-angiosperm tracheophytes (lycophytes, monilophytes, and gymnosperms [26]) combined. He identified seven classes in angiosperms, of which four were angiosperm specific. The evolution of novel meristem types [13, 28, 29] in angiosperms has, therefore, been associated with their rise to dominance. The discovery of the organization of stem cells and their derivatives in R. carbonica demonstrates that the diversity of developmentally distinct root meristem types that existed before the origin of angiosperms [27] (Figure 4E) but are now extinct was greater than previously described. It also shows that extant root meristem organization represents a subset of the diversity that has existed since roots first evolved.
Experimental Procedures
The 139 thin sections of Carboniferous coal balls from the Oxford University Herbaria and the University of Oxford Natural History Museum were inspected for root meristems. The original L. oldhamia root apex described by Stopes and Watson [3] and Weiss [7] also was re-examined courtesy of the Manchester Museum, The University of Manchester (Thin section R646). Meristems were imaged with an Olympus BX50 microscope and quantitatively characterized using Fiji [30]. To quantitatively characterize the cell shape, cell area, and cell division pattern of R. carbonica, a line drawing was made of the 988 cells that constitute the distal portion of the apex (Figure 2C) and the 405 cells representing the development of the ground tissues (Figures 3B and 3C). Line drawings also were made of the cells in the distal portion of Apex 76.1 and L.oldhamia apices (Figures 2A and 2B).
Author Contributions
A.J.H. and L.D. designed the project. A.J.H. carried out the analyses with assistance from J.G.D. A.J.H., J.G.D., and L.D. wrote the manuscript.
Acknowledgments
A.J.H. was funded by a Doctoral Training Partnership Scholarship from the Biotechnology and Biological Research Council (BB/J014427/1). J.G.D. was funded by the Mexican Scientific and Technological Council (CONACyT grant 206843) and DGAPA-PASPA-UNAM for sabbatical support. This research was funded by a European Research Council advanced award (EVO-500) to L.D. We are grateful to the Oxford University Museum of Natural History, Oxford University Herbaria, the University of Manchester, Manchester Museum, Dr. N.J. Hetherington, and Mrs. C. Kirchhelle (Oxford University) for technical assistance. We are grateful to Professor P. Donoghue, Professor A.M. Hetherington, Dr. C.J. Harrison, Dr. C.M. Berry, and Dr. V.A.S. Jones for helpful discussions. L.D. is grateful to Ms. I. Marston for insights early in this project. We are grateful to A. Tomescu and two anonymous reviewers for insightful comments on the manuscript.
Published: June 2, 2016
Footnotes
Supplemental Information includes a Supplemental Discussion and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2016.04.072.
Supplemental Information
References
- 1.Clowes F.A.L. Blackwell; 1961. Apical Meristems. [Google Scholar]
- 2.Scott A.C., Rex G. The formation and significance of Carboniferous coal balls. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985;311:123–137. [Google Scholar]
- 3.Stopes M.C., Watson D.M.S. On the present distribution and origin of the calcareous concretions in coal seams, known as “Coal Balls.”. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1909;200:262–273. [Google Scholar]
- 4.Halket A.C. The rootlets of “Amyelon radicans”, Will.; their anatomy, their apices and their endophytic fungus. Ann. Bot. 1930;44:865–905. [Google Scholar]
- 5.Halket A.C. A note on the origin of lateral roots and the structure of the root-apex of Lyginopteris oldhamia. New Phytol. 1932;31:279–283. [Google Scholar]
- 6.Osborn T.G.B. The lateral roots of Amyelon radicans, Will., and their mycorhiza. Ann. Bot. 1909;23:603–611. [Google Scholar]
- 7.Weiss F.E. The root-apex and young root of Lyginodendron. Mem. Proc. Manch. Lit. Philos. Soc. 1913;57:1–8. [Google Scholar]
- 8.Dennis R.L. A developmental study of roots of presumed seed fern origin from the upper Pennsylvanian of Illinois. Trans. Ill. State Acad. Sci. 1969;61:146–156. [Google Scholar]
- 9.Ehret D.L., Phillips T.L. Psaronius root systems – morphology and development. Palaeontographica Abteilung B. 1977;161:147–164. [Google Scholar]
- 10.Strullu-Derrien C., McLoughlin S., Philippe M., Mørk A., Strullu D.G. Arthropod interactions with bennettitalean roots in a Triassic permineralized peat from Hopen, Svalbard Archipelago (Arctic) Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012;348–349:45–58. [Google Scholar]
- 11.Shishkova S., Rost T.L., Dubrovsky J.G. Determinate root growth and meristem maintenance in angiosperms. Ann. Bot. 2008;101:319–340. doi: 10.1093/aob/mcm251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janczewski E.D. Recherches sur l’accroisement terminal des racines dans les Phanerogames. Ann. Des. Sci. Nat. Bot. Ser. 5. 1874;20:162–201. [Google Scholar]
- 13.Schüepp O. Band 4. Gebrüder Borntraeger; 1926. (Meristeme. Handbuch der Pflanzenanatomie). [Google Scholar]
- 14.Pillai A. Root apical organization in gymnosperms—some cycads and Ginkgo biloba. Proc. Indian Acad. Sci. B. 1963;57:211–222. [Google Scholar]
- 15.Pillai A. Root apical organization in gymnosperms—some conifers. Bull. Torrey Bot. Club. 1964;91:1–13. [Google Scholar]
- 16.Pillai A. Root apical organization in gymnosperms: root apex of Ephedra foliata, with a suggestion on the possible evolutionary trend of root apical structures in gymnosperms. Planta. 1966;70:26–33. doi: 10.1007/BF00539907. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox H. Growth studies of the root of incense cedar, Libocedrus decurrens. I. The origin and development of primary tissues. Am. J. Bot. 1962;49:221–236. [Google Scholar]
- 18.Allen G.S. Embryogeny and the development of the apical meristoms of Pseudotsuga; late embryogeny. Am. J. Bot. 1947;34:73–80. [PubMed] [Google Scholar]
- 19.von Guttenberg H. Band 8, Teil 4. Gebrüder Borntraeger; 1961. (Grundzüge der Histogenese höherer Pflanzen. II. Die Gymnospermen. Handbuch der Pflanzenanatomie). [Google Scholar]
- 20.Milindasuta B.-E. Developmental anatomy of coralloid roots in cycads. Am. J. Bot. 1975;62:468–472. [Google Scholar]
- 21.Bogar G.D., Smith F.H. Anatomy of seedling roots of Pseudotsuga menziesii. Am. J. Bot. 1965;52:720–729. [Google Scholar]
- 22.Voronin N.S. Evolution of the primary structures in plant roots. Proc. State Pedagog. Inst. Kaluga. 1964;13:3–179. [Google Scholar]
- 23.Voronin N.S. Apical meristems of the root in gymnosperms and the principles of their graphical interpretation. Bot. Zhur Moscow. 1969;54:67–76. [Google Scholar]
- 24.Schüepp O. Untersuchungen über Wachstum und Formwechsel von Vegetationspunkten. Jb. Wiss. Bot. 1917;57:17–79. [Google Scholar]
- 25.Evert R. Third Edition. John Wiley & Sons; 2006. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development. [Google Scholar]
- 26.Qiu Y.-L., Li L., Wang B., Chen Z., Knoop V., Groth-Malonek M., Dombrovska O., Lee J., Kent L., Rest J. The deepest divergences in land plants inferred from phylogenomic evidence. Proc. Natl. Acad. Sci. USA. 2006;103:15511–15516. doi: 10.1073/pnas.0603335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raven J.A., Edwards D. Roots: evolutionary origins and biogeochemical significance. J. Exp. Bot. 2001;52:381–401. doi: 10.1093/jexbot/52.suppl_1.381. [DOI] [PubMed] [Google Scholar]
- 28.Heimsch C., Seago J.L., Jr. Organization of the root apical meristem in angiosperms. Am. J. Bot. 2008;95:1–21. doi: 10.3732/ajb.95.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Groot E.P., Doyle J.A., Nichol S.A., Rost T.L. Phylogenetic distribution and evolution of root apical meristem organization in dicotyledonous angiosperms. Int. J. Plant Sci. 2004;165:97–105. [Google Scholar]
- 30.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.