Summary
Streptophytes colonized the land some time before 470 million years ago [1, 2, 3]. The colonization coincided with an increase in morphological and cellular diversity [4, 5, 6, 7]. This increase in diversity is correlated with a proliferation in transcription factors encoded in genomes [8, 9, 10]. This suggests that gene duplication and subsequent diversification of function was instrumental in the generation of land plant diversity. Here, we investigate the diversification of the streptophyte-specific Lotus japonicus ROOTHAIRLESS LIKE (LRL) transcription factor (TF) [11, 12] subfamily of basic loop helix (bHLH) proteins by comparing gene function in early divergent and derived land plant species. We report that the single Marchantia polymorpha LRL gene acts as a general growth regulator required for rhizoid development, a function that has been partially conserved throughout multicellular streptophytes. In contrast, the five relatively derived Arabidopsis thaliana LRL genes comprise two antagonistically acting groups of differentially expressed genes. The diversification of LRL genes accompanied the evolution of an antagonistic regulatory element controlling root hair development.
Highlights
-
•
The LRL bHLH transcription factor family diversified during land plant evolution
-
•
Expression of individual LRL genes was gradually restricted to specific domains
-
•
In derived lineages, there are two sets of antagonistically acting LRL genes
-
•
LRL gene function is partially conserved between streptophyte algae and angiosperms
Breuninger et al. show that the LRL transcription factor family of angiosperms is derived from a single-copy gene in early diverging plant lineages. The single-copy MpLRL gene acts as a general growth regulator in liverworts. LRL function diversified into two antagonistically acting groups of proteins active in root hair development in angiosperms.
Results and Discussion
LRL Gene Number Increased in Land Plants
To identify LRL-related (group XI basic helix loop helix) gene sequences from early diverging land plants and streptophyte algae, we performed BLAST searches on Marchantia polymorpha and Chara braunii sequences using Arabidopsis thaliana AtLRL sequences as queries. Single transcripts, Cb_bHLHtranscript1 (GenBank accession number KX037431) and Mp_bHLHtranscript1 (KX037432), were identified in each species encoding proteins with basic loop helix (bHLH) and LRL domains similar to those found in PpLRL1 of Physcomitrella patens and AtLRL3 of A. thaliana (Figure S1) and were therefore designated MpLRL and CbLRL, respectively. These genes are most likely single-copy genes in C. braunii and M. polymorpha because a single band was detected in Southern blots of gDNA hybridized with a probe containing the LRL domain sequence (Figure S1). In flowering land plants, LRL transcription factors (TFs) form three well-supported clades (XIa, XIb, and XIc, termed here class I, II, and III [12]). Comparison of MpLRL with other LRL sequences demonstrated that MpLRL and PpLRL are placed outside these three clades suggesting that LRL TFs diversified after the divergence of bryophytes and flowering plants (Figure S1). The CbLRL is placed among the class I LRL sequences on a long branch (Figure S1). No LRL-related sequences were identified in Chlamydomonas reinhardtii or Ostreococcus tauri or in the filamentous streptophyte Klebsomidium flaccidum suggesting that LRL TFs evolved among complex streptophyte algae. Taken together, these data indicate that the LRL proteins constitute a highly conserved transcription factor family, which diversified during streptophyte evolution from a most likely single-copy gene in the most ancestral groups of Charales and liverworts to a gene family with several members belonging to different monophyletic clades in flowering plants.
LRL Gene Expression Is Restricted to Specific Domains in Arabidopsis
To assess whether diversification of the LRL gene family was accompanied by changes in gene expression, we compared expression patterns of LRL genes in C. braunii, M. polymorpha and A. thaliana. In situ hybridization in C. braunii showed that LRL mRNA was present in most tissues of the plant; CbLRL mRNA was detected in the nodes, internodes, and the oogonium surrounding the egg cell and zygotes (Figures 1A, 1G, and S1). In M. polymorpha, highest levels of MpLRL mRNA were detected in the meristematic notch by in situ hybridization (Figures 1B, 1C, and S1). Highest fluorescence levels in the meristematic notch was observed in plants transformed with proMpLRL:NLS-3xYFP (Figures 1D, 1H, and 1I) indicating that the promoter was most strongly active in the same cells in which the mRNA was detected. Lower levels of YFP fluorescence were detected throughout the thallus (Figures 1E, 1J, 1F, 1K, and S1). The relatively high expression in the notch and lower expression in the thallus is different from what is observed with ubiquitously expressed reporters (compare Figure 1D with Figure S1J), indicating that the MpLRL expression heterogeneity is not caused by differences in cell densities along the thallus. Together, these data indicate that MpLRL is more highly expressed in the vicinity of the meristem than elsewhere in the thallus. This preferential expression in some domains is also observed in another early diverging land plant, the moss P. patens [12].
Figure 1.
The LRL Expression Pattern Diversifies into Specific Domains
(A and G) Whole-mount in situ of CbLRL. Gene expression was detected in all tissue types analyzed. (A) Part of thallus consisting of a main axis and whorled branchlets with reproductive organs and oogonium with unfertilized egg cell. (G) Fertilized zygote surrounded by oogonial tube cells. Scale bars, 1 mm (A) and 100 μm (G).
(B and C) Whole-mount in situ hybridization of MpLRL. Strongest gene expression was detected in meristematic tissues. (B) Dorsal view; (C) ventral view. Scale bars, 100 μm.
(D and I) Dorsal view of a M. polymorpha thallus expressing proMpLRL:NLS-3xYFP. (D) YFP fluorescence of dorsal thallus. (I) Overlay of bright-field and YFP fluorescence. Note the higher fluorescence signal in the meristematic notch. Scale bars, 5 mm.
(E, F, J, and K) Close-up view of a meristematic notch in M. polymorpha expressing proMpLRL:NLS-3xYFP on dorsal and ventral side, respectively. (E and F) YFP signal only and (J and K) showing the overlay of YFP fluorescence and bright field. Note the relatively weaker signal outside of the meristematic notch. Scale bars, 500 μm.
(H) Overlay of bright-field image and YFP fluorescence of M. polymorpha gemma expressing proMpLRL:NLS-3xYFP. Scale bar, 100 μm.
(L–P″) Confocal images of proAtLRL1 (L), proAtLRL2 (M), proAtLRL3 (N), proAtLRL4 (O), and proAtLRL5 (P) promoter fusions to NLS-3xYFP within the root hair zone (RHZ), elongation zone (EZ; L′–P′), and the root apical meristem (RAM; L″–P″). VS, vertical section; HS, horizontal section.
(Q) Root hair expression of AtLRL genes. Only the promoter of AtLRL4 was not detected in root hair cells (RH).
(R) Table summarizing AtLRL expression domains in the root. + and – indicate expression with –, negative; +, positive; ++, strong. E, epidermis; RH, root hair; C, cortex; En, endodoermis; P, pericylcle; S, stele.
See also Figures S1 and S2.
We determined the spatial expression pattern of each of the five A. thaliana LRL genes using promoter:YFP fusions. The fluorescence patterns revealed that each of the promoters is active in distinct though overlapping domains. Summed together these expression domains include cells of most tissue types (Figure S2; summary in Figure 1R). In the root, the differences between the AtLRL gene expression patterns were most pronounced in the root apical meristem (RAM) and elongation zone (EZ; Figures 1L′–1P″). The promoters of AtLRL1 and AtLRL4 were active early in the development of the provascular tissue and endodermis, respectively (Figures 1L″, 1L′, O″, and O′). proAtLRL3 was active in every cell and tissue (Figures 1N″ and 1N′); neither proAtLRL2 nor proAtLRL5 were active in the RAM (Figures 1M″ and 1P″). Later, in the root-hair zone (RHZ), proAtLRL1 and proAtLRL3 were active in every cell, while the proAtLRL2 was active in most cells except pericycle and endodermis (Figures 1L–1N and 1Q). proAtLRL4 was active in all tissues except the epidermis in the root hair zone, whereas proAtLRL5 activity was detected in all tissues (Figures 1O–1Q). Taken together, these data indicate that single-copy LRL genes are broadly expressed in C. braunii and M. polymorpha plants, while the different A. thaliana LRL genes are expressed in distinct but overlapping domains. This suggests that LRL genes function throughout the plant in streptophyte algae and became progressively more restricted to specific tissues in land plants. In derived land plant taxa, multiple LRL genes are expressed in distinct domains, suggesting that different genes have distinct though overlapping functions.
MpLRL Function Is Required for Dorsal Thallus and Rhizoid Development
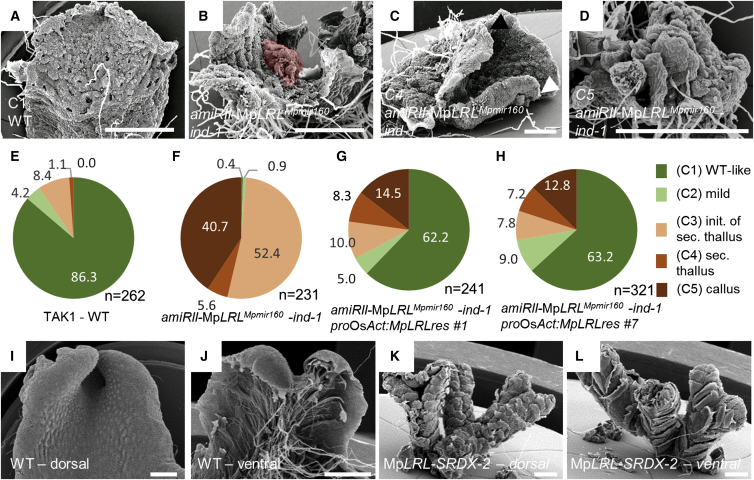
To investigate whether changes in gene expression patterns reflect changes in gene function, we compared M. polymorpha and A. thaliana LRL gene function. We generated two different inducible artificial microRNA (amiR) constructs to reduce MpLRL activity [13] using the ethanol inducible AlcR/AlcA system [14] in which the induced expression could be monitored with an inducible AlcA:NLS-3xCFP reporter on the same T-DNA. This construct, AlcA:amiR-MpLRLMpmir160-AlcA:NLS-3xCFP-proOsAct:AlcR (the expression pattern of proOsAct is shown in Figure S1), was transformed and two lines for each amiR-MpLRLMpmir160 sequence were established—amiRI-MpLRLMpmir160-ind-1 and amiRI-MpLRLMpmir160-ind-2; amiRII-MpLRLMpmir160-ind-1 and amiRII-MpLRLMpmir160-ind-2. Each line expressed CFP and developed a defective phenotype only upon ethanol induction (20% ethanol vapor; Figure S3). Induction of the expression of the amiR-MpLRLMpmir160 microRNAs in these lines caused growth defects in gemmae—vegetative propagules of M. polymorpha—which we classified into five categories (C1–C5): (C1) wild-type like plants; (C2) plants with undifferentiated patches of tissue outgrowth on the dorsal thallus; (C3) plants with larger outgrowths on the dorsal thallus indicating the initiation of secondary, ectopic thallus (Figure 2B); (C4) plants with a fully grown ectopic secondary thallus (Figure 2C); and (C5) the entire dorsal side of the plant was covered with rhizoid-less callus like tissue (Figure 2D). Using these categories, we quantified the phenotypic variation in one amiRI-MpLRLMpmir160-ind-1 and one amiRII-MpLRLMpmir160-ind-1 line. Both lines developed a significant number of callus-like phenotypes (C5 phenotype), but the frequency of the phenotypes depended on the amiR-MpLRLMpmir160 sequences used (Figure S3). amiRI-MpLRLMpmir160-ind-1 plants generally form secondary thalli (C4 phenotype), while up to 46.9% of meristems in lines carrying the amiRII-MpLRLMpmir160-ind-1 construct developed callus like tissue (C5 phenotype). All defects could be complemented by double-transforming the amiRII-MpLRLMpmir160-ind-1 line with an amiRII-MpLRLMpmir160-resistant MpLRL cDNA, MpLRLres, driven by the constitutive proOsAct promoter. The majority of meristems that developed on two independent MpLRL knockdown lines transformed with the MpLRLres gene developed wild-type features (compare Figure 2F with Figures 2G and 2H). To independently determine the function of MpLRL, we fused the MpLRL cDNA to the EAR repressor domain SRDX [15, 16] and expressed this fusion under the control of proOsAct promoter. The growth of the dorsal thallus of proOsAct:MpLRL-SRDX plants was severely impaired, resulting in the development of plants without dorsal characteristics and prominent ventral scales without rhizoids (Figures 2K, 2L, and S3). Severe morphological defects developed in 2-week-old gemma expressing the MpLRL-SRDX fusion using an ethanol inducible MpLRL-SRDX gene construct (compare MpLRL-SRDXind-1 and MpLRL-SRDXind-2 in Figure S3). These plants were morphologically similar to the severe C4 and C5 phenotypes observed in amiRII-MpLRLMpmir160-ind-1 lines. Taken together, the absence of rhizoids in the most severe phenotypes of the amiR-MpLRLMpmir160 and MpLRL-SRDX lines indicates that MpLRL positively regulates rhizoid development. These results indicate that MpLRL is a general growth regulator and required for dorsal development in gemmae.
Figure 2.
MpLRL Is a Growth Regulator Required for Both Dorsal Thallus and Rhizoid Development
(A–D) Phenotypes of plants grown from gemmae transformed with the inducible MpLRLamiRMp160II transgene in inductive conditions compared to wild-type. (A) Wild-type plants with category (C1) phenotype, (B) plants with initiating outgrowth (colored patch) on the dorsal thallus indicative for an outgrowing thallus (C3), (C) plants with secondary thallus formed on the dorsal side (C4, arrowheads indicate meristems: white arrow, primary thallus; black arrow, secondary thallus), and (D) plants with only rhizoid-less callus like tissue developing on the dorsal thallus (C5).
(E and F) Quantification of meristematic phenotypes of MpLRLamiRII-ind-1 grown under inductive conditions. (E) Wild-type and (F) MpLRLamiRII-ind-1.
(G and H) Quantification of meristematic phenotypes of two independent double-transformants of proOsAct:MpLRLres in the MpLRLamiRII-ind-1. Transformant line #1 (G) and transformant line #7 (H). Note the large number of relatively wild-type-like meristems and the small number of the strong, category C5 phenotypes.
(I and J) Fully grown wild-type M. polymorpha thalli. (I) Dorsal view and (J) ventral view are shown.
(K and L) Fully grown M. polymorpha thalli transformed with the proOsAct:MpLRL-SRDX fusion. (K) Dorsal view; (L) ventral view. Note the lack of dorsal-like tissue types in (K) and the lack of rhizoids in (L).
Scale bars, 500 μm. See also Figure S3.
The Two Classes of AtLRL Genes Act Antagonistically
To define the function of LRL genes in angiosperms, we generated gain- and loss-of-function lines for each class of LRL genes in A. thaliana. Angiosperm class I LRL genes are positive regulators of root hair development [11, 12]; Atlrl1-2 Atlrl3-1 double mutants initiate root hairs that do not elongate (Figure 3B). Furthermore, longer root hairs develop in plants that constitutively overexpress AtLRL1, AtLRL2, or AtLRL3 (Figures 3D–3F, 3I, and S4); plants transformed with 35S:AtLRL1 or 35S:AtLRL3 developed root hairs that were 899 ± 70 (mean ± SD) μm and 823 ± 27 μm (Figure 3D, 3F, and 3I) and were longer than wild-type root hairs (474 ± 47 μm; Figure 3A). Mutants with reduced activity of class II LRL genes—Atlrl5-1T-DNA 35S:AtLRL4amiR-I or Atlrl5-1T-DNA 35S:AtLRL4amiR-II plants—were indistinguishable from wild-type despite a significant decrease in steady-state levels of AtLRL4 and AtLRL5 mRNA levels (Figures 3C and S4). However, root hairs did not develop on wild-type plants transformed with either 35S:AtLRL4 or 35S:AtLRL5 gene constructs demonstrating that AtLRL4 and AtLRL5 repress root hair elongation (Figures 3G, 3H, and 3I). We conclude that class I and class II LRL genes have antagonistic functions in the regulation of root hair development.
Figure 3.
AtLRL Class I and Class II Act Antagonistically
(A–C) Root hair development in AtLRL loss-of-function mutants. (A) Wild-type root hair growth, (B) Atlrl1-2; Atlrl3-1 double mutant (class I AtLRL mutant) with severe root hair growth defects, (C) Atlrl5-1; 35S:AtLRL4amiRI double mutant (class II AtLRL mutant), which is morphologically indistinguishable from wild-type.
(D–H) Root hair development in AtLRL gain-of-function mutants. (D–F) Class I overexpression leads to increased root hair elongation. (D) 35S:AtLRL1, (E) 35S:AtLRL2, (F) 35S:AtLRL3. (G and H) Class II overexpression leads to decreased root hair elongation. (G) 35S:AtLRL4 and (H) 35S:AtLRL5.
(I) Quantification of root hair length in LRL-overexpressing lines. Student’s t test p values <0.05 are marked with asterisks.
(J) qPCR analysis of class I AtLRL gene expression in two independent 35:AtLRL4 and 35S:AtLRL5 transformants. Note the lower steady-state levels of AtLRL3 mRNA in both overexpressing lines and decreased steady state of AtLRL1 and AtLRL2 mRNA in AtLRL5-overexpressing lines.
Scale bars, 500 μm. See also Figure S4.
To test whether the class I and class II LRL genes reciprocally regulate each other’s expression, we determined the steady-state levels of all AtLRL mRNAs in lines overexpressing each of the LRL genes. We could not detect changes of AtLRL4 or AtLRL5 steady-state mRNA levels in plants that overexpress AtLRL1, AtLRL2, or AtLRL3 (Figure S4). By contrast, steady-state levels of AtLRL3 mRNA were significantly reduced in 35S:AtLRL4 and 35S:AtLRL5 backgrounds (Figures 3J and S4). These data suggest that class II LRL genes repress the expression of the class I AtLRL3 gene. We conclude that the two classes of AtLRL act antagonistically during root hair development and class II LRL genes have the potential to act as transcriptional repressors.
CbLRL Can Substitute for the Loss of LRL Gene Function in M. polymorpha
If LRL gene function was conserved after C. braunii and M. polymorpha diverged from a common ancestor, we predicted that expression of CbLRL would suppress the phenotypic defect caused by decreased MpLRL activity in Mplrl knockdown plants. We transformed the inducible MpLRL knockdown (amiRII-MpLRLMpmir160-ind-1) plant with CbLRL cDNA under the control of a constitutive promoter. While defective gemmae developed on 92.2% of the amiRII-MpLRLMpmir160-ind-1 lines in inducing conditions (Figure 4H), the majority of gemmae in amiRII-MpLRLMpmir160-ind-1; proOsAct:CbLRL plants were wild-type (67.9%; Figure 4J). This suggests that CbLRL can substitute for MpLRL function in M. polymorpha. In contrast, transformation of the amiRII-MpLRLMpmir160-ind-1 line with the proOsAct:NLS-YFP control construct did not restore wild-type development (1.7% developed wild-type phenotypes; Figure 4I). To determine whether relatively derived LRL genes can substitute for loss of MpLRL gene function, we expressed the AtLRL1 (class I) and AtLRL4 (class II) cDNAs using the proOsACT promoter in the background with decreased MpLRL activity. Only 27.3% and 29.7% of proOsAct:AtLRL1; amiRII-MpLRLMpmir160-ind-1 and proOsAct:AtLRL4; amiRII-MpLRLMpmir160-ind-1 lines developed wild-type gemma (Figures 4K and 4L). This indicates that the A. thaliana LRL genes cannot complement the defect in MpLRL loss-of-function lines as effectively as CbLRL. These data support the hypothesis that angiosperm LRL genes have functionally diverged since M. polymorpha and A. thaliana last shared a common ancestor.
Figure 4.
Conservation of LRL Gene Function among Streptophytes
(A and A′) Root hair development in Col-0 wild-type (A) and the Atlrl1-2;Atlrl3-1 double mutant (A′). Root hairs of the double mutant did not elongate.
(B–C′) Complementation of the Atlrl1-2;Atlrl3-1 double mutant with a AtLRL1 (B and B′) or AtLRL3 (C and C′) cDNA, respectively, driven by either proAtLRL1 (B and C) or proAtLRL3 (B′ and C′). Root hair growth was restored with all promoter-cDNA combinations.
(D and D′) Complementation of the Atlrl1-2;Atlrl3-1 double mutant with a AtLRL4 cDNA driven by either proAtLRL1 (D) or proAtLRL3 (D′). Complementation of the root hair growth defect was not detected.
(E and E′) Complementation of the Atlrl1-2;Atlrl3-1 double-mutant phenotype using a MpLRL cDNA driven by either proAtLRL1 (E) or proAtLRL3 (E′). Partial restoration of root hair growth using either promoter.
(F and F′) Complementation of the Atlrl1-2;Atlrl3-1 double-mutant phenotype with a CbLRL cDNA driven by either proAtLRL1 (F) or proAtLRL3 (F′). Partial complementation could be detected using the proAtLRL1 promoter.
(G and H) Distribution of phenotype classes of M. polymorpha plants grown for 2 weeks from gemmae in 20% EtOH vapor of wild-type (G) and MpLRLamiRII-ind-1 (H).
(I–L) Distribution of phenotype classes of MpLRLamiRII-ind-1 grown in 20% EtOH vapor and double-transformed with (I) proOsAct:NLS-YFP, (J) proOsAct:CbLRL, (K) proOsAct:AtLRL1, and (L) proOsAct:AtLRL4. Note the reduction of the strong class C5 phenotype in (J)–(L) compared to (I) and (H).
AtLRL Class I and Class II Genes Have Diverged Functions
We tested the ability of CbLRL and MpLRL to restore root hair development in the Atlrl1-2 Atlrl3-1 mutant background. Wild-type root hair growth was restored when double mutants were transformed with proAtLRL1:AtLRL1 and proAtLRL3:AtLRL3 (Figures 4B–4C′, positive controls). Only partial restoration of root hair growth was observed in proAtLRL1:CbLRL; Atlrl1-2 Atlrl3-1 or proAtLRL1:MpLRL; Atlrl1-2 Atlrl3-1 or proAtLRL3:MpLRL; Atlrl1-2 Atlrl3-1 lines (Figures 4E–4F′). This is consistent with the hypothesis that A. thaliana LRL proteins have functionally diverged from LRL proteins in early diverging plants, although some aspects of the ancestral function have been conserved. Since expression of AtLRL4 negatively regulate root hair development, and both AtLRL1 and AtLRL3 positively regulate root hair development, we predicted that expression of AtLRL4 in the AtLRL1 or AtLRL3 domains of the Atlrl1-2 Atlrl3-1 double mutant would not restore growth. As predicted, expression of proAtLRL1:AtLRL4 or proAtLRL3:AtLRL4 in Atlrl1-2 Atlrl3-1 double mutants did not restore root hair elongation (Figures 4D and 4D′). This supports the hypothesis that AtLRL1 and AtLRL3 are functionally diverged from AtLRL4.
Taken together, these data support the hypothesis that LRL proteins have diversified during the course of land plant evolution. A single LRL gene controls thallus and rhizoid development throughout the early diverging land plant M. polymorpha, a function that partially has been conserved since C. braunii and land plants diverged from a common ancestor. Gene duplication followed by functional diversification gave rise to two classes of antagonistically acting proteins expressed in distinct but overlapping domains in relatively derived plants such as A. thaliana. This established a novel negative regulatory element controlling root hair outgrowth. Antagonistic regulation is critical in gene regulatory networks facilitating negative feedback or incoherent feedforward regulation [17]. It is therefore conceivable that the diversification of LRL gene function was an innovation that contributed robustness to the network, critical for the integration of internal signals with changing environmental conditions in derived groups of land plants.
Author Contributions
Conceptualization, H.B. and L.D.; Methodology, H.B.; Investigation, H.B., A.T., and S.S.; Resources, H.S. and T.N.; Writing – Original Draft, H.B. and L.D.; Writing – Review & Editing, H.B. and L.D.; Funding Acquisition, L.D.; Supervision, L.D.
Acknowledgments
We thank Kimitzune Ishizaki for providing us with Tak-1 and Tak-2 strains and John Bowman for providing protocols and advice for the artificial microRNA design. We thank Michael Lenhard for providing vectors containing the AlcA/AlcR inducible system. We thank all lab members for critically reading this manuscript. We thank Sandy Hetherington for helpful discussions regarding the phylogeny. This project was funded by an ERC Advanced Grant (EVO500; project no. 25028) to L.D.
Published: June 2, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, and one dataset and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2016.04.060.
Accession Numbers
The accession numbers for the CbLRL and MpLRL sequences reported in this paper are GenBank: KX037431 and KX037432, respectively. The accession numbers for the A. thaliana LRL genes reported in this paper are TAIR: AtLRL1 At2g24260, AtLRL2 At4g30980, AtLRL3 At5g58010, AtLRL4 At1g03040, and AtLRL5 At4g02590.
Supplemental Information
References
- 1.Clarke J.T., Warnock R.C.M., Donoghue P.C.J. Establishing a time-scale for plant evolution. New Phytol. 2011;192:266–301. doi: 10.1111/j.1469-8137.2011.03794.x. [DOI] [PubMed] [Google Scholar]
- 2.Bateman R.M., Crane P.R., DiMichele W.A., Kenrick P.R., Rowe N.P., Speck T., Stein W.E. Early evoltuion of land plants: phylogeny, physiology, and ecology of the primary terrestrial radiation. Annu. Rev. Ecol. Syst. 1998;29:263–292. [Google Scholar]
- 3.Wellman C.H., Osterloff P.L., Mohiuddin U. Fragments of the earliest land plants. Nature. 2003;425:282–285. doi: 10.1038/nature01884. [DOI] [PubMed] [Google Scholar]
- 4.Bowman J.L. Walkabout on the long branches of plant evolution. Curr. Opin. Plant Biol. 2013;16:70–77. doi: 10.1016/j.pbi.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Dolan L. Body building on land: morphological evolution of land plants. Curr. Opin. Plant Biol. 2009;12:4–8. doi: 10.1016/j.pbi.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Jones V.A.S., Dolan L. The evolution of root hairs and rhizoids. Ann. Bot. (Lond.) 2012;110:205–212. doi: 10.1093/aob/mcs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle J.A. Phylogenetic analyses and morphological evolution in land plants. In: Ambrose B.A., Purugganan M., editors. Annual Plant Reviews Volume 45: The Evolution of Plant Form. John Wiley and Sons; 2013. pp. 1–50. [Google Scholar]
- 8.Pires N., Dolan L. Early evolution of bHLH proteins in plants. Plant Signal. Behav. 2010;5:911–912. doi: 10.4161/psb.5.7.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang D., Weiche B., Timmerhaus G., Richardt S., Riaño-Pachón D.M., Corrêa L.G., Reski R., Mueller-Roeber B., Rensing S.A. Genome-wide phylogenetic comparative analysis of plant transcriptional regulation: a timeline of loss, gain, expansion, and correlation with complexity. Genome Biol. Evol. 2010;2:488–503. doi: 10.1093/gbe/evq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rensing S.A. Gene duplication as a driver of plant morphogenetic evolution. Curr. Opin. Plant Biol. 2014;17:43–48. doi: 10.1016/j.pbi.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Karas B., Amyot L., Johansen C., Sato S., Tabata S., Kawaguchi M., Szczyglowski K. Conservation of lotus and Arabidopsis basic helix-loop-helix proteins reveals new players in root hair development. Plant Physiol. 2009;151:1175–1185. doi: 10.1104/pp.109.143867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tam T.H.Y., Catarino B., Dolan L. Conserved regulatory mechanism controls the development of cells with rooting functions in land plants. Proc. Natl. Acad. Sci. USA. 2015;112:E3959–E3968. doi: 10.1073/pnas.1416324112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flores-Sandoval E., Dierschke T., Fisher T.J., Bowman J.L. Efficient and inducible use of artificial microRNAs in Marchantia polymorpha. Plant Cell Physiol. 2016;57:281–290. doi: 10.1093/pcp/pcv068. [DOI] [PubMed] [Google Scholar]
- 14.Maizel A., Weigel D. Temporally and spatially controlled induction of gene expression in Arabidopsis thaliana. Plant J. 2004;38:164–171. doi: 10.1111/j.1365-313X.2004.02027.x. [DOI] [PubMed] [Google Scholar]
- 15.Hiratsu K., Matsui K., Koyama T., Ohme-Takagi M. Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J. 2003;34:733–739. doi: 10.1046/j.1365-313x.2003.01759.x. [DOI] [PubMed] [Google Scholar]
- 16.Heyl A., Ramireddy E., Brenner W.G., Riefler M., Allemeersch J., Schmülling T. The transcriptional repressor ARR1-SRDX suppresses pleiotropic cytokinin activities in Arabidopsis. Plant Physiol. 2008;147:1380–1395. doi: 10.1104/pp.107.115436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma W., Trusina A., El-Samad H., Lim W.A., Tang C. Defining network topologies that can achieve biochemical adaptation. Cell. 2009;138:760–773. doi: 10.1016/j.cell.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.