Abstract
Background
Athletes who return to sport participation after anterior cruciate ligament reconstruction (ACLR) have a higher risk of a second anterior cruciate ligament injury (either reinjury or contralateral injury) compared with non–anterior cruciate ligament–injured athletes.
Hypotheses
Prospective measures of neuromuscular control and postural stability after ACLR will predict relative increased risk for a second anterior cruciate ligament injury.
Study Design
Cohort study (prognosis); Level of evidence, 2.
Methods
Fifty-six athletes underwent a prospective biomechanical screening after ACLR using 3-dimensional motion analysis during a drop vertical jump maneuver and postural stability assessment before return to pivoting and cutting sports. After the initial test session, each subject was followed for 12 months for occurrence of a second anterior cruciate ligament injury. Lower extremity joint kinematics, kinetics, and postural stability were assessed and analyzed. Analysis of variance and logistic regression were used to identify predictors of a second anterior cruciate ligament injury.
Results
Thirteen athletes suffered a subsequent second anterior cruciate ligament injury. Transverse plane hip kinetics and frontal plane knee kinematics during landing, sagittal plane knee moments at landing, and deficits in postural stability predicted a second injury in this population (C statistic = 0.94) with excellent sensitivity (0.92) and specificity (0.88). Specific predictive parameters included an increase in total frontal plane (valgus) movement, greater asymmetry in internal knee extensor moment at initial contact, and a deficit in single-leg postural stability of the involved limb, as measured by the Biodex stability system. Hip rotation moment independently predicted second anterior cruciate ligament injury (C = 0.81) with high sensitivity (0.77) and specificity (0.81).
Conclusion
Altered neuromuscular control of the hip and knee during a dynamic landing task and postural stability deficits after ACLR are predictors of a second anterior cruciate ligament injury after an athlete is released to return to sport.
Keywords: anterior cruciate ligament reconstruction, kinetics, kinematics, postural stability
Anterior cruciate ligament (ACL) rupture is a devastating injury that is linked to short-term functional deficits and significant long-term morbidity, including premature development of osteoarthritis and significant progressive disability, despite surgical or nonsurgical intervention.27,40,41,47,54 With respect to short-term outcomes, estimates of the overall likelihood that an athlete will incur a subsequent ACL injury (either reinjury or contralateral) after return to sport participation after ACL reconstruction (ACLR) range between 1 in 4 (25%) to 1 in 17 (6%) athletes.47,49,58
The risk of subsequent ACL injury is significantly higher compared with risk of initial ACL injury,47,49,51,58 especially in young, active individuals.28,48,51,58 A prospective, multi-center study from the Multicenter Orthopaedic Outcome Network (MOON) group58 reported a rate of 1 of 17 (6%) second ACL injuries within the short timespan of 2 years after initial injury. This represents a substantially higher injury rate compared with initial ACL injuries, reported to occur at a risk of 1 in 60 to 100,17,36 even in a “high-risk” population of young, female athletes. In studies with longer term follow-up, the rates of second ACL injuries are even higher. In a retrospective case series, Salmon et al49 found that 12% of their sample sustained a second ACL injury within 5 years after primary ACLR. In a 10-year follow-up of this cohort, the rate of injury was greater, as 1 in 3.7 individuals had suffered a second ACL injury.47 Shelbourne et al51 also reported that younger patients were at higher risk, as 17% of patients under the age of 18 years sustained a second ACL injury, while only 4% over the age of 25 years sustained a second injury.
These findings underscore the significantly increased risk for a second ACL injury, especially when resuming sport participation after initial ACLR. Despite the significantly higher risk of second ACL injury, information regarding risk factors associated with second ACL injuries is scarce. In the few studies that have reported such information,7,49 investigations have been limited to clinical assessments and have only evaluated unmodifiable risk factors, such as subjective ratings, demographics, and concomitant injuries. Risk of second ACL injury is greatest in individuals with a history of an initial ACL injury caused by contact with another player and with return to cutting and pivoting sport activities.49 Identification of modifiable factors predictive of second ACL injury is necessary to effectively reduce this high risk of second injury and subsequent sequelae.
Modifiable risk factors potentially predictive of initial ACL injuries have been identified in female athletes. In a prospective, controlled cohort study, Hewett et al21 investigated the effect of biomechanical and neuromuscular measures on risk of ACL injury in uninjured female athletes. Of the 205 female athletes evaluated and followed during the sport season, 9 athletes (4.4%) suffered an ACL injury. Comparison of the prospectively measured variables of the injured and noninjured athletes generated a predictive model for first ACL injury. The most predictive variable for ACL injury was lower extremity frontal plane loads at the knee, which were significantly correlated to hip adduction moments in athletes with ACL injury. In addition, athletes who went on to ACL injury also demonstrated significant side-to-side differences in lower extremity biomechanics, as well as reduced relative lower extremity flexor activation during landing, relative to those who did not sustain ACL injury. Further, 4 of the 9 athletes who sustained ACL injury identified during the original study incurred a second ACL injury within a 5-year period. Although it is reasonable to assume that factors associated with initial ACL injury may remain predictive of second ACL injury, this assumption has yet to be definitively supported. Prospective studies may help to identify modifiable risk factors of subsequent ACL injury in order to develop targeted rehabilitation interventions to reduce this risk.
The purpose of the present study was to identify predictors of a second ACL injury after ACLR in a young, athletic sample. Our hypothesis was that after primary ACLR, prospectively measured deficits in neuromuscular control at the hip and knee and in postural stability would predict second ACL injury. In addition, we hypothesized that these measures would predict future injury with high sensitivity and specificity.
METHODS
Participants
A prospective, case-cohort design was used to identify the predictors of a second ACL injury (ipsilateral or contralateral) after primary ACLR and return to sport. Fifty-six young athletes (35 female, 21 male) who recently sustained an ACL injury, underwent surgical reconstruction, completed rehabilitation, and were released to return to their prior level of activity were recruited to participate in this study (Table 1). Twenty-five participants had an ACLR using bone–patellar tendon–bone autograft tissue, 27 had an ACLR using hamstring tendon graft, and 4 underwent an ACLR with allograft tissue. Inclusion criteria required the participant to be between the ages of 10 and 25 years, have no history of ACL injury, and no history of bilateral lower extremity or lower back injury during the previous 12 months. In addition, the participant was expected to return to a pivoting or cutting (level 1 or 2) sport at least 50 hours per year. Daniel et al10 described a level 1 sport as jumping, pivoting, or hard cutting such as basketball, football, and soccer and a level 2 sport as one requiring lateral motion, but less jumping or hard cutting than level 1, such as baseball, racket sports, or skiing. Patients were excluded if they had elected not to return to pivoting or cutting sports or were not released by both their physician and physical therapist to return to their preinjury level of participation. Involved and uninvolved limbs were operationally identified with respect to their initial injury.
TABLE 1.
Participant Demographic Dataa
| Participants Enrolled Total (N = 56)
|
Initial ACLR (n=43)
|
Second ACL Injury (n = 13)
|
|||||
|---|---|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | Mean | ± SD | P Value | |
| Age, y | 16.41 | 2.97 | 16.60 | 3.29 | 15.77 | 1.36 | .19 |
| Height, cm | 167.26 | 11.67 | 167.02 | 12.37 | 168.08 | 9.40 | .78 |
| Weight, kg | 66.82 | 18.16 | 66.72 | 19.44 | 67.14 | 13.77 | .94 |
| BMI | 23.54 | 4.48 | 23.50 | 4.66 | 23.68 | 4.02 | .90 |
ACLR, anterior cruciate ligament reconstruction; SD, standard deviation; P value, difference between initial ACLR group and second ACL injury group, independent t test; BMI, body mass index.
The study was approved by the Institutional Review Boards and informed consent was obtained from all participants and guardians (if applicable) before testing. Height and weight and other demographic data were collected from all participants. All participants then underwent a full 3-dimensional biomechanical analysis of movement during a drop vertical jump (DVJ), an assessment of postural stability, and an assessment of anterior-posterior (A-P) knee laxity. In the 12-month period after initial testing, 13 patients (11 females, 2 males) sustained a subsequent ACL injury (10 contralateral, 3 ipsilateral). Characteristics of the study sample, by injury status, are presented in Table 1 and details about their second injury are outlined in Appendix 1 (see online Appendix for this article at http://ajs.sagepub.com/supplemental/).
Testing Protocol
Drop Vertical Jump Maneuver
Each participant underwent 3-dimensional biomechanical motion analysis during a DVJ. Trials were collected with EVaRT (Version 4, Motion Analysis Corporation, Santa Rosa, California) using a 10-camera motion analysis system (Eagle cameras, Motion Analysis Corporation) sampled at 240 Hz. Before testing, each participant was first instrumented with 37 retroreflective markers (Figure 1). A static trial in a standing, neutral position was used to align the participant with the laboratory coordinate system and to serve as a reference point for subsequent kinematic analysis. For the DVJ, the participant was positioned on top of a 31-cm box and executed 3 trials. The participant dropped off the box, landed with each foot onto separate force platforms (AMTI, Watertown, Massachusetts), and then immediately executed a maximal effort vertical jump toward an overhead target (Figure 2). Data from each force platform were sampled at 1200 Hz and synchronized with the motion analysis system. These methods have been published previously,13 and we have demonstrated high reliability in obtaining variables of interest in individuals following ACLR.45
Figure 1.
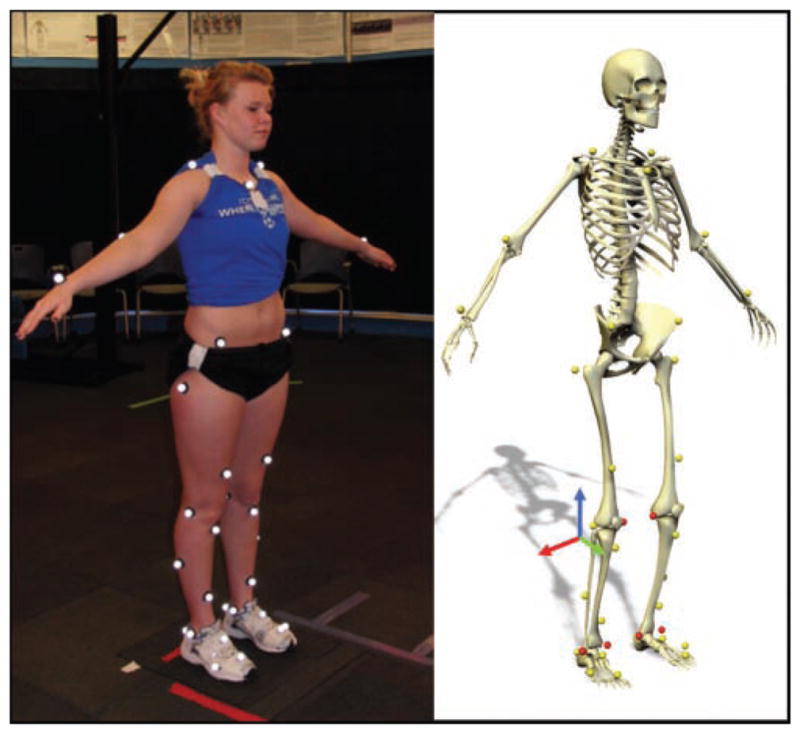
Picture of participant with the identification of the 37 retroreflective marker locations used for 3-dimensional motion analysis.
Figure 2.
Diagrammatic skeletal representation of drop vertical jump maneuver used in this study.
Kinematic and Kinetic Analysis
Data analysis was performed in Visual3D (Version 4.0, C-Motion, Inc, Germantown, Maryland) and MATLAB (Version 7, The Mathworks, Inc, Natick, Massachusetts). Initial contact of each limb was defined when the vertical ground-reaction force first exceeded 10 N. The landing phase was defined from initial contact to the lowest point of the body’s center of mass. Lower extremity kinematic and kinetic variables were calculated during this phase and the mean of the 3 landing trials was used for subsequent data analysis.
Marker trajectories were filtered through a low-pass Butterworth digital filter at a cutoff frequency of 12 Hz. Hip joint centers were estimated based on the work of Bell et al,3 while the knee and ankle joint centers were identified as the midpoint between the medial and lateral knee and ankle joint markers, respectively. The joint centers were used for 3-dimensional measures as well as an assessment of 2-dimensional frontal plane knee motion during landing. All other kinematic analyses were calculated using previously described methods21 with high reliability.12 Hip flexion, hip adduction, hip internal rotation, knee extension, knee adduction, and knee internal rotation kinematics were described as positive values.12
Inverse dynamics were used to calculate sagittal, frontal, and transverse plane knee and hip moments from the kinematic and force plate data.57 Vertical ground-reaction force data were filtered through a low-pass Butterworth filter at a cutoff frequency of 12 Hz. Net internal moments are described and represent the body’s reaction to the external load on each joint. Hip extensor, hip abductor, hip external rotator, knee flexor, knee abductor, and knee external rotator moments were described as positive values. Peak knee and hip moments were calculated for the landing phase of the DVJ. In addition, to better understand the neuromuscular contributions at the onset of landing when injury often occurs,25 net moments were calculated at initial contact and the net impulse (area under the moment curve) during the first 10% of the landing phase of the DVJ was also reported. All kinetic and power measures were normalized by body weight (kg). Raw values for both the involved and uninvolved limbs in addition to side-to-side differences in all kinematic and kinetic variables were calculated for data analysis.
Postural Stability Analysis
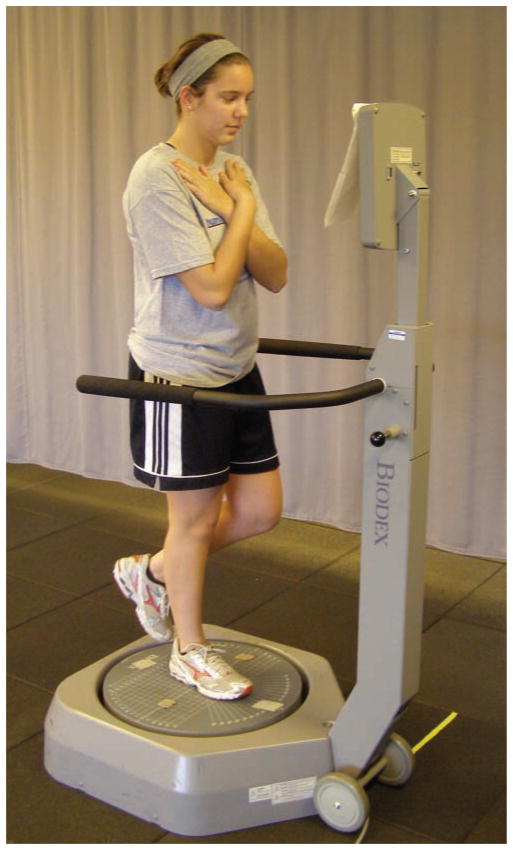
All participants had their single limb, dynamic postural stability assessed on both the involved and uninvolved limb using the Balance System SD (Biodex, Shirley, New York). The participant was positioned and balanced centrally on a single limb in the center of the dynamic, unstable platform (Figure 3). The individual stood with the test limb in slight flexion (less than 10μ), the contralateral limb flexed, and both arms crossed with hands resting on the contralateral shoulder. The participant was instructed to maintain a stable posture on the platform for 20 seconds while the stability system was set at a level 4 stability setting. The individual repeated this 20-second trial 3 times on each limb. Limb testing order was randomized. During each trial, the Balance System recorded movement of the platform away from a level position in degrees of deflection. Data were generated representing overall stability as well as deviations in the A-P and medial-lateral direction. Higher instability values represent decreased ability to maintain a stable platform and more altered postural stability by the participant. These methods have been previously reported with high reliability.46
Figure 3.
Participant during the assessment of postural stability using the Biodex SD Stability System. The individual is in a unilateral stance position with the contralateral limb flexed and both arms crossed at the chest. The participant is tested with eyes open, but receives no visual feedback on performance during the testing session.
Anterior-Posterior Knee Laxity
Participants had their A-P knee laxity assessed with a CompuKT (MedMetric Corporation, San Diego, California). Individuals were positioned supine with the tested knee resting on a standard wedge, flexed to 20μ. The CompuKT was affixed to the distal shank and A-P translation was assessed at 2 force levels (89 N and 134 N). All testing was executed by a single tester (M.V.P.), who has demonstrated high reliability in prior studies.37
Injury Surveillance
Participants were enrolled in the study between June 2007 and October 2008. After the initial testing session, each individual was contacted monthly by e-mail or phone for 12 months. At the time of each contact, the participant reported any knee injuries since their return to sports and the number of athlete-exposures in which the athlete participated since the last date of contact. An athlete-exposure was defined as participation in games and practice sessions of a pivoting or cutting sport with their team where the athlete was at risk for ACL injury. All second ACL injuries represented noncontact or indirect contact injuries (contact at a location other than the lower extremity),32 as there was an absence of any direct contact to the athlete’s knee. Second ACL injury was confirmed with arthroscopy, MRI, or significant change (>3 mm) on the assessment of A-P knee laxity on the CompuKT.
Statistical Analysis
Two group comparisons were conducted using the Student t test or Kruskal-Wallis test for normal and nonnormally distributed variables, respectively. Based on our hypothesis, and prior work identifying predictors of an initial ACL injury, specific biomechanical variables were considered in these analyses. The variables selected were based on the current clinical knowledge, literature reviews, and expert opinions from biomechanical and sports medicine areas. These variables included kinematics, kinetics, power, and vertical ground-reaction force at the hip and knee at specific time points during the landing phase of the DVJ maneuver. Further, Z-scores corresponding to the normalized variables were calculated using the standardized deviation method. The standardized deviation method produces standard variables with a sample mean of 0 and standard deviation of 1. Differences between involved and uninvolved legs on their corresponding measures were calculated, and used to assess for asymmetry between the 2 legs. The standardized Z-score of the difference measure was also computed.
Multivariable logistic regression, using variables with P values of <.10 from the 2-group comparisons, was then used to identify the most predictive variables of the second ACL injury. The P value of .10 was used so that potentially important predictors would not be excluded from this comprehensive data set.24 The selected variables from the Student t test and Kruskal-Wallis analyses were grouped into 2 different sets: 1 set including biomechanical measures obtained from involved and uninvolved legs only, and the other set including differences between involved and uninvolved legs. Our rationale was to identify the most important predictors from each set, while avoiding the potential problem of excluding an important predictor because of its strong correlation with another predictive variable that has already been included in the model. Only variables that were significant at P < .05 remained in the final model for each set. Finally, the 2 sets of predictive variables remaining in the final models were combined and entered into a final multivariable stepwise logistic regression analysis to determine a best predictive final model. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) from the last final model were reported. The corresponding receiver operating curve (ROC) was plotted, and the area under the ROC statistics was reported.
RESULTS
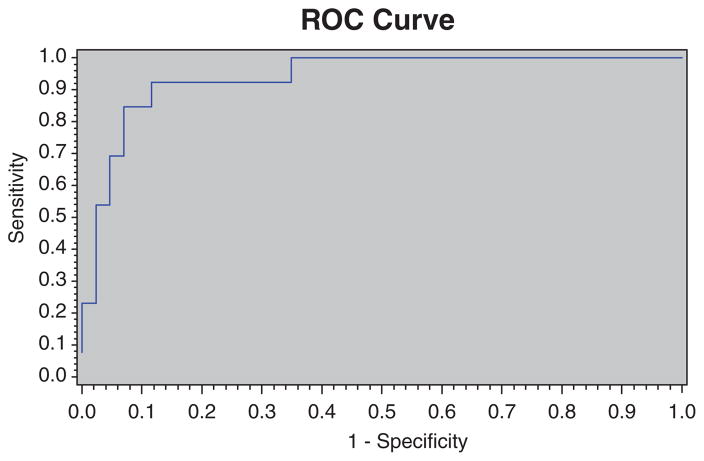
Multivariable logistic regression identified 4 variables that combined to predict a second ACL injury after ACLR and return to sports with an area under the ROC of 0.94 (Figure 4). These 4 variables included biomechanical measures during the DVJ task and deficits of involved limb postural stability. During landing, prediction variables included the uninvolved limb transverse plane hip net moment impulse, the 2-dimensional frontal plane knee joint range of motion, and asymmetries in sagittal plane knee moments at initial contact. This integrated model was both highly sensitive (0.92) and specific (0.88) for prediction of second ACL injury risk.
Figure 4.
The corresponding receiver operating curve (ROC) plotted for the overall model. Multivariable logistic regression identified 4 variables that predicted a second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sports. The area under the ROC statistic is shown with an area under the ROC of 0.94.
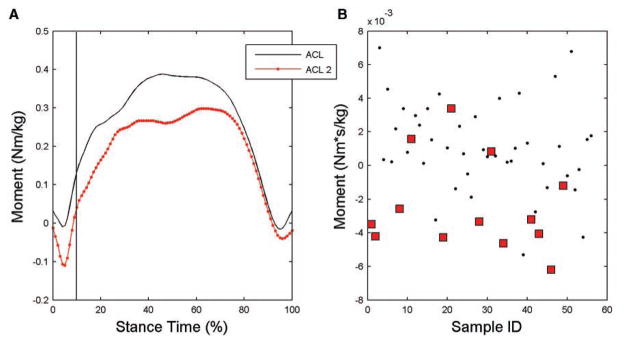
Univariate analyses indicated that the uninvolved limb transverse plane hip net moment impulse during the initial 10% of landing was associated with increased risk of second ACL injury, with an area under the ROC of 0.81 and both high sensitivity (0.77) and specificity (0.81). In the final multivariable logistic regression model, this variable was identified as the strongest predictor of second ACL injury (Figure 5). Participants who sustained a second ACL injury demonstrated a significantly different hip transverse plane moment impulse compared with those who did not incur a second ACL injury (P < .001; Figure 6). The impulse during the initial 10% of stance was representative of a net hip internal rotator moment in the participants who sustained a second ACL injury (−2.4 × 10−3 Nms/kg) compared with a net external rotator moment on the uninvolved limb of participants who did not incur a second ACL injury (1.1 × 10−3 Nms/kg; Figure 6). Greater hip external rotator moments may act to restrain hip internal rotation motion during this phase of landing. Specifically, participants with less hip external rotator moment during early landing were over 8 times (OR = 8.4; 95% CI = 2.1, 33.3) more likely to sustain a second ACL injury than those with greater hip external rotation moment (Table 2).
Figure 5.
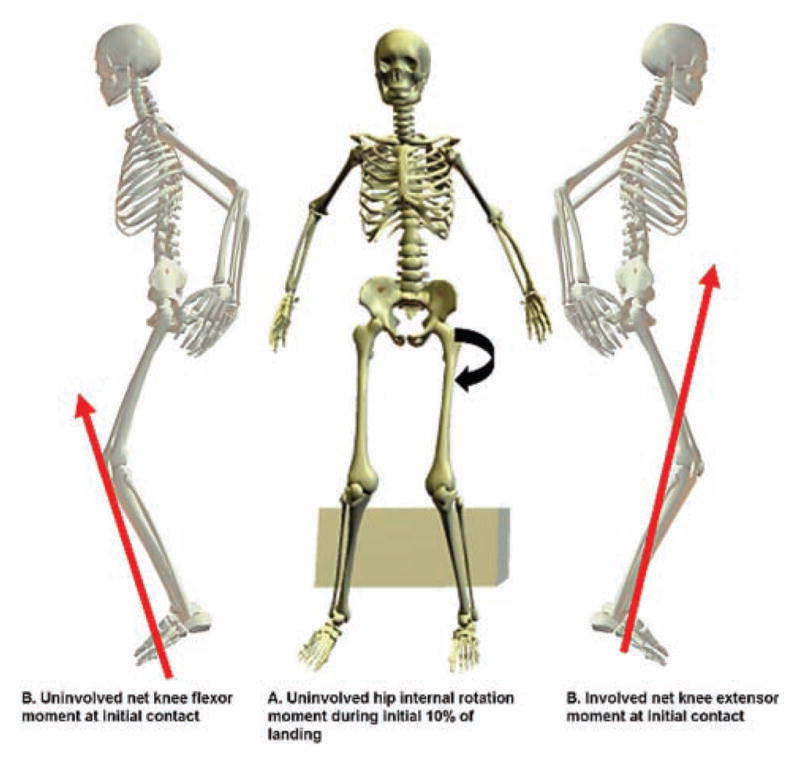
Example of second anterior cruciate ligament (ACL) injury predictors in a patient with history of a right ACL injury and reconstruction. A, participants who sustained a second ACL injury had a contralateral hip internal rotation moment during the first 10% of the landing cycle compared with an external rotation moment in the group of patients who did not suffer a second injury. B, participants who sustained a second ACL injury had a side-to-side difference in sagittal plane knee moment at initial contact with a knee extensor moment on the contralateral limb and a knee flexor moment in the involved limb. All moments are described as internal moments.
Figure 6.
A, internal hip rotation moment averaged over stance phase for the anterior cruciate ligament (ACL) and second ACL injured groups. B, scatter plot of internal hip rotation moment impulse for ACL (dots) and second ACL (squares) injured groups. Impulse was calculated during the initial 10% of stance.
TABLE 2.
Multivariable Model Odds Ratio Estimates
| Variable | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Uninvolved hip rotation net moment impulse (initial 10% of landing) | 8.4 | 2.1, 33.3 |
| 2-dimensional frontal plane knee motion during landing | 3.5 | 1.3, 9.9 |
| Side-to-side difference in sagittal plane knee moment at initial contact | 3.3 | 1.2, 8.8 |
| Postural stability on involved limb | 2.3 | 1.1, 4.7 |
Two-dimensional frontal plane knee joint range of motion during the landing phase of the involved limb, in addition to hip kinetics, demonstrated significantly greater angular displacement in the group that suffered a second ACL injury compared with the ACLR patients who did not suffer a second injury (P = .03; Figure 7). The second injury group demonstrated an average of 16.2μ of total frontal plane (valgus) movement, while the remaining patients translated 12.1μ in the frontal plane. Participants with an increase in frontal plane motion were over 3 times as likely (OR = 3.5; 95% CI = 1.3, 9.9) to incur a second ACL injury than those with reduced frontal plane motion.
Figure 7.
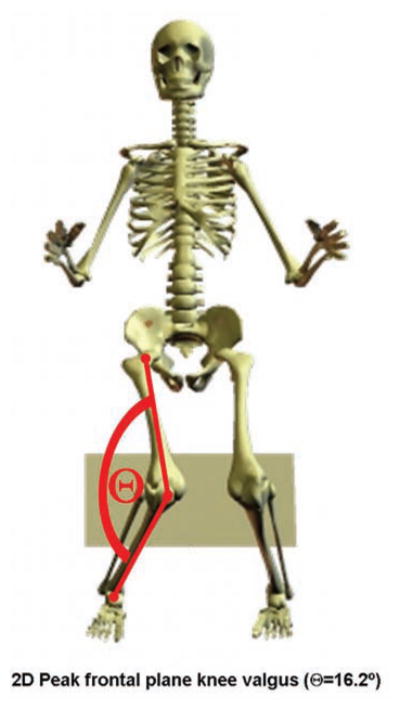
Example of second anterior cruciate ligament (ACL) injury predictors in a patient with history of a right ACL injury and reconstruction. Participants who sustained a second ACL injury had increased 2-dimensional peak frontal plane knee motion during the landing phase of the drop vertical jump.
Side-to-side differences in sagittal plane knee moment at initial contact were significantly different between groups (P = .03; Figure 5). The second injury group demonstrated a 4.1-fold greater asymmetry in internal knee extensor moment at initial contact when compared with the cohort of patients who did not suffer additional injury. Repeated-measure 2 × 2 analysis of variance showed a significant side (initial involved vs initial uninvolved) × group (initial ACLR vs second ACL injury) interaction (P = .03) of sagittal plane knee moment at initial contact of landing. Post hoc analysis demonstrated that the internal knee extensor moment of the uninvolved limb at the point of initial contact in the second injury group (−2.8 × 10−2 Nm/kg) was significantly lower than the involved limb of the second injury group (9.5 × 10−2 Nm/kg) and both involved (11.4 × 10−2 Nm/kg) and uninvolved (8.4 × 10−2 Nm/kg) limbs in the first injury group, which demonstrated a net knee flexor moment at the point of initial contact (Figure 8). Participants who demonstrated asymmetry in this variable were over 3 times as likely (OR = 3.3; 95% CI = 1.2, 8.8) to incur a second ACL injury than those who demonstrated symmetry in sagittal plane knee moments at the point of initial contact.
Figure 8.
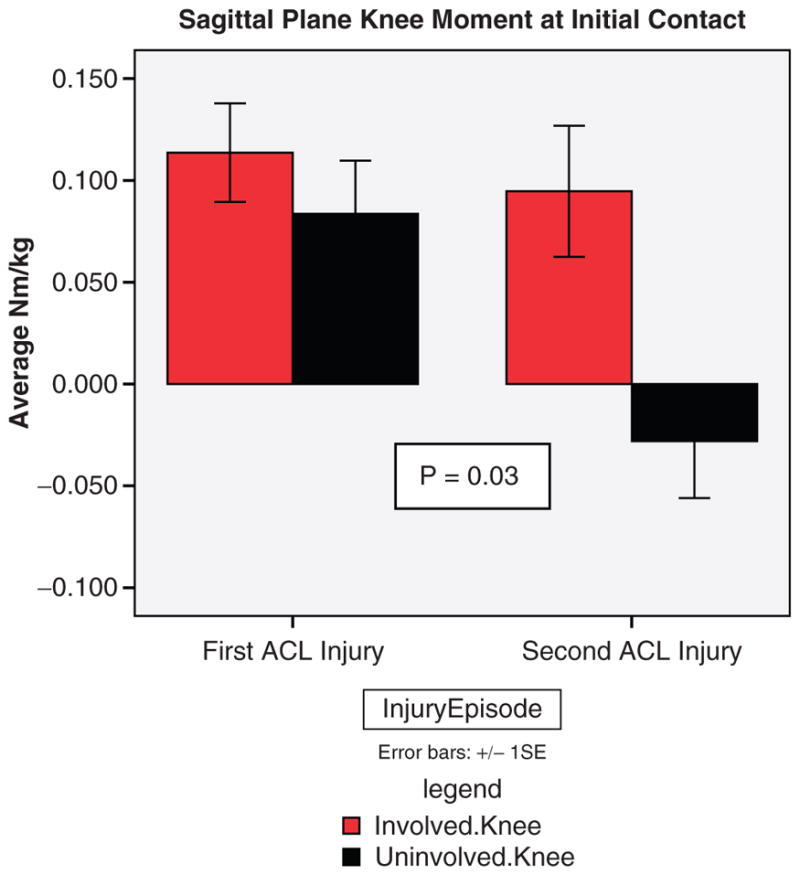
Sagittal plane knee moments at initial contact for the involved and uninvolved limb of the initial anterior cruciate ligament (ACL) injury group and the second ACL injury group.
Participants with deficits in single-leg postural stability of the involved limb, as measured by the Biodex stability system, were twice as likely (OR = 2.3; 95% CI = 1.1, 4.7) to incur a second injury as compared with those who did not demonstrate single-leg stability of the involved limb. The involved limb of the second injury group demonstrated a mean degree of deflection on the overall stability score of 4.07° ± 2.06°, while the stability score on the involved limb of the first injury group was 3.63° ± 1.58°. Additional variables, including graft type and A-P knee laxity, were evaluated in the statistical analysis; however, they were not predictive of a second ACL injury in this cohort.
DISCUSSION
The findings of the current study support the tested hypothesis that altered neuromuscular control patterns during landing and deficits in postural stability predict subsequent ACL injuries in a sample of athletes at the time of return to sport after initial ACLR. Specifically, transverse plane net moment impulse at the hip, dynamic frontal plane knee range of motion, side-to-side differences in sagittal plane knee moment at initial contact, and deficits in postural stability predicted a second ACL injury with both high sensitivity and specificity. To our knowledge, this represents the first report of biomechanical and neuromuscular factors predictive of a second ACL injury that are potentially modifiable by rehabilitative intervention. These findings are consistent with several studies that describe altered movement patterns and impairments either predictive of initial ACL injury21 in an uninjured population or demonstrated by patients after initial ACLR.9,22,33,45
Proximal Neuromuscular Control of Frontal Plane Motion
In this study, frontal plane range of motion of the lower extremity and the neuromuscular contributions that control these movements during the deceleration phase of landing from a DVJ were identified as strong predictors of future ACL injury in a cohort of athletes after ACLR. The position of dynamic valgus alignment of the lower extremity has been described in several studies as a body position in which the knee joint collapses medially. This position represents a combination of hip adduction, hip internal rotation, knee flexion, knee abduction, and tibial rotation.21,25,50 This position has been shown to increase strain on the ACL in a cadaveric model15,31 and, in a prospective cohort study by Hewett et al,21 to predict future ACL injury in a cohort of female high school athletes. Prior reports of dynamic valgus have analyzed this motion through 3-dimensional analyses.21,35,50
Although 3-dimensional analysis is often described as a gold standard for kinematic evaluation, recent evidence indicates that 2-dimensional analysis of frontal plane knee motion correlates with 3-dimensional measures of this motion.35 In addition, 2-dimensional video analysis was used to identify a frontal plane knee position as a common mechanism of injury in female athletes at the time of ACL injury.5,25,44 Our findings indicated that a similar high-risk position during a DVJ task, identified in 2 dimensions, predicted a second ACL injury. It is likely that 2-dimensional assessment of frontal plane motion is a representation of contributions of hip abduction, hip rotation, and knee abduction, which collectively increase risk for ACL injury. There is high clinical utility of a 2-dimensional analysis of frontal plane motion that could identify a risk factor for future ACL injury after ACLR. Current criteria utilized to assess an athlete’s readiness to return to sports after ACLR may include clinical measures of range of motion, strength, and success on functional performance tests.39,55 Video analysis of frontal plane motion during an athletic maneuver, such as the DVJ, appears to be an appropriate tool to include in an assessment of an athlete’s readiness to safely return to sports.
Frontal plane motion of the knee during the landing phase of a DVJ is potentially controlled by neuromuscular contribution at the hip, knee, and ankle. Several studies have investigated the relationship of hip strength and hip kinematics during dynamic tasks such as landing, running, and stair climbing,6,52,53,56 particularly in the presence of patellofemoral injury. Souza and Powers52 assessed hip kinematics during the DVJ task in patients with and without patellofemoral abnormality and reported increased hip internal rotation motion during the DVJ and a coinciding deficit in hip extensor strength as measured on an isokinetic dynamometer in patellofemoral patients compared with controls. McLean et al34 noted an association between hip internal rotation kinematics and knee valgus moment during athletic tasks, a position known to increase strain on the ACL.15 Data from the current study indicated that a deficit in net hip muscle external rotation torque during the early phase of landing was a strong predictor of future ACL injury, either when examined in the presence of other potential risk factors or when examined independently, when it predicted ACL injury risk with an area under the ROC curve of 0.81.
The clinical implications of these findings of hip muscle external rotation torque deficits are significant. The current results indicate that interventions targeted to address impaired hip strength have the potential to reduce second ACL injury after ACLR. Functional anatomical analyses indicate that the gluteus maximus is a strong hip extender and external rotator43 while in a flexed hip posture.42 During the DVJ, the average hip flexion angle starts at approximately 25° to 30° of flexion at initial contact and increases to 55° of peak flexion.12 Considering gluteus maximus function, and the findings of Souza and Powers,53 which indicate a relationship between hip extensor muscle performance and hip internal rotation kinematics with the DVJ task, an intervention focused on hip external rotation and hip extension strength and recruitment may assist in the reduction of dynamic frontal plane knee motion. Essentially, gluteus maximus strengthening and enhanced recruitment after ACLR may have the potential to reduce future ACL injury in this population. In addition to increased hip strength, neuromuscular training focused on plyometrics and landing techniques, such as squat jumps, scissor jumps, and tuck jumps, has the potential to improve gluteal muscle recruitment and correct abnormal frontal plane mechanics during the DVJ.14 Previously, altered frontal plane motion has been shown to predict ACL injury in a healthy population.21 Our findings now indicate that altered frontal plane motion may predict a second ACL injury after ACLR. Current evidence indicates that neuromuscular interventions have the potential to improve jumping mechanics and, in turn, reduce the ACL injury rate in a healthy population.16,18–20,30 Therefore, implementation of these interventions at the end stages of rehabilitation after ACLR may have the potential to also reduce second ACL injury rates.
Asymmetries in Sagittal Plane Moments During Landing
Side-to-side differences in neuromuscular control of the knee during dynamic, athletic tasks was theorized to be a potential risk factor for ACL injury in a healthy population.22 Similarly, these results indicate that side-to-side asymmetries in neuromuscular variables persist following ACLR, which is consistent with our earlier work, which found side-to-side differences in vertical ground-reaction force and loading rate that persisted for up to 2 years after ACLR.45 Our current findings indicate that limb asymmetries identified after ACLR are predictive of future ACL injury, specifically asymmetries in sagittal plane knee moments. Injuries to the ACL often occur with the knee at low knee flexion angles.4,29 In addition, Lloyd26 suggested that cocontraction of the quadriceps and hamstrings assists in providing up to 80% of the resistance to frontal plane movement at the knee. Therefore, the ability of the knee to symmetrically activate the sagittal plane musculature to assist in frontal plane stability during a bipedal task, at a time when the knee is in a high-risk position of low flexion, is critical. The asymmetry in sagittal plane knee moments at initial contact, as demonstrated by the participants who subsequently suffered a second ACL injury, indicates that an imbalance in force absorption strategies during the onset of the DVH task may increase future injury risk.38
Deficits in Postural Stability
Balance or postural stability is defined as an individual’s ability to maintain the center of mass over the base of support. The deviation of the center of mass away from the base of support during dynamic athletic activities has recently been identified by several authors as a mechanism that increases valgus load on the ACL.23,25,44 This increase in frontal plane load through a poorly controlled trunk is a potential risk factor for ACL injury in a healthy population of female athletes. Zazulak et al59,60 identified core proprioception and kinesthetic awareness as a predictor of knee and ACL injury in a population of previously uninjured collegiate female athletes. In these studies, female athletes with decreased core proprioception and kinesthetic awareness were more likely to suffer a knee injury. Dynamic postural stability assessment is a method to objectively quantify deviations in balance from a neutral point and may be a simple method to quantify an individual’s ability to control movement of the center of mass in an objective and dynamic fashion. Prior studies have demonstrated deficits in postural stability after ACL injury,1,2,22 an improvement in postural stability and knee kinematics with perturbation training after ACL injury,8,11 and an improvement in postural stability with neuromuscular training after ACLR.22,61 The current findings of the association of postural deficits with increased risk of second ACL injury indicate that the presence of altered involved limb single-leg balance after ACLR may limit the ability of the athlete to control the movement of the center of mass over the base of support during dynamic athletic activities and subsequently increase the risk of knee injury. Intervention strategies throughout the postoperative course that target improved postural stability and control of the center of mass may help minimize subsequent ACL injury risk after ACLR. These can include double- and single-leg balance activities on stable and unstable surfaces, with and without perturbations.
Potential Study Limitations
Although the predictive model of second ACL injury after ACLR presented has high sensitivity and specificity, we recognize the potential limitations that should be considered relative to the interpretation of the current study results. A relatively small sample cohort of 56 young, athletic individuals was prospectively enrolled in our study, which could potentially limit the generalizability of the results to other populations. However, the 13 second ACL injuries observed in our study, which exceeded our a priori power analysis of 7.7 ACL injuries needed to meet sufficient statistical power, increased our confidence that these results may be applicable to the young, athletic population, despite the relatively small initial enrollment of 56 patients. Another potential limitation of the study is the potential for inequitable distribution of graft type among the sample. Specifically, there were very few participants with allograft tissue ACLR compared to the number of participants who had ACLR using autograft patellar tendon and hamstrings graft material. Although it was not the primary aim of the study to identify graft source as a potential risk factor for future ACL injury and graft type did not influence our predictive model, prior evidence7 indicates that allograft tissue may be a predictor of ipsilateral retear of an ACLR graft. As a result of our low number of participants with an allograft reconstruction, we cannot state with confidence that allograft tissue was not a predictor of a second injury. Finally, the aims of this study were focused on biomechanical variables in patients after ACLR in an attempt to create a predictive model of who would suffer a second ACL injury. An interesting analysis would include a comparison of these groups to a healthy group who never suffered a lower extremity injury.
SUMMARY AND CONCLUSION
Biomechanical Measures and Postural Control Deficits Predict Second ACL Injury
Current evidence indicates a high incidence of second ACL injury after ACLR, especially in populations who return to sport. However, potential mechanisms underlying this increased risk have not been identified in a prospective cohort study. Our study prospectively evaluated biomechanical and neuromuscular variables during the landing phase of a DVJ, in addition to assessing dynamic postural stability at the time the athlete was released to return to sport, to determine if deficits in these variables were predictive of a second ACL injury. The study findings indicate that net hip rotation moment impulse, frontal plane knee range of motion during landing, asymmetries in sagittal plane knee moments at initial contact, and postural stability are collectively a strong predictor of a second ACL injury after ACLR with high sensitivity (0.92) and specificity (0.88). In addition, net hip rotation moment impulse, in isolation, is also a good predictor of second ACL injury with moderate to high sensitivity (0.78) and specificity (0.81).
The identification of high-risk variables that predict future ACL injury, at the time of patient discharge to return to sports after ACLR, is the first step in the development of more efficacious interventions, and more appropriate discharge criteria, for the determination of when an athlete should return to sport after ACL injury rehabilitation. With these key identified variables that predict athletes at high risk for future ACL injury, more valid criteria can now be developed and implemented, as necessary criteria to be released to return to sports or activity. Ultimately, it follows logically to pursue testing of specific rehabilitation interventions to determine if targeted interventions could also be modified to address these specific impairments in hip external rotator muscle strength and recruitment and thus decrease the likelihood of future ACL injury.
Acknowledgments
The authors would like to acknowledge funding support from NFL Charities, the University of Cincinnati University Research Council (URC), and the National Institutes of Health/NAIMS Grant R01-AR049735, R01-AR055563, R01-AR056259, F32 AR 055844 and the Cincinnati Children’s Hospital Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number UL1RR026314.
We would like to thank Dr. Robert Heidt and the staff at Wellington Orthopaedic and Sports Medicine Center, Dr. Keith Kenter and the staff of the University of Cincinnati Sports Medicine, Dr. Eric Wall and the staff of and Cincinnati Children’s Hospital Division of Orthopaedics, Thomas Palmer and the athletes within the Northern Kentucky University Athletic Department and Theresa Behan and the athletes within the Thomas More College Athletic Department for their participation in this study. We would also like to thank Rebecca Reder, Michael Allen and the Ortho/Sports Physical Therapy Team at the Cincinnati Children’s Hospital Medical Center.
Footnotes
The Cincinnati Children’s Hospital Medical Center Institutional Review Board and Rocky Mountain University of Health Professions approved this study.
One or more authors has declared a potential conflict of interest:
References
- 1.Beard DJ, Dodd CA, Trundle HR, Simpson AH. Proprioception enhancement for anterior cruciate ligament deficiency: a prospective randomised trial of two physiotherapy regimes. J Bone Joint Surg Br. 1994;76(4):654–659. [PubMed] [Google Scholar]
- 2.Beard DJ, Kyberd PJ, Dodd CA, Simpson AH, O’Connor JJ. Proprioception in the knee. J Bone Joint Surg Br. 1994;76(6):992–993. [PubMed] [Google Scholar]
- 3.Bell AL, Pedersen DR, Brand RA. A comparison of the accuracy of several hip center location prediction methods. J Biomech. 1990;23(6):617–621. doi: 10.1016/0021-9290(90)90054-7. [DOI] [PubMed] [Google Scholar]
- 4.Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 5.Boden BP, Torg JS, Knowles SB, Hewett TE. Video analysis of anterior cruciate ligament injury: abnormalities in hip and ankle kinematics. Am J Sports Med. 2009;37(2):252–259. doi: 10.1177/0363546508328107. [DOI] [PubMed] [Google Scholar]
- 6.Bolgla LA, Malone TR, Umberger BR, Uhl TL. Hip strength and hip and knee kinematics during stair descent in females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2008;38(1):12–18. doi: 10.2519/jospt.2008.2462. [DOI] [PubMed] [Google Scholar]
- 7.Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case-control study. Am J Sports Med. 2009;37(12):2362–2367. doi: 10.1177/0363546509340633. [DOI] [PubMed] [Google Scholar]
- 8.Chmielewski TL, Rudolph KS, Snyder-Mackler L. Development of dynamic knee stability after acute ACL injury. J Electromyogr Kinesiol. 2002;12(4):267–274. doi: 10.1016/s1050-6411(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 9.Colby SM, Hintermeister RA, Torry MR, Steadman JR. Lower limb stability with ACL impairment. J Orthop Sports Phys Ther. 1999;29(8):444–451. doi: 10.2519/jospt.1999.29.8.444. discussion 452–454. [DOI] [PubMed] [Google Scholar]
- 10.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient: a prospective outcome study. Am J Sports Med. 1994;22(5):632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald GK, Axe MJ, Snyder-Mackler L. The efficacy of perturbation training in nonoperative anterior cruciate ligament rehabilitation programs for physically active individuals. Phys Ther. 2000;80(2):128–140. [PubMed] [Google Scholar]
- 12.Ford KR, Myer GD, Hewett TE. Reliability of landing 3D motion analysis: implications for longitudinal analyses. Med Sci Sports Exerc. 2007;39(11):2021–2028. doi: 10.1249/mss.0b013e318149332d. [DOI] [PubMed] [Google Scholar]
- 13.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35(10):1745–1750. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- 14.Ford KR, Myer GD, Smith RL, Byrnes RN, Dopirak SE, Hewett TE. Use of an overhead goal alters vertical jump performance and biomechanics. J Strength Cond Res. 2005;19(2):394–399. doi: 10.1519/15834.1. [DOI] [PubMed] [Google Scholar]
- 15.Fung DT, Zhang LQ. Modeling of ACL impingement against the inter-condylar notch. Clin Biomech (Bristol, Avon) 2003;18(10):933–941. doi: 10.1016/s0268-0033(03)00174-8. [DOI] [PubMed] [Google Scholar]
- 16.Gilchrist J, Mandelbaum BR, Melancon H, et al. A randomized controlled trial to prevent noncontact anterior cruciate ligament injury in female collegiate soccer players. Am J Sports Med. 2008;36(8):1476–1483. doi: 10.1177/0363546508318188. [DOI] [PubMed] [Google Scholar]
- 17.Gomez E, DeLee JC, Farney WC. Incidence of injury in Texas girls’ high school basketball. Am J Sports Med. 1996;24(5):684–687. doi: 10.1177/036354659602400521. [DOI] [PubMed] [Google Scholar]
- 18.Hewett TE, Ford KR, Myer GD. Anterior cruciate ligament injuries in female athletes: part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34(3):490–498. doi: 10.1177/0363546505282619. [DOI] [PubMed] [Google Scholar]
- 19.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes: a prospective study. Am J Sports Med. 1999;27(6):699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 20.Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: part 1, mechanisms and risk factors. Am J Sports Med. 2006;34(2):299–311. doi: 10.1177/0363546505284183. [DOI] [PubMed] [Google Scholar]
- 21.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 22.Hewett TE, Paterno MV, Myer GD. Strategies for enhancing proprioception and neuromuscular control of the knee. Clin Orthop Relat Res. 2002;402:76–94. doi: 10.1097/00003086-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417–422. doi: 10.1136/bjsm.2009.059162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosmer D, Lemeshow S. Applied Logistic Regression. New York, NY: John Wliey and Sons Inc; 1989. [Google Scholar]
- 25.Krosshaug T, Nakamae A, Boden BP, et al. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35(3):359–367. doi: 10.1177/0363546506293899. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd DG. Rationale for training programs to reduce anterior cruciate ligament injuries in Australian football. J Orthop Sports Phys Ther. 2001;31(11):645–654. doi: 10.2519/jospt.2001.31.11.645. discussion 661. [DOI] [PubMed] [Google Scholar]
- 27.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 28.Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91(10):2321–2328. doi: 10.2106/JBJS.H.00539. [DOI] [PubMed] [Google Scholar]
- 29.Malinzak RA, Colby SM, Kirkendall DT, Yu B, Garrett WE., Jr A comparison of knee joint motion patterns between men and women in selected athletic tasks. Clin Biomech (Bristol, Avon) 2001;16(5):438–445. doi: 10.1016/s0268-0033(01)00019-5. [DOI] [PubMed] [Google Scholar]
- 30.Mandelbaum BR, Silvers HJ, Watanabe D, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing the incidence of ACL injuries in female athletes: two-year follow up. Am J Sports Med. 2005;33(7):1003–1010. doi: 10.1177/0363546504272261. [DOI] [PubMed] [Google Scholar]
- 31.Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13(6):930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 32.Marshall SW, Padua D, McGrath M. Incidence of ACL injury. In: Hewett TE, Schultz SJ, Griffin LY, editors. Understanding and Preventing Noncontact ACL Injuries. Champaign, IL: Human Kinetics; 2007. pp. 5–30. [Google Scholar]
- 33.Mattacola CG, Perrin DH, Gansneder BM, Gieck JH, Saliba EN, McCue FC., 3rd Strength, functional outcome, and postural stability after anterior cruciate ligament reconstruction. J Athl Train. 2002;37(3):262–268. [PMC free article] [PubMed] [Google Scholar]
- 34.McLean SG, Huang X, van den Bogert AJ. Association between lower extremity posture at contact and peak knee valgus moment during sidestepping: implications for ACL injury. Clin Biomech (Bristol, Avon) 2005;20(8):863–870. doi: 10.1016/j.clinbiomech.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 35.McLean SG, Walker K, Ford KR, Myer GD, Hewett TE, van den Bogert AJ. Evaluation of a two dimensional analysis method as a screening and evaluation tool for anterior cruciate ligament injury. Br J Sports Med. 2005;39(6):355–362. doi: 10.1136/bjsm.2005.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messina DF, Farney WC, DeLee JC. The incidence of injury in Texas high school basketball: a prospective study among male and female athletes. Am J Sports Med. 1999;27(3):294–299. doi: 10.1177/03635465990270030401. [DOI] [PubMed] [Google Scholar]
- 37.Myer GD, Ford KR, Paterno MV, Nick TG, Hewett TE. The effects of generalized joint laxity on risk of anterior cruciate ligament injury in young female athletes. Am J Sports Med. 2008;36(6):1073–1080. doi: 10.1177/0363546507313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myer GD, Paterno MV, Ford KR, Hewett TE. Neuromuscular training techniques to target deficits before return to sport after anterior cruciate ligament reconstruction. J Strength Cond Res. 2008;22(3):987–1014. doi: 10.1519/JSC.0b013e31816a86cd. [DOI] [PubMed] [Google Scholar]
- 39.Myer GD, Paterno MV, Ford KR, Quatman CE, Hewett TE. Rehabilitation after anterior cruciate ligament reconstruction: criteria based progression through the return to sport phase. J Orthop Sports Phys Ther. 2006;36(6):385–402. doi: 10.2519/jospt.2006.2222. [DOI] [PubMed] [Google Scholar]
- 40.Myklebust G, Bahr R. Return to play guidelines after anterior cruciate ligament surgery. Br J Sports Med. 2005;39(3):127–131. doi: 10.1136/bjsm.2004.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myklebust G, Holm I, Maehlum S, Engebretsen L, Bahr R. Clinical, functional, and radiologic outcome in team handball players 6 to 11 years after anterior cruciate ligament injury: a follow-up study. Am J Sports Med. 2003;31(6):981–989. doi: 10.1177/03635465030310063901. [DOI] [PubMed] [Google Scholar]
- 42.Neumann DA. Kinesiology of the hip: a focus on muscular actions. J Orthop Sports Phys Ther. 2010;40(2):82–94. doi: 10.2519/jospt.2010.3025. [DOI] [PubMed] [Google Scholar]
- 43.Newman D. Kinesiology of the Musculoskeletal System: Foundations for Physical Rehabilitation. St Louis, MO: Mosby; 2002. [Google Scholar]
- 44.Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 45.Paterno MV, Ford KR, Myer GD, Heyl R, Hewett TE. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sport Med. 2007;17(4):258–262. doi: 10.1097/JSM.0b013e31804c77ea. [DOI] [PubMed] [Google Scholar]
- 46.Paterno MV, Myer GD, Ford KR, Hewett TE. Neuromuscular training improves single-limb stability in young female athletes. J Orthop Sports Phys Ther. 2004;34(6):305–316. doi: 10.2519/jospt.2004.34.6.305. [DOI] [PubMed] [Google Scholar]
- 47.Pinczewski LA, Lyman J, Salmon LJ, Russell VJ, Roe J, Linklater J. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. Am J Sports Med. 2007;35(4):564–574. doi: 10.1177/0363546506296042. [DOI] [PubMed] [Google Scholar]
- 48.Rauh MJ, Macera CA, Ji M, Wiksten DL. Subsequent injury patterns in girls’ high school sports. J Athl Train. 2007;42(4):486–494. [PMC free article] [PubMed] [Google Scholar]
- 49.Salmon L, Russell V, Musgrove T, Pinczewski L, Refshauge K. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(8):948–957. doi: 10.1016/j.arthro.2005.04.110. [DOI] [PubMed] [Google Scholar]
- 50.Schmitz RJ, Shultz SJ, Nguyen AD. Dynamic valgus alignment and functional strength in males and females during maturation. J Athl Train. 2009;44(1):26–32. doi: 10.4085/1062-6050-44.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37(2):246–251. doi: 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- 52.Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12–19. doi: 10.2519/jospt.2009.2885. [DOI] [PubMed] [Google Scholar]
- 53.Souza RB, Powers CM. Predictors of hip internal rotation during running: an evaluation of hip strength and femoral structure in women with and without patellofemoral pain. Am J Sports Med. 2009;37(3):579–587. doi: 10.1177/0363546508326711. [DOI] [PubMed] [Google Scholar]
- 54.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilk KE, Arrigo C, Andrews JR, Clancy WG. Rehabilitation after anterior cruciate ligament reconstruction in the female athlete. J Athl Train. 1999;34(2):177–193. [PMC free article] [PubMed] [Google Scholar]
- 56.Willson JD, Ireland ML, Davis I. Core strength and lower extremity alignment during single leg squats. Med Sci Sports Exerc. 2006;38(5):945–952. doi: 10.1249/01.mss.0000218140.05074.fa. [DOI] [PubMed] [Google Scholar]
- 57.Winter D. Biomechanics and Motor Control of Human Movement. New York, NY: John Wiley; 1990. [Google Scholar]
- 58.Wright RW, Dunn WR, Amendola A, et al. Risk of tearing the intact anterior cruciate ligament in the contralateral knee and rupturing the anterior cruciate ligament graft during the first 2 years after anterior cruciate ligament reconstruction: a prospective MOON cohort study. Am J Sports Med. 2007;35(7):1131–1134. doi: 10.1177/0363546507301318. [DOI] [PubMed] [Google Scholar]
- 59.Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. Deficits in neuromuscular control of the trunk predict knee injury risk: a prospective biomechanical-epidemiologic study. Am J Sports Med. 2007;35(7):1123–1130. doi: 10.1177/0363546507301585. [DOI] [PubMed] [Google Scholar]
- 60.Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. The effects of core proprioception on knee injury: a prospective biomechanical-epidemiogical study. Am J Sports Med. 2007;35(3):368–373. doi: 10.1177/0363546506297909. [DOI] [PubMed] [Google Scholar]
- 61.Zech A, Hubscher M, Vogt L, Banzer W, Hansel F, Pfeifer K. Neuromuscular training for rehabilitation of sports injuries: a systematic review. Med Sci Sports Exerc. 2009 Sep 2; doi: 10.1249/MSS.0b013e3181a3cf0d. Epub ahead of print. [DOI] [PubMed] [Google Scholar]